Abstract
Cold seeps and hydrothermal vents are deep-sea reducing environments that are characterized by lacking oxygen and photosynthesis-derived nutrients. Most animals acquire nutrition in cold seeps or hydrothermal vents by maintaining epi- or endosymbiotic relationship with chemoautotrophic microorganisms. Although several seep- and vent-dwelling animals hosting symbiotic microbes have been well-studied, the genomic basis of adaptation to deep-sea reducing environment in nonsymbiotic animals is still lacking. Here, we report a high-quality genome of Chiridota heheva Pawson & Vance, 2004, which thrives by extracting organic components from sediment detritus and suspended material, as a reference for nonsymbiotic animal’s adaptation to deep-sea reducing environments. The expansion of the aerolysin-like protein family in C. heheva compared with other echinoderms might be involved in the disintegration of microbes during digestion. Moreover, several hypoxia-related genes (Pyruvate Kinase M2, PKM2; Phospholysine Phosphohistidine Inorganic Pyrophosphate Phosphatase, LHPP; Poly(A)-specific Ribonuclease Subunit PAN2, PAN2; and Ribosomal RNA Processing 9, RRP9) were subject to positive selection in the genome of C. heheva, which contributes to their adaptation to hypoxic environments.
Similar content being viewed by others
Introduction
Echinodermata is a phylum of marine animals comprising 5 extant classes, including Holothuroidea (feather star, subphylum Pelmatozoa), Asteriodea and Ophiuroidea (starfish and brittle star, subphylum Asterozoa), and Echinoidea and Holothuroidea (sea urchin and sea cucumber, subphylum Echinozoa)1. Adult echinoderms are characterized by having a body showing pentameral symmetry, a water vascular system with external tube feet (podia), and an endoskeleton consisting of calcareous ossicles2. Echinoderms exhibit a high divergence in morphology, from the star-like architecture in Asteroidea to the worm-like architecture in Holothuroidea3,4.
Compared with other echinoderms, holothurians have a unique body architecture and evolutionary history. The worm-like body of the holothurian preserves the pentameral symmetry structurally along the oral–aboral axis5. In addition, holothurians have a soft and stretchable body, in which the ossicles are greatly reduced in size2. The order Apodida is a group of holothurians that are found in both shallow-water and deep-sea environments6. Phylogenetic analyses showed that Apodida is sister to other orders of Holothuroidea7,8. Apodid holothurians lack tube feet and complex respiratory trees, making them morphologically distinct from other holothurians2. In contrast to other classes of Echinodermata, which experienced a severe evolutionary bottleneck during the Permian-Triassic mass extinction interval, Holothuroidea did not experience family-level extinction through the interval. The deposit-feeding lifestyle of holothurians conferred a selective advantage during the primary productivity collapse of the Permian–Triassic mass-extinction9. As the genomes of only two shallow-water holothurians (Apostichopus japonicus and Parastichopus parvimensis) have been assembled and analyzed10,11,12, it is critical to study the genomes of more holothurians to dissect their special morphological characteristics and evolutionary history.
Cold seeps are areas where methane, hydrogen sulfide, and other hydrocarbons seep or emanate as gas from deep geologic sources13. Hydrocarbon-fluid seepage from cold seeps is completely devoid of O2 and comprises high levels of sulfides. After reacting with sulfides contained in the fluid, any free O2 is removed from the deep-sea water. Thus, cold seeps are characterized by high hydrostatic pressure, low temperature, lack of oxygen, and photosynthesis-derived nutrients, and high concentrations of reducing chemicals14. Chemosynthetic microbes oxidize the reduced chemicals contained in the hydrocarbon fluids to produce energy and fix carbon into organic matter, which supports a large amount of invertebrates, including tubeworms, mussels, clams, and gastropods15. Most of these macrobenthos depend on the epi- or endosymbiotic relationships with chemoautotrophic microorganisms for nutrition14,16,17. Recent genomic analyses have revealed the genetic basis of adaptation in several seep- and vent-dwelling macrobenthos hosting symbiotic bacteria18,19,20,21. However, the genomic basis of nutrient acquisition and hypoxic adaptation in cold seep-dwelling nonsymbiotic animals is still lacking with only one reported genome22.
Echinoderms are a rare component of deep-sea chemosynthetic ecosystems23. Chiridota heheva Pawson & Vance, 2004 (Apodida: Chiridotidae) is one of the few echinoderms that occupies all three types of chemosynthetic ecosystems (hydrothermal vent, cold seep, and organic fall)24. This suggests that the species is well adapted to deep-sea reducing environments. Unlike most cold seep- and hydrothermal vent-dwelling species, C. heheva does not host chemosynthetic bacteria6. It derives nutrients from a variety of sources, extracting organic components from sediment detritus, suspended material, and wood fragments when available6,25. The cosmopolitan distribution and special lifestyle of C. heheva make it an ideal model to study adaptation to deep-sea reducing environments in nonsymbiotic animals.
Here, we assembled and annotated a high-quality genome of C. heheva collected from the Haima cold seep in the South China Sea. Evolutionary analysis revealed that the ancestor of C. heheva diverged from the ancestors of two shallow-water holothurians (A. japonicus and P. parvimensis) approximately 429 Ma ago. Additionally, demographic analysis suggested that C. heheva might have colonized the current habitat in the early Miocene. Comparative genomic analysis showed that the aerolysin-like protein (ALP) family was significantly expanded in C. heheva compared with other echinoderms. The expansion of the ALP family might be involved in the disintegration of microbes during digestion, which in turn facilitated its adaptation to cold seep environments. Moreover, several hypoxia-related genes were subject to positive selection in the genome of C. heheva, which contributes to their adaptation to hypoxic environments.
Results and discussion
Characterization and genome assembly of C. heheva
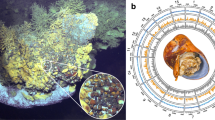
The sequenced sample was collected at a depth of 1385 meters using manned submersible Shenhaiyongshi from the Haima cold seep in the South China Sea (16° 73.228′ N, 110° 46.143′ E) (Fig. 1). We sequenced the sample’s genome on the Nanopore and Illumina sequencing platforms. In total, 42.43 Gb of Nanopore reads and 49.19 Gb of Illumina reads were obtained (Supplementary Tables 1 and 2). Species identity of the sequenced individual was first determined according to its morphological characteristics. In addition, we assembled the mitochondrial genome of the individual using Illumina reads. The sequence identity between the published C. heheva mitochondrial genome and our assembled genome was 99.74%, which confirmed the species identity of the sequenced individual26. Based on the k-mer distribution of Illumina reads, the size of the C. heheva genome was estimated to be 1.23 Gb with a high heterozygosity of 2% (Supplementary Fig. 1 and Supplementary Table 3). The C. heheva genome was assembled into 4609 contigs, with a total size of 1.107 Gb and contig N50 of 1.22 Mb (Table 1). We determined the completeness of the assembled genome by running benchmarking universal single-copy orthologs (BUSCO) and sequencing quality assessment tool (SQUAT) software. BUSCO analysis with metazoan (obd10) gene set showed that the assembled C. heheva genome contained 89.6% complete single-copy orthologs (Supplementary Table 4). Additionally, 91.1% of Illumina reads could be aligned to the assembled genome with high confidence in SQUAT assessment (Supplementary Table 5). These results indicate the high integrity of our assembled genome.
Genome annotation
Repetitive elements represented 624.38 Mb in the C. heheva genome assembly (Supplementary Table 6). Long interspersed nuclear elements (LINEs) were the largest class of annotated transposable elements (TEs), making up 9.72% of the genome. DNA transposons, which were the second largest class of TEs, represented 33.59 Mb (3.03%) of the genome. Additionally, the C. heheva genome comprised a large proportion (38.39%) of unclassified interspersed repeats. Comparative genomic analysis among C. heheva and other echinoderms revealed that the C. heheva genome consisted of the largest number of TEs (Fig. 2a, b; Supplementary Table 7). Repetitive elements constituted 56.40% of the C. heheva genome, and they accounted for 26.68% and 25.02% of the genomes of A. japonicus and P. parvimensis, respectively. The differences in the repeat content were close to the size differences between the genomes of C. heheva and the other two holothurians. This suggests that repeats contributed to the size differences among the genomes of these three holothurians. Notably, the proportion of LINEs in the C. heheva genome was substantially higher than that in the genomes of other echinoderms (Fig. 2b). Kimura distance-based copy-divergence analysis identified a recent expansion of LINEs in the C. heheva genome (Fig. 2c). Protein-coding genes were identified in the genome of C. heheva through a combination of ab initio and homology-based protein-prediction approaches. In total, we derived 36,527 gene models in the C. heheva genome. The structure of predicted genes in C. heheva is slightly different to that of other previously sequenced echinoderm genomes. With longer exons and introns, genes in C. heheva are longer than the ones in A. japonicus (Supplementary Table 8).
a Comparison of the occurrence and composition of repetitive elements in the genomes of 7 echinoderms. b Comparison of the proportion of repetitive elements, retrotransposon, and long interspersed nuclear elements (LINEs) in the genomes of 7 echinoderms. The proportions of repetitive elements and LINEs are higher in the genome of C. heheva than that in other echinoderms. c Transposable element-accumulation profile in C. heheva genome. A recent burst of LINEs was observed in C. heheva.
Phylogenomic analysis and demographic inference
With more than 1400 extant species, Holothuroidea is one of the largest classes in the phylum Echinodermata1. In addition, holothurians are well adapted to diverse marine environments, with habitats ranging from shallow intertidal areas to hadal trenches27,28. However, due to the lack of body fossils, evolutionary study of Holothuroidea is more difficult than other classes of Echinodermata. To investigate the evolutionary history of C. heheva, a maximum-likelihood (ML) phylogenetic tree was reconstructed using single-copy orthologs of C. heheva and 16 other deuterostomes (Supplementary Fig. 2). Chiridota heheva appeared sister to two other holothurians. In addition, divergence times were determined among 7 echinoderms that had whole-genome sequences (Fig. 3a). The divergence time of A. japonica and other echinoderms was estimated to be approximately 539 million years (Ma), which is generally consistent with the fossil records29,30. The ancestor of Chiridota heheva diverged from the ancestors of two shallow-water holothurians (A. japonicus and P. parvimensis) approximately 429 Ma ago. As Apodida is the basal taxon in Holothuroidea, these results support the view that holothurians had evolved by the Early Ordovician31.
a A species tree of 7 echinoderm species. In total, 988 single-copy orthologs were used to reconstruct the phylogenetic tree. The divergence time between species pairs was listed above each node, and 95% confidence interval of the estimated divergence time was denoted as blue bar. The numbers of protein families that were significantly expanded (red) and contracted (blue) (P < 0.05) in each species are denoted beside the species names. b Demographic history of C. heheva (blue) and A. japonicus (red). The changes of ancestral-population size of C. heheva and A. japonicus were inferred using the PSMC method. Time in history was estimated by assuming a generation time of 3 years and a mutation rate of 1.0 × 10−8.
To better investigate the evolution of holothurians, we inferred the histories of ancestral-population sizes of C. heheva and A. japonicus using the pairwise sequential Markovian coalescent (PSMC) method (Fig. 3b). Chiridota heheva experienced a decline in population size approximately 21 Ma ago. Ocean temperature increased slowly between the late Oligocene and early Miocene (21–27 Ma ago) after long-term cooling from the end of the Eocene32,33. Furthermore, species diversity within Echinodermata started to increase in the early Miocene34,35. These results indicate that C. heheva might have colonized the current habitat in the early Miocene. A decline in ancestral-population size in A. japonicus started in the late Miocene (approximately 8 Ma ago). Chiridota heheva also experienced a moderate decline in population size in the early Pliocene. Additionally, the oceans experienced a decrease in temperature during the late Miocene (7–5.4 Ma ago)36. These results suggest that global cooling and environmental changes in the late Miocene were an important driver of demographic changes in both shallow-water and deep-sea holothurians.
Hox/ParaHox gene clusters
Apodida do not have tube feet or complex respiratory trees, which are commonly found in other holothurians37. It has been demonstrated that Hox genes play a critical role in embryonic development38. In addition, previous studies proposed that the presence/absence and expression pattern of Hox genes might contribute to morphological patterning and embryonic development in echinoderms10,11. Therefore, to determine whether Hox genes contribute to morphological divergence in Holothuroidea, we identified Hox gene clusters and their evolutionary sister complex, the ParaHox gene cluster, in the genomes of C. heheva and 6 other echinoderms (Supplementary Fig. 3). A Hox cluster and a ParaHox cluster could be identified in the genomes of all 7 species. The gene composition and arrangement of both Hox and ParaHox clusters were highly consistent between the genomes of C. heheva and A. japonicus, suggesting that Hox/ParaHox genes do not control the development of tube feet and respiratory trees in Apodida. Hox4 and Hox6 were missing in the genomes of both C. heheva and A. japonicus, which is inconsistent with the view that the loss of Hox4 or Hox6 in echinoderms is a lineage-specific event5.
NLR repertoire in C. heheva
NACHT and leukine-rich, repeat-containing proteins (NLRs) are important components of pathogen-recognition receptors (PRRs) involved in animal innate immune systems, which can perceive pathogen-associated molecular patterns (PAMPs) of viruses and bacteria39. The bona fide NLRs contain a NACHT (NAIP, CIITA, HET-E, and TP1) domain, which belongs to the signal transduction ATPases with numerous domain (STAND) superfamilies, and a series of C-terminal leukine-rich repeats (LRRs)40,41. The Pfam hidden Markov model (HMM) search combined with phylogenetic analysis approach identified only 53 NLRs in C. heheva (Supplementary Table 9), compared with a largely expanded set of 203 NLRs in purple-sea urchin (Strongylocentrotus purpuratus)42. Chiridota heheva contained 24 NLRs with one or more N-terminal death/DED domain, 22 NACHT-only NLRs, 6 NLRs with other domains, including the immunoglobulin V-set domain, which was not identified in sea-urchin NLRs, and only one NLR with LRRs (Supplementary Table 9). Taken together, these results indicate that the C. heheva NLR repertoire shows different abundances and structural complexities than the sea urchin.
We performed phylogenetic analysis of C. heheva NLRs and other representative NLRs of metazoans, including humans, Amphimedon queenslandica, S. purpuratus, Acropora digitifera, Nematostella vectensis, Pinctada fucata, Capitella teleta, mollusks, and arthropods43. We found that the majority of C. heheva NLRs form a monophyletic lineage with sea-urchin NLRs (Fig. 4), supporting the lineage-specific evolution of NLRs in Echinodermata44. Given that human IPAF (ice protease-activating factor) and NAIP (neuronal apoptosis-inhibitory protein) proteins were reported to have originated before the evolution of vertebrates44, one C. heheva NLR clustering with these two proteins indicates that this NLR may have an ancient independent origin (Fig. 4).
The unrooted phylogenetic tree was reconstructed based on the NACHT-domain sequences using a maximum-likelihood method. The values near the nodes are ultrafast bootstrap (UFBoot) values. NLRs from different types of species are highlighted by branches of different colors. The species name is shown near the corresponding lineage.
Gene-family evolution
We performed gene-family analysis based on the phylogenetic tree of 7 echinoderms (Fig. 3a). Compared with other echinoderms, 66 gene families were expanded, and 25 gene families were contracted in C. heheva (P < 0.05) (Supplementary Data 1 and Supplementary Table 10). Several significantly expanded gene families are involved in the processes of cell cycle progression, protein folding, and ribosome assembly. As high hydrostatic pressure causes cell cycle delay and affects protein folding45,46, expansion of these families may have contributed to the adaptation of C. heheva to cold seep environments.
Aerolysins, which are pore-forming toxins (PFTs), were first characterized as virulence factors in the pathogenic bacterium Aeromonas hydrophyla47,48. As typical pore-forming proteins, aerolysin and related proteins are found in a large variety of species and possess diverse functions49. ALPs in eukaryotes originated from recurrent horizontal gene transfer (HGT)50. ALPs of the same origin have similar functions, while the ones of different origins possess diverse functions50. The ALPs were significantly expanded in the genome of C. heheva (7 copies) compared with other echinoderms (0 or 1 copy) (P < 0.05) (Supplementary Data 1). To investigate the possible origin and function of C. heheva ALPs, we reconstructed the phylogenetic tree of ALPs in echinoderms and diverse species. Chiridota heheva ALPs did not cluster with ALPs from other echinoderms. Additionally, these two groups of ALPs were clustered with aerolysins from distinct groups of bacteria (Fig. 5). This suggests that ALPs from C. heheva and other echinoderms have different origins. Chiridota heheva ALPs form a clade with ALPs from stony corals (Stylophora pistillata, Pocillopora damicornis, and Orbicella faveolata) and sea anemones (Nematostella vectensis and Ecaiptasia diaphana). This indicates that ALPs from C. heheva, stony corals, and sea anemones might have the same origin and similar biological functions. It was shown that ALPs from hydra and sea anemones (N. vectensis) are involved in prey disintegration after predation by lysing cells through pore formation on membranes50,51. The microbial communities of cold seeps are very different from those of other seafloor ecosystems52. Moreover, some of these microbes have unique cellular structures that might be difficult to disintegrate53, which impedes nutrient acquisition of C. heheva from free-living microbes of cold seeps. Therefore, the expansion of the ALP family might have contributed to microbe digestion in C. heheva, which in turn facilitated its adaptation to cold seep environments.
The unrooted phylogenetic tree was reconstructed using a maximum-likelihood method. The values near the nodes are ultrafast bootstrap (UFBoot) values. ALPs from different types of species are highlighted by branches of different colors. The species name is shown near the corresponding lineage. ALPs from C. heheva do not cluster with ALPs from other echinoderms (A. japonicus, P. parvimensis), but with the ones from sea anemones (N. vectensis, E. diaphana).
Positively selected genes
To better understand the genetic basis of its adaptation to a deep-sea reducing environment, we searched for positively selected genes (PSGs) in C. heheva. Compared with 6 other echinoderms, 27 PSGs were identified in the C. heheva genome (Supplementary Table 11). Four hypoxia-related genes (pyruvate kinase M2, PKM2; phospholysine phosphohistidine inorganic pyrophosphate phosphatase, LHPP; poly(A)-specific ribonuclease subunit PAN2, PAN2; and ribosomal RNA processing 9, RRP9) were identified as PSGs in C. heheva54,55,56,57. PKM2 promotes transactivation of HIF-1 target genes by directly interacting with the HIF-1α subunit. In addition, the transcription of the PKM2 gene is activated by HIF-1. This positive-feedback loop increases glycolysis and lactate production and decreases oxygen consumption under hypoxic conditions54. LHPP interacts with PKM2 to induce ubiquitin-mediated degradation of PKM2 and impede the glycolysis and respiration under hypoxia55. Thus, selection against these two interacting genes (PKM2 and LHPP) might play a key role in the hypoxic adaptation in C. heheva. Interestingly, the LHPP was also subject to positive selection in cetaceans, which are hypoxia-tolerant mammals58. Furthermore, both C. heheva and cetaceans have the same amino acid substitution at position 118 of the LHPP protein (Fig. 6), which indicates a possible convergent evolution in the LHPP during the adaptation of cetaceans and C. heheva to hypoxic environments. A positively charged amino acid (histidine, H) in two shallow-water holothurians is substituted to a negatively charged one (aspartic acid, D) in C. heheva at this position, which might cause a conformation change that contributes to the hypoxic adaptation in C. heheva. To study the potential underlying structural effects of this substitution, we predicted the three-dimensional structures of LHPP from echinoderms (Supplementary Fig. 4). The substitution, which is located in an α-helix, does not change the conformation of LHPP. The effect of the substitution of the LHPP protein needs to be further investigated.
A large number of metazoans reside in cold seeps and hydrothermal vents, which are challenging environments with high concentration of toxic compounds and chronic hypoxia59,60. Several physiological and molecular modifications of the respiratory system have been identified to cope with hypoxia in organisms living in these environments60. The concentration of hemoglobin, which is an oxygen-binding protein, is higher in seep- and vent-dwelling species than closely related species living in well-oxygenated environments60. In addition, hemoglobins from deep-sea organisms have higher affinity for oxygen than hemoglobins from shallow-water relatives61. This facilitates seep- and vent-dwelling species to thrive in the extreme environments by improving the efficiency of oxygen transportation. Four hypoxia-related genes (PKM2, PAN2, LHPP, and RRP9) were identified to be positively selected in C. heheva. PKM2 increases glycolysis and decreases oxygen consumption by promoting transactivation of HIF-1 target genes through directly interacting with the HIF-1α subunit under hypoxic conditions54. This suggests that animals living in deep-sea chemosynthetic environments might also adapt to hypoxic conditions through reprogramming glucose metabolism. Intriguingly, LHPP gene was subjected to positive selection in both C. heheva and cetaceans. This indicates a possible convergent evolution, in which echinoderms and mammals utilize similar strategies to cope with hypoxic challenges.
Methods
Sample collection and genome sequencing
The C. heheva sample used in this study was collected using manned submersible Shenhaiyongshi from the Haima cold seep in the South China Sea (16° 73.228′ N, 110° 46.143′ E, 1385 m deep) on August 2, 2019. The C. heheva individuals were kept in an enclosed sample chamber placed in the sample basket of the submersible. Once the samples were brought to the upper deck of the mothership, the muscle of the individuals was dissected, cut into small pieces, and immediately stored at −80 °C. The samples were then transported to Sun Yat-sen University on dry ice and stored at −80 °C until use.
To construct Nanopore sequencing library, high-molecular-weight genomic DNA was prepared by the CTAB method. The quality and quantity of the DNA were measured via standard agarose-gel electrophoresis and with a Qubit 4.0 Fluorometer (Invitrogen). Sequencing library was constructed and sequenced by Nanopore PromethION platform (Oxford Nanopore Technologies). Additionally, DNA was extracted to construct Illumina sequencing library. The quality and quantity of the DNA were measured via standard agarose-gel electrophoresis and with a Qubit 2.0 Fluorometer (Invitrogen). Sequencing library was constructed and sequenced by Illumina Novaseq platform (Illumina).
Mitochondrial and nuclear genome assembly
Low-quality (reads with ≥10% unidentified nucleotide and/or ≥ 50% nucleotides having phred score < 5) and sequencing-adapter-contaminated Illumina reads were filtered and trimmed with Fastp (v0.21.0)62 to obtain high-quality Illumina reads, which were used in the following analyses. Mitochondrial genome of C. heheva was assembled using the two-step mode of mitoZ (v2.4)63 with the high-quality Illumina reads. The assembled genome was annotated using mitoZ (v2.4) with parameter “–clade Echinodermata”.
The size and heterozygosity of C. heheva genome were estimated using high-quality Illumina reads by k-mer frequency-distribution method. The number of k-mers and the peak depth of k-mer sizes at 17 was obtained using Jellyfish (v2.3.0)64 with the -C setting. Genome size was estimated based on the k-mer analysis as described previously65. The heterozygosity of C. heheva genome was determined by fitting the k-mer distribution of Arabidopsis thaliana using Kmerfreq implemented in SOAPdenovo2 (r242)66.
Low-quality Nanopore reads were filtered using custom Python script. Two draft-genome assemblies were generated using filtered Nanopore reads with Shasta (v0.4.0)67 and WTDBG2 (v2.5)68, respectively. The contigs of the two draft assemblies were subject to error correction using filtered Nanopore reads with Racon (v1.4.16)69 three times. The corrected contigs were then polished with high-quality Illumina reads with Pilon (v1.23)70 three times. The error-corrected contigs of Shasta assembly and WTDBG2 assembly were assembled into longer sequences using quickmerge (v0.3)71. The merged contigs were subject to error correction using filtered Nanopore reads with Racon three times, and then using high-quality Illumina reads with Pilon three times. As the heterozygosity of C. heheva genome is high, haplotypic duplications in the assembled genome were identified and removed using purge_dups (v1.2.3)72. The completeness and quality of the assembly was evaluated using BUSCO (v4.0.5)73 against the conserved Metazoa dataset (obd10), and SQUAT with high-quality Illumina reads74.
Genome annotation
Repetitive elements in the assembly were identified by de novo predictions using RepeatMasker (v4.1.0) (https://www.repeatmasker.org/). A de novo repeat library for C. heheva was built using RepeatModeler (v2.0.1)75. To identify repetitive elements, sequences from the C. heheva assembly were aligned to the de novo repeat library using RepeatMasker (v4.1.0). Additionally, repetitive elements in C. heheva genome assembly were identified by homology searches against known repeat databases using RepeatMasker (v4.1.0). A repeat landscape of C. heheva genome was obtained using an R script that was modified from https://github.com/ValentinaBoP/TransposableElements. To compare the proportion and composition of repetitive elements among the genomes of echinoderms, genome sequences of Strongylocentrotus purpuratus (GCA_000002235.3), Lytechinus variegatus (GCA_000239495.2), Acanthaster planci (GCA_001949165.1), and Anneissia japonica (GCA_011630105.1) were downloaded from NCBI. Genome sequence of Parastichopus parvimensis was downloaded from echinobase (http://bouzouki.bio.cs.cmu.edu/Echinobase/PpDownloads, retrieved September 2021). Repetitive elements in the genomes of these species were identified by homology searches against known repeat databases using RepeatMasker (v4.1.0). The proportion and composition of repetitive elements of Apostichopus japonicus was obtained from Li et al. (2018)11.
We applied a combination of homology-based and de novo predication methods to build consensus-gene models for the C. heheva genome assembly. For homology-based gene prediction, protein sequences of Helobdella robusta, Lytechinus variegatus, Strongylocentrotus purpuratus, Dimorphilus gyrociliatus, Apostichopus japonicus and Acanthaster planci were aligned to the C. heheva genome assembly using tblastn. The exon–intron structures then were determined according to the alignment results using GenomeThreader (v1.7.0)76. In addition, de novo gene prediction was performed using Augustus (v3.3.2)77, with the parameters obtained by training the software with protein sequences of Drosophila melanogaster and Parasteatoda tepidariorum. Two sets of gene models were integrated into a nonredundant consensus-gene set using EvidenceModeler (v1.1.1)78. To identify functions of the predicted proteins, we aligned the C. heheva protein models against NCBI NR, trEMBL, and SwissProt database using blastp (E-value threshold: 10−5), and against eggNOG database79 using eggNOG-Mapper80. In addition, KEGG annotation of the protein models was performed using GhostKOALA81.
Phylogenomic analysis
Protein sequences of 15 metazoan species (A. planci, S. purpuratus, Lytechinus variegatus, A. japonicus, Anneissia japonica, Saccoglossus kawalevskii, Branchiostoma floridae, Ciona intestinalis, Danio rerio, Gallus gallus, H. robusta, Mus musculus, Pelodiscus sinensis, Petromyzon marinus, and Xenopus laevis) proteins were downloaded from NCBI. Protein sequences of Parastichopus parvimensis were downloaded from Echinobase12. OrthoMCL (v2.0.9)82 was applied to determine and cluster gene families among these 16 metazoan species and C. heheva. Gene clusters with >100 gene copies in one or more species were removed. Single-copy othologs in each gene cluster were aligned using MAFFT (v7.310)83. The alignments were trimmed using ClipKit (v1.1.3)84 with “gappy” mode. The phylogenetic tree was reconstructed with the trimmed alignments using a maximum-likelihood method implemented in IQ-TREE2 (v2.1.2)85 with H. robusta as outgroup. The best-fit substitution model was selected by using ModelFinder algorithm86. Branch supports were assessed using the ultrafast bootstrap (UFBoot) approach with 1000 replicates87.
To estimate the divergent time among echinoderms, single-copy orthologs were identified among A. japonica, A. planci, A. japonicus, P. parvimensis, C. heheva, L. variegatus, and S. purpuratus after running OrthoMCL pipeline as mentioned above. Single-copy orthologs were aligned using MAFFT (v7.310), trimmed using ClipKit (v1.1.3) with ‘gappy’ mode, and concatenated using PhyloSuite (v1.2.2)88. Divergent time among 7 echinoderms were estimated using the concatenated alignment with MCMCtree module of the PAML package (v4.9)89. MCMCtree analysis was performed using the maximum-likelihood tree that was reconstructed by IQ-TREE2 as a guide tree and calibrated with the divergent time obtained from TimeTree database (minimum = 193 million years and soft maximum = 350 million years between L. variegatus and S. purpuratus)90.
Demographic inference of C. heheva and A. japonicus
Paired-end Illumina reads of A. japonicus were downloaded from NCBI SRA database11. The reads of A. japonicus were filtered and trimmed with fastp (v0.21.0). The Illumina clean reads of C. heheva and A. japonicus were aligned to the respective reference-genome assembly using BWA (v0.7.17)91 with “mem” function. Genetic variants were identified using Samtools (v1.9)92. Whole-genome consensus sequence was generated with the genetic variants using Samtools (v 1.9). PSMC (v0.6.5)93 was used to infer the demographic history of C. heheva and A. japonicus using the whole genome consensus sequences. The substitution mutations rate and generation time of C. heheva and A. japonicus was set to 1.0 × 10−8 and 3 years according to the previous study of A. planci94.
Homeobox gene analysis
Homeobox genes in C. heheva genome were identified by following the procedure described previously95. Homeodomain sequences, which were retrieved from HomeoDB database (http://homeodb.zoo.ox.ac.uk)96, were aligned to C. heheva genome assembly using tbalstn. Sequences of the candidate homeobox genes were extracted based on the alignment results. The extracted sequences were aligned against NCBI NR and HomeoDB database to classify the homeobox genes.
Identification of NOD-like receptors (NLRs) in C. heheva
We used HMMER (v3.1)97 to search against the proteome of C. heheva with the HMM profile of NACHT domain (PF05729) retrieved from Pfam 34.0 as the query and an e cutoff value of 0.01. Proteins identified by the HMM search were retrieved from the proteome and aligned with 964 representative proteins from eukaryotes and prokaryotes98, and other representative metazoan NLRs43 using hmmalign method implemented in HMMER (v3.1) based on the STAND NTPase domain. The alignment was refined by manual editing. The large-scale phylogenetic analysis was performed using an approximate maximum likelihood method implemented in FastTree99. Representative SWACOS and MalT NTPases were used as outgroups98. Significant hit clustering with metazoan NLRs was regarded as NLRs, and protein-domain organizations were annotated through hmmscan method implemented in HMMER (v3.1).
Phylogenetic analysis of Chiridota NLRs and other representative metazoan NLRs
To explore the evolutionary relationships among C. heheva NLRs and other representative metazoan NLRs, we reconstructed the phylogenetic tree of NLRs. The NACHT domains of C. heheva NLRs and representative metazoan NLRs were aligned using MAFFT (v7.310), and then refined by manual editing. The representative metazoan NLRs were chosen from literature43. The phylogenetic tree was reconstructed using a maximum-likelihood method implemented in IQ-TREE2 (v2.1.2). The best-fit substitution model was selected by using ModelFinder algorithm. Branch supports were assessed using the UFBoot approach with 1000 replicates.
Gene-family expansion and contraction analysis
r8s (v1.7)100 was applied to obtain the ultrametric tree of 7 echinoderm species, which is calibrated with the divergent time between A. planci and S. purpuratus (541 mya) obtained from TimeTree database. CAFÉ (v5)101 was applied to determine the significance of gene-family expansion and contraction among 7 echinoderm species based on the ultrametric tree and the gene clusters determined by OrthoMCL (v2.0.9). The divergence time reported by TimeTree database might not be precise as it is a consensus of divergence times estimated in previous studies. Therefore, we repeated the analysis twice, in which the divergence time between A. planci and S. purpuratus was set to 461 mya and 495 mya according to the previous studies, respectively102,103. All the three analyses had the same result.
We used HMMER (v3.3.2) to search against NCBI nonredundant protein database (accessed on July 2021) with the HMM profile of aerolysin domain (PF01117) retrieved from Pfam 34.0 as the query and a e cutoff value of 0.001. Proteins identified by the HMM search were retrieved and filtered for the ones that have less than 75 residues. The filtered proteins were aligned with aerolysin-like proteins (ALPs) from C. heheva, A. japonicas, and P. parvimensis using MAFFT (v7.310) and trimmed using ClipKit (v1.1.3) with ‘gappy’ mode. The phylogenetic tree was reconstructed using a maximum-likelihood method implemented in IQ-TREE2 (v2.1.2). The best-fit substitution model was selected by using ModelFinder algorithm. Branch supports were assessed using the UFBoot approach with 1000 replicates.
Identification and analysis of positively selected genes
Branch-site models implemented in the codeml module of the PAML package is widely used to identify positively selected genes (PSGs). Thus, we identified PSGs in the C. heheva genome within the single-copy orthologs among 7 echinoderm species, based on the branch-site models using GWideCodeML (v1.1)104. C. heheva was set as the ‘foreground’ phylogeny, and the other species were set as the ‘background’ phylogeny. An alternative branch site model (Model = 2, NSsites = 2, and fix_omega = 0) and a neutral branch site model (Model = 2, NSsites = 2, fix_omega = 1, and omega = 1) were tested. Genes with Bayesian empirical Bayes (BEB) sites > 90% and a corrected P-value < 0.1 were identified to have been subject to positive selection.
To investigate LHPP gene evolution, sequences of LHPP from 8 mammals (Odobenus rosmarus, Orcinus orca, Lipotes vexillifer, Tursiops truncates, Physeter catodon, Balaenoptera acutorostrata, Mus musculus, and Homo sapiens) and 7 echinoderms (A. japonica, A. planci, A. japonicus, P. parvimensis, C. heheva, L. variegatus, and S. purpuratus) were aligned using MAFFT (v7.310). To reconstruct the phylogenetic tree, OrthoMCL (v2.0.9)82 was applied to determine and cluster gene families among these 15 species. Gene clusters with >100 gene copies in one or more species were removed. Single-copy othologs in each gene cluster were aligned using MAFFT (v7.310)83. The alignments were trimmed using ClipKit (v1.1.3)84 with “gappy” mode. The phylogenetic tree was reconstructed with the trimmed alignments using a maximum-likelihood method implemented in IQ-TREE2 (v2.1.2)85. H. robusta was used as outgroup. The best-fit substitution model was selected by using ModelFinder algorithm86. The three-dimensional structure of a protein provides important information for understanding its biochemical function and interaction properties in molecular detail. In this study, the three-dimensional structure of four LHPP proteins from (O. orca, H. sapiens, A. japonicus and C. heheva) was generated through homology modeling using the SWISS-MODEL workspace (http://swissmodel.expasy.org/workspace/)105.
Statistics and reproducibility
Alpha levels of 0.05 were regarded as statistically significant throughout the study, unless otherwise specified.
Data availability
Raw reads and genome assembly are accessible in NCBI under BioProject number PRJNA752986. Assembled genome sequences are accessible under Whole Genome Shotgun project number JAIGNY000000000. Raw reads and genome assembly are also available at the CNGB Sequence Archive (CNSA) of China National GeneBank DataBase (CNGBdb) with accession number CNP0002134. The genome assembly, related annotation files, and source files for generating figures can be accessed through Figshare106 at https://doi.org/10.6084/m9.figshare.15302229.
Code availability
Custom script used in this study is available at Figshare106 (https://doi.org/10.6084/m9.figshare.15302229). Versions and parameters for other software packages used in this study are described in the reporting summary and elsewhere in the “Methods.”
References
Pawson, D. L. Phylum Echinodermata. Zootaxa 1668, 749–764 (2007).
Pechenik, J. A. Biology of the Invertebrates (McGraw-Hill, 2015).
Smith, A. B., Zamora, S. & Alvaro, J. J. The oldest echinoderm faunas from Gondwana show that echinoderm body plan diversification was rapid. Nat. Commun. 4, 1385 (2013).
Mooi, R. & David, B. Radial symmetry, the anterior/posterior axis, and Echinoderm Hox genes. Annu. Rev. Ecol. Evol. S 39, 43–62 (2008).
Li, Y. et al. Genomic insights of body plan transitions from bilateral to pentameral symmetry in Echinoderms. Commun. Biol. 3, 371 (2020).
Pawson, D. L. & Vance, D. J. Chirodota heheva, new species, from western Atlantic deep-sea cold seeps and anthropogenic habits (Echinodermata: Holothuroidea: Apodida). Zootaxa 534, 1–12 (2004).
Miller, A. K. et al. Molecular phylogeny of extant Holothuroidea (Echinodermata). Mol. Phylogenet Evol. 111, 110–131 (2017).
Lacey, K. M. J., McCormack, G. P., Keegan, B. F. & Powell, R. Phylogenetic relationships within the class holothuroidea, inferred from 18S rRNA gene data. Mar. Biol. 147, 1149–1154 (2005).
Twitchett, R. J. & Oji, T. Early Triassic recovery of echinoderms. C. R. Palevol. 4, 531–542 (2005).
Zhang, X. J. et al. The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biol. 15, e2003790 (2017).
Li, Y. L. et al. Sea cucumber genome provides insights into saponin biosynthesis and aestivation regulation. Cell Discov. 4, ARTN 29 (2018).
Arshinoff, B. I. et al. Echinobase: leveraging an extant model organism database to build a knowledgebase supporting research on the genomics and biology of echinoderms. Nucleic Acids Res., https://doi.org/10.1093/nar/gkab1005 (2021).
Suess, E. Marine cold seeps and their manifestations: geological control, biogeochemical criteria and environmental conditions. Int J. Earth Sci. 103, 1889–1916 (2014).
Levin, L. A. in Oceanography and Marine Biology (eds Gibson, R. J. A. & Gordon, J. D. M.) 11–56 (CRC Press, 2005).
Vanreusel, A. et al. Biodiversity of cold seep ecosystems along the European margins. Oceanography 22, 110–127 (2009).
Petersen, J. M. & Dubilier, N. Methanotrophic symbioses in marine invertebrates. Environ. Microbiol. Rep. 1, 319–335 (2009).
Van Dover, C. L., German, C. R., Speer, K. G., Parson, L. M. & Vrijenhoek, R. C. Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295, 1253–1257 (2002).
Li, Y. et al. Genomic adaptations to chemosymbiosis in the deep-sea seep-dwelling tubeworm Lamellibrachia luymesi. BMC Biol. 17, 91 (2019).
Sun, J. et al. Adaptation to deep-sea chemosynthetic environments as revealed by mussel genomes. Nat. Ecol. Evol. 1, 121 (2017).
Sun, Y. et al. Genomic signatures supporting the symbiosis and formation of chitinous tube in the deep-sea tubeworm Paraescarpia echinospica. Mol. Biol. Evol., https://doi.org/10.1093/molbev/msab203 (2021).
Sun, J. et al. The Scaly-foot Snail genome and implications for the origins of biomineralised armour. Nat. Commun. 11, 1657 (2020).
Liu, R. et al. De Novo genome assembly of Limpet Bathyacmaea lactea (Gastropoda: Pectinodontidae): the first reference genome of a deep-sea gastropod endemic to cold seeps. Genome Biol. Evol. 12, 905–910 (2020).
Tunnicliffe, V. The nature and origin of the modern hydrothermal vent fauna. Palaios 7, 338–350 (1992).
Thomas, E. A. et al. Chiridota heheva-the cosmopolitan holothurian. Mar. Biodivers. 50, 110 (2020).
Carney, R. S. Stable isotope trophic patterns in echinoderm megafauna in close proximity to and remote from Gulf of Mexico lower slope hydrocarbon seeps. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 1965–1971 (2010).
Sun, S., Sha, Z. & Xiao, N. The first two complete mitogenomes of the order Apodida from deep-sea chemoautotrophic environments: New insights into the gene rearrangement, origin and evolution of the deep-sea sea cucumbers. Comp. Biochem Physiol. Part D Genomics Proteomics 39, 100839 (2021).
Smirnov, A. V., Gebruk, A. V., Galkin, S. V. & Shank, T. New species of holothurian (Echinodermata: Holothuroidea) from hydrothermal vent habitats. J. Mar. Biol. Assoc. 80, 321–328 (2000).
Jamieson, A. The Hadal zone: life in the Deepest Ocean (Cambridge University Press, 2015).
Smith, A. B. in Echinoderm Phylogeny and Evolutionary Biology (eds Paul, C. R. C. & Smith, A. B.) 85–97 (Clarendon Press, 1988).
Bottjer, D. J., Davidson, E. H., Peterson, K. J. & Cameron, R. A. Paleogenomics of echinoderms. Science 314, 956–960 (2006).
Reich, M. The oldest synallactid sea cucumber (Echinodermata: Holothuroidea: Aspidochirotida). Palaeontol. Z. 84, 541–546 (2010).
Zachos, J., Flower, B. & Paul, H. Orbitally paced climate oscillations across the oligocene/miocene boundary. Nature 388, 567–570 (1997).
Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001).
Oyen, C. W. & Portell, R. W. Diversity patterns and biostratigraphy of Cenozoic echinoderms from Florida. Palaeogeogr. Palaeocl. 166, 193–218 (2001).
Kroh, A. Climate changes in the early to middle miocene of the central paratethys and the origin of its echinoderm fauna. Palaeogeogr. Palaeocl. 253, 169–207 (2007).
Herbert, T. D. et al. Late Miocene global cooling and the rise of modern ecosystems. Nat. Geosci. 9, 843–847 (2016).
Barnes, R. D. Invertebrate Zoology (Holt-Sauders International, 1982).
Pearson, J. C., Lemons, D. & McGinnis, W. Modulating Hox gene functions during animal body patterning. Nat. Rev. Genet. 6, 893–904 (2005).
Lange, C. et al. Defining the origins of the NOD-Like receptor system at the base of animal evolution. Mol. Biol. Evol. 28, 1687–1702 (2011).
Ausubel, F. M. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6, 973–979 (2005).
Leipe, D. D., Koonin, E. V. & Aravind, L. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J. Mol. Biol. 343, 1–28 (2004).
Hibino, T. et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol. 300, 349–365 (2006).
Yuen, B., Bayes, J. M. & Degnan, S. M. The characterization of sponge NLRs provides insight into the origin and evolution of this innate immune gene family in animals. Mol. Biol. Evol. 31, 106–120 (2014).
Zhang, Q., Zmasek, C. M. & Godzik, A. Domain architecture evolution of pattern-recognition receptors. Immunogenetics 62, 263–272 (2010).
George, V. T., Brooks, G. & Humphrey, T. C. Regulation of cell cycle and stress responses to hydrostatic pressure in fission yeast. Mol. Biol. Cell 18, 4168–4179 (2007).
Yancey, P. H. & Siebenaller, J. F. Co-evolution of proteins and solutions: protein adaptation versus cytoprotective micromolecules and their roles in marine organisms. J. Exp. Biol. 218, 1880–1896 (2015).
Dal Peraro, M. & van der Goot, F. G. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92 (2016).
Abrami, L., Fivaz, M. & van der Goot, F. G. Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol. 8, 168–172 (2000).
Szczesny, P. et al. Extending the aerolysin family: from bacteria to vertebrates. PLoS ONE 6, e20349 (2011).
Moran, Y., Fredman, D., Szczesny, P., Grynberg, M. & Technau, U. Recurrent horizontal transfer of bacterial toxin genes to eukaryotes. Mol. Biol. Evol. 29, 2223–2230 (2012).
Sher, D., Fishman, Y., Melamed-Book, N., Zhang, M. & Zlotkin, E. Osmotically driven prey disintegration in the gastrovascular cavity of the green hydra by a pore-forming protein. FASEB J. 22, 207–214 (2008).
Ruff, S. E. et al. Global dispersion and local diversification of the methane seep microbiome. Proc. Natl Acad. Sci. USA 112, 4015–4020 (2015).
Katayama, T. et al. Isolation of a member of the candidate phylum ‘Atribacteria’ reveals a unique cell membrane structure. Nat. Commun. 11, 6381 (2020).
Luo, W. B. et al. Pyruvate kinase M2 Is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011).
Chen, W. J. et al. LHPP impedes energy metabolism by inducing ubiquitin-mediated degradation of PKM2 in glioblastoma. Am. J. Cancer Res. 11, 1369–1390 (2021).
Bett, J. S. et al. The P-body component USP52/PAN2 is a novel regulator of HIF1A mRNA stability. Biochem J. 451, 185–194 (2013).
Benita, Y. et al. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 37, 4587–4602 (2009).
Tian, R. et al. Adaptive evolution of energy metabolism-related genes in hypoxia-tolerant mammals. Front. Genet. 8, 205 (2017).
Nakayama, N., Obata, H. & Gamo, T. Consumption of dissolved oxygen in the deep Japan Sea, giving a precise isotopic fractionation factor. Geophys. Res. Lett., 10.1029/2007GL029917 (2007).
Hourdez, S. & Lallier, F. H. Adaptations to hypoxia in hydrothermal-vent and cold-seep invertebrates. Rev. Environ. Sci. Biotechnol. 6, 143–159 (2007).
Hourdez, S. & Weber, R. E. Molecular and functional adaptations in deep-sea hemoglobins. J. Inorg. Biochem. 99, 130–141 (2005).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Meng, G., Li, Y., Yang, C. & Liu, S. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47, e63 (2019).
Marcais, G. & Kingsford, C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 27, 764–770 (2011).
Star, B. et al. The genome sequence of Atlantic cod reveals a unique immune system. Nature 477, 207–210 (2011).
Luo, R. et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1, 18 (2012).
Shafin, K. et al. Nanopore sequencing and the Shasta toolkit enable efficient de novo assembly of eleven human genomes. Nat. Biotechnol. 38, 1044–1053 (2020).
Ruan, J. & Li, H. Fast and accurate long-read assembly with wtdbg2. Nat. Methods 17, 155–158 (2020).
Vaser, R., Sovic, I., Nagarajan, N. & Sikic, M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 27, 737–746 (2017).
Walker, B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 9, e112963 (2014).
Chakraborty, M., Baldwin-Brown, J. G., Long, A. D. & Emerson, J. J. Contiguous and accurate de novo assembly of metazoan genomes with modest long read coverage. Nucleic Acids Res. 44, e147 (2016).
Guan, D. et al. Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics 36, 2896–2898 (2020).
Simao, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Yang, L. A., Chang, Y. J., Chen, S. H., Lin, C. Y. & Ho, J. M. SQUAT: a sequencing quality assessment tool for data quality assessments of genome assemblies. BMC Genomics 19, 238 (2019).
Flynn, J. M. et al. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl Acad. Sci. USA 117, 9451–9457 (2020).
Gremme, G., Brendel, V., Sparks, M. E. & Kurtz, S. Engineering a software tool for gene structure prediction in higher organisms. Inf. Softw. Tech. 47, 965–978 (2005).
Stanke, M., Schoffmann, O., Morgenstern, B. & Waack, S. Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinformatics 7, 62 (2006).
Haas, B. J. et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 9, R7 (2008).
Huerta-Cepas, J. et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314 (2019).
Huerta-Cepas, J. et al. Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Mol. Biol. Evol. 34, 2115–2122 (2017).
Kanehisa, M., Sato, Y. & Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 428, 726–731 (2016).
Li, L., Stoeckert, C. J. Jr. & Roos, D. S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13, 2178–2189 (2003).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Steenwyk, J. L., Buida, T. J. 3rd, Li, Y., Shen, X. X. & Rokas, A. ClipKIT: a multiple sequence alignment trimming software for accurate phylogenomic inference. PLoS Biol. 18, e3001007 (2020).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 35, 518–522 (2018).
Zhang, D. et al. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 20, 348–355 (2020).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Kumar, S., Stecher, G., Suleski, M. & Hedges, S. B. TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 34, 1812–1819 (2017).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Li, H. & Durbin, R. Inference of human population history from individual whole-genome sequences. Nature 475, 493–496 (2011).
Hall, M. R. et al. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature 544, 231–234 (2017).
Marletaz, F., Peijnenburg, K. T. C. A., Goto, T., Satoh, N. & Rokhsar, D. S. A new Spiralian phylogeny places the enigmatic arrow worms among Gnathiferans. Curr. Biol. 29, 312 (2019). +.
Zhong, Y. F., Butts, T. & Holland, P. W. HomeoDB: a database of homeobox gene diversity. Evol. Dev. 10, 516–518 (2008).
Eddy, S. R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011).
Urbach, J. M. & Ausubel, F. M. The NBS-LRR architectures of plant R-proteins and metazoan NLRs evolved in independent events. Proc. Natl Acad. Sci. USA 114, 1063–1068 (2017).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
Sanderson, M. J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 (2003).
De Bie, T., Cristianini, N., Demuth, J. P. & Hahn, M. W. CAFE: a computational tool for the study of gene family evolution. Bioinformatics 22, 1269–1271 (2006).
Rouse, G. W. et al. Fixed, free, and fixed: The fickle phylogeny of extant Crinoidea (Echinodermata) and their Permian-Triassic origin. Mol. Phylogenet. Evol. 66, 161–181 (2013).
Peterson, K. J., Cotton, J. A., Gehling, J. G. & Pisani, D. The Ediacaran emergence of bilaterians: congruence between the genetic and the geological fossil records. Philos. Trans R. Soc. Lond. B Biol. Sci. 363, 1435–1443 (2008).
Macias, L. G., Barrio, E. & Toft, C. GWideCodeML: a python package for testing evolutionary hypotheses at the genome-wide level. G3 10, 4369–4372 (2020).
Bordoli, L. et al. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 4, 1–13 (2009).
Zhang, L. The genome of an apodid holothuroid (Chiridota heheva) provides insights into its adaptation to a deep-sea reducing environment, v8. Figshare, https://doi.org/10.6084/m9.figshare.15302229 (2022).
Acknowledgements
We thank Dr. Kang Ding and Dr. Zhimin Jian for leading the expedition of TS12-02, the crew of research vessel (R/V) Tansuoyihao, the pilot team of the manned submersible Shenhaiyongshi, and the onboard diving scientists for their technical support during the cruise. We gratefully acknowledge the National Supercomputing Center in Guangzhou for provision of computational resources. This study was supported by National Natural Science Foundation of China (No. 31900309), GuangDong Basic and Applied Basic Research Foundation (No. 2019A1515011644), Innovation Group Project of Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai) (No. 311021006), and National Innovation and Entrepreneurship Training Project for College Student of China (No. 20201126). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Author information
Authors and Affiliations
Contributions
M.W and J.G.H. conceived of the project and designed research; J.H. collected the sample; P.T., L.Z, Y.M., G.T., Q.C., and Q.Z. assembled and annotated the genome; L.Z., J.H., Z.G., M.W., S.Q., and H.-Y.Z. performed the evolutionary analyses; M.W., G.H., and J.G.H. wrote the paper with contribution from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Martin Schlegel, Saoirse Foley, and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary handling editor: Caitlin Karniski. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., He, J., Tan, P. et al. The genome of an apodid holothuroid (Chiridota heheva) provides insights into its adaptation to a deep-sea reducing environment. Commun Biol 5, 224 (2022). https://doi.org/10.1038/s42003-022-03176-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-022-03176-4
This article is cited by
-
Sea cucumbers: an emerging system in evo-devo
EvoDevo (2024)
-
New evidence for a cosmopolitan holothurian species at deep-sea reducing environments
Marine Biodiversity (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.