Abstract
We combined conventional evidence from longitudinal data in UK Biobank and genetic evidence from Mendelian randomization (MR) approach to infer the causality between sleep behaviors and fracture risk. We found that participants with insomnia showed 6.4% higher risk of fracture (hazard ratio [HR] = 1.064, 95% CI = 1.038–1.090, P = 7.84 × 10−7), falls and bone mineral density (BMD) mediated 24.6% and 10.6% of the intermediary effect; the MR analyses provided the consistent evidence. A U-shape relationship was observed between sleep duration and fracture risk (P < 0.001) with the lowest risk at sleeping 7–8 h per day. The excessive daytime sleepiness and “evening” chronotype were associated with fracture risk in observational study, but the association between chronotype and fracture did not show in MR analyses. We further generated a sleep risk score (SRS) with potential risk factors (i.e., insomnia, sleep duration, chronotype, and daytime sleepiness). We found that the risk of fracture increased with an increasing SRS (HR = 1.087, 95% CI = 1.065–1.111, P = 1.27 × 10−14). Moreover, 17.4% of the fracture cases would be removed if all participants exhibited a healthy sleep pattern. In conclusion, insomnia had a causal effect on fracture, falls had a larger intermediary effect than BMD in this association. Individuals with fracture risk could benefit from the intervention on unhealthy sleep pattern.
Similar content being viewed by others
Introduction
Globally, fracture is a primary cause of disability in adulthood, of which fragile fracture is the major type in the elderly1. In 2000, the estimated number of new osteoporotic fractures worldwide was 9.0 million for people aged 50 years or more, and Europe accounted for the highest number of fractures (34.8%)2. The hip fractures accounted for 18.2% (1.63 million) of all fractures2, and by 2050, the estimated number of new hip fractures worldwide will increase to 6.26 million cases3. According to the report of epidemiology and burden of osteoporosis in the 27 countries of the European Union (EU27), the healthcare cost of fragility fractures was estimated to reach €47 billion for people aged 50 years or more in 20254. Thus, the primary prevention of osteoporotic fractures is important to reduce the cost burden to society.
Both genetic and environmental factors contribute to the risk of fracture5. Several genome-wide association studies have identified dozens of genome-wide significant fracture loci6,7,8. All loci were associated with bone mineral density (BMD), suggesting that the lower BMD was the major risk factor for fracture6. Besides, environmental factors, such as smoking9, abdominal obesity10, and heavy drinking11, were also found to be associated with the increased fracture risk. Recently, increasing evidence from animal studies has put forward the importance of the circadian system and sleep in the process of bone resorption and formation12,13. For example, the clock gene-knockout rats, which cannot regulate the circadian rhythms, displayed abnormal bone mass13. Despite these findings, only several observational studies have investigated the relationship between sleep behaviors and fracture risk, but the results were conflicting. For instance, a recent cohort study from the Women’s Health Initiative observed that short sleep duration, but not long sleep duration, was associated with increased risk of fracture14. However, other studies found that long sleep duration15, self-reported napping15, nocturnal hypoxemia16, and premature awakening17 were associated with an increased risk of fracture. Besides, another study reported that insomnia was associated with the risk of falls, but not hip fracture18.
While there were some explanations for the controversial findings, unknown confounding factors in an observational study could be one reason. With the public availability of genetic data for sleep traits, the Mendelian randomization (MR) approach, which is a method that applies genetic variants as instrumental variables (IVs) for the exposure of interest, could strengthen the causal inference on the relationship between exposure and outcome (i.e., fracture)19, Since the genetic alleles are randomly assorted during conception, MR analyses are less susceptible to confounding factors19. Therefore, in this study, we first conducted a prospective observational study to investigate the relationship of insomnia, sleep duration, excessive daytime sleepiness, snoring, and chronotype with fracture risk within the UK Biobank dataset, then, with the summary GWAS data of fracture, we implemented MR approach to detect the causal relationship between these sleep traits and fracture risk. We finally developed a sleep risk score, which integrated the potential fracture-risk sleep traits, and examined the association between sleep risk score and fracture.
Results
Insomnia and fracture risk
After excluding participants with missing data on the main covariates at baseline (n = 23,688), 13,002 out of the 398,073 participants (3.27%) developed an incident fracture during a median of 7.96 years of follow-up (Supplementary Table 1). In primary analysis (model 0), an association was observed between insomnia and incident fracture, with a 6.4% higher risk (hazard ratio [HR]: 1.064, 95% confidence interval [95% CI]: 1.038–1.090, P = 7.84 × 10−7) (Fig. 1). Further adjustments for BMD (model 1) and falls (model 2) attenuated the estimated hazard ratios for the association between insomnia and the incidence of fracture (HR: 1.059, 95% CI: 1.033–1.086, P = 5.63 × 10−6 for model 1; HR: 1.041, 95% CI: 1.016–1.067, P = 0.001 for model 2) (Fig. 1). Based on the fully adjusted model (model 3), insomnia was associated with incident fracture, with a 3.7% higher risk (HR: 1.037, 95% CI: 1.012–1.063, P = 0.004) (Fig. 1). In addition, we conducted a series of analyses to assess the mediating role of several covariates (i.e., BMD and falls) on the observed associations. The results from the mediation analysis showed that 24.6% and 10.7% of the intermediary effect of insomnia on the risk of fracture was mediated by falls and BMD, respectively (Supplementary Table 2 and Supplementary Table 3). These results suggested that the mediating effect of falls was larger than BMD in the pathway between insomnia and fracture risk. Findings stratified by sex were consistent with the pooled results in model 0 (HR: 1.078, 95% CI: 1.037–1.121, P = 1.47 × 10−4 for male; HR: 1.049, 95% CI: 1.016–1.082, P = 0.004 for female) (Fig. 1). Besides, the PAR% for fracture was estimated as 11.3%, suggesting that 11.3% of the fracture cases could be removed if insomnia as a risk factor was removed.
The 95% confidence interval was presented in the error bar. Model 0 was adjusted for confounders, including age, sex, body mass index, education, smoking, alcohol consumption, physical activity, cognitive impairment, depression, and the use of glucocorticoid medication, benzodiazepines, and antidepressants; Model 1 = Model 0 + BMD; Model 2 = Model 0 + falls; Model 3 = Model 0 + BMD + falls. * the P-value of the intercept term. BMD bone mineral density, CI confidence interval, HR hazard ratio, IVW inverse-variance weighted, MR Mendelian randomization, MR-PRESSO MR pleiotropy residual sum and outlier, OR odds ratio.
In two-sample MR, genetically predicted insomnia was associated with increased risk of fracture (OR: 1.059, 95% CI: 1.028–1.090, P = 8.97 × 10−5) with the IVW approach. In the weighted median method, the magnitude of the causal association estimate was similar (Fig. 1). MR-Egger regression showed no evidence of directional pleiotropy (P for intercept = 0.711). After excluding one outlier, the causal association estimate was consistently using the MR-PRESSO test (OR: 1.056, 95% CI: 1.027–1.086, P = 1.94 × 10−4) (Fig. 1). The causality between insomnia and the incident fracture was replicated by one-sample MR analysis (HR: 1.061, 95% CI: 1.003–1.123, P = 0.040) (Fig. 1). Although evidence from two-sample MR analyses did not support the association between genetically predicted insomnia and BMD (OR: 1.015, 95% CI: 0.995–1.035, P = 0.146), there was a causal relationship between insomnia and risk of falls (OR: 1.014, 95% CI: 1.006–1.022, P = 0.001), and to some degree supported the hypothesis that the mediating effect of falls was larger than BMD. The bidirectional MR analysis did not suggest the effects of fracture on insomnia (OR: 0.960, 95% CI: 0.896–1.028, P = 0.244 for IVW random-effects model).
Sleep duration and fracture risk
In this prospective study, there was evidence of a U-shape association between sleep duration and fracture risk in model 0 (P < 0.001 for nonlinearity), with the lowest risk of fracture at 7–8 h per day of sleep duration (Fig. 2a, b and Supplementary Fig. 1a–d). Compared with those who slept 7 or 8 h per night, participants who were short (less than 7 h of sleep) and long sleepers (more than 8 h of sleep) had increased risk of fracture in model 0 (HR: 1.154, 95% CI: 1.109–1.202, P = 2.70 × 10−12; HR: 1.099, 95% CI:1.031–1.172, P = 0.004, respectively) (Table 1). We found that the effect of short or long sleep duration on fracture risk was attenuated by additionally adjusting for BMD and falls (model 1, model 2, and model 3) (Table 1). Falls and BMD were estimated to mediate 19.0% and 12.7% of the effect of sleep duration on fracture in observational analyses (Supplementary Table 4 and Supplementary Table 5). The results were consistent in the stratified analysis by sex (Supplementary Table 6). Interestingly, compared with participants who slept 7–8 h per night, short and long sleep duration were estimated to explain a similar percentage (5.20%) of the population risk of developing a fracture.
a Sleeping 7 h per day and b sleeping 8 h per day. Hazard ratios are indicated by blue solid lines and the 95% confidence intervals by blue shaded areas. The red line denotes the harzard ratio of one. In all these analyses, models were adjusted for risk factors for fracture, including age, sex, body mass index, education, smoking, alcohol consumption, physical activity, cognitive impairment, depression, and the use of glucocorticoid medication, benzodiazepines, and antidepressants.
In the two-sample MR, we found that genetically determined increased sleep duration was inversely associated with fracture risk (OR: 0.997, 95% CI: 0.995–0.999, P = 0.004) (Table 1). After excluding one outlier, similar findings were observed in MR-PRESSO test (OR: 0.997, 95% CI: 0.995–0.999, P = 0.009) (Table 1). The evidence from the MR-Egger regression also did not support the presence of directional pleiotropy (P = 0.229 for intercept). Furthermore, findings from two-sample MR, which genetically predicted sleep duration, were associated with a decreased risk of falls (OR: 0.999, 95% CI: 0.999–0.999, P < 0.001), but not with BMD (OR: 1.000, 95% CI: 0.998–1.002, P = 0.624). Consistently, the bidirectional MR analyses did not find evidence to support the effect of fracture on sleep duration (OR: 1.000, 95% CI: 0.967–1.034, P = 0.992 for IVW random-effect model).
Snoring, excessive daytime sleepiness, chronotype, and fracture risk
In the observational study (model 0), we found that snoring was associated with a decreased risk of fracture (HR: 0.951, 95% CI: 0.914–0.989, P = 0.012) (Supplementary Table 7). However, while digging into our data, we were aware that participants with snoring were less likely to have insomnia and abnormal sleep duration (Supplementary Table 8). Then, we conducted sensitivity analyses stratified by insomnia symptoms and sleep duration and found the participants free of insomnia and with normal sleep duration, and found that the association between snoring and fracture risk was not statistically significant (HR: 1.063, 95% CI: 0.965–1.171, P = 0.218) (Supplementary Table 7). Similarly, evidence from one-sample MR and two-sample MR did not support this association (OR: 1.214, 95% CI: 0.770–1.913, P = 0.402 for two-sample MR; HR: 2.134, 95% CI: 0.932–4.887, P = 0.073 for one-sample MR in model 1) (Supplementary Table 7). These results suggested that since participants with snoring were more likely in the low-risk group for other sleep factors (i.e., never/rarely had insomnia and had normal sleep duration), the observational estimates may not reflect the causal effects of snoring on fracture risk.
In multivariable Cox regression (model 0), we found statistically significant associations of daytime sleepiness with the risk of fracture (HR: 1.076, 95% CI: 1.039–1.113, P = 3.20 × 10−5), and being morning preference was a protective factor for fracture risk (HR: 0.963, 95% CI: 0.944–0.982, P = 1.79 × 10−4) (Supplementary Table 9). However, in the two-sample MR, there was no evidence of the association of chronotype (OR: 0.986, 95% CI: 0.954–1.020, P = 0.425), which was consistent with results from the one-sample MR (HR: 0.979, 95% CI: 0.916–1.047, P = 0.538) (Supplementary Table 9).
Multivariable MR analyses for sleep behaviors
In the multivariable MR analyses, where insomnia, sleep duration, snoring, and chronotypes were modeled, we found a direct effect of insomnia on fracture risk (OR: 1.055, 95% CI: 1.027–1.085, P = 1.36 × 10−4), and a negative direct effect of sleep duration (OR: 0.997, 95% CI: 0.995–0.999, P = 0.011), which is consistent with univariable MR results. These data, taken together, suggested independent causal effects of insomnia and sleep duration on fracture risk.
The sleep risk score and fracture risk
Finally, we included four potential risk factors (i.e., insomnia, sleep duration, chronotype, and daytime sleepiness) to develop a sleep risk score (SRS). Each participant received a score of 1 for each of the following sleep behaviors: insomnia (“sometimes” or “usually”), abnormal sleep duration (less than 7 h per day or more than 8 h per day), late chronotype (“evening” or “evening than morning”), and frequent daytime sleepiness (“often” or “all the time”) (Table 2). All these component scores were summed to generate an SRS, ranging from 0 to 4. The higher scores indicated poor sleep quality.
By generating the sleep risk score, we assessed the joint effect of sleep behaviors on fracture risk and found that the risk of fracture increased significantly with an increasing SRS (i.e., poor sleep quality) (HR: 1.087, 95% CI: 1.065–1.111, P = 1.27 × 10−14) (Fig. 3). For both vertebral and nonvertebral fracture, such association remained significant (HR: 1.152, 95% CI: 1.011–1.313, P-value = 0.033 for vertebral fracture; HR: 1.086, 95% CI: 1.063–1.110, P-value = 7.71 × 10−14 for nonvertebral fracture). When stratified by sex, the effect estimates of the association between sleep risk score and fracture risk in men were slightly larger than in women (HR: 1.101, 95% CI: 1.065–1.139, P = 2.01 × 10−8 for male; HR: 1.069, 95% CI: 1.040–1.099, P = 1.88 × 10−6 for female) (Fig. 3). Besides, the PAR% for fracture was estimated as 17.4%, suggesting that 17.4% of incident fracture cases in this study would be removed if all participants had been in healthy sleep behaviors.
The 95% confidence interval was presented in the error bar. Model 0 was adjusted for confounders, including age, education, sex, smoking, alcohol consumption, physical activity, body mass index, and the use of glucocorticoid, benzodiazepines, and antidepressants; Model 1 = Model 0 + BMD; Model 2 = Model 0 + falls; Model 3 = Model 0 + BMD + falls. BMD bone mineral density, CI confidence interval, IVW inverse-variance weighted, MR Mendelian randomization, MR-PRESSO MR pleiotropy residual sum and outlier, OR odds ratio.
Based on the baseline characteristics, participants with healthy sleep quality (i.e., lower sleep risk score) have a decreased risk of falls and higher BMD (Table 2). For example, in the individuals who had SRS = 0, 12.8% of them had falls, compared with 31.3% in SRS = 4 group (Table 2). After adjusting multiple covariates (i.e., model 0), we found that participants with poor sleep quality have an increased risk of falls and reduced BMD (OR: 1.254, 95% CI: 1.241–1.266, P-value < 2 × 10−16 for falls, and β-coefficient = −0.004, SE = 2.59 × 10−4, P-value < 2 × 10−16 for BMD). We then further included BMD and falls as mediators in the adjusted model and found that the magnitude of the associations of SRS with fracture risk was attenuated (HR: 1.054, 95% CI: 1.031–1.077, P = 2.24 × 10−6 for model 3) (Fig. 3). The mediation analyses showed that 18.2% and 12.2% of the intermediary effect of SRS on the risk of fracture was mediated by falls and BMD (Supplementary Table 10 and Supplementary Table 11).
Discussion
In this study, using a large-scale UK Biobank dataset, we investigated the association of five sleep behaviors (insomnia, sleep duration, snoring, daytime sleepiness, and chronotype) with fracture risk. Our findings suggest that unhealthy sleep patterns could result in a higher risk of fracture, and 17.4% of all fracture cases could be removed if all participants exhibited healthy sleep patterns. To some extent, falls had a larger intermediary effect than BMD in the association between sleep and fracture. Both the observational study and MR analyses consistently suggested a causal effect of insomnia on the fracture risk. Further, a U-shape relationship was observed between sleep duration and fracture risk, with the lowest risk of fracture at 7–8 h per day of sleep duration.
Previous observational studies examining the associations between insomnia and fracture risk were few, and existing evidence was inconsistent. For example, one study that included 34,163 nursing home residents found no association between insomnia and the risk of hip fracture (OR: 0.99, 95% CI: 0.77–1.26)18. However, due to the limited validity of the insomnia Minimum Data Set items used in the analysis, it is possible that their conclusion was prone to bias20. In contrast, the results from another cohort study in the Women’s Health Initiative (WHI) suggested an association between insomnia and an elevated risk of total fracture (HR: 1.03, 95% CI: 1.01–1.06) in multivariable models14, which agreed with our findings. Furthermore, using genetic variants associated with insomnia phenotype, we found strong evidence for the causal effect of insomnia on fracture risk.
Recent epidemiological studies have reported a J-shaped or reverse J-shaped association between sleep duration and fracture risk, and the evidence is not consistent. For example, a cohort study including 157,306 women found that, compared with subjects sleeping 7 h per night, short sleepers (≤5 h per night) had a 12% (95% CI: 5–20%) increased odds of all fractures, but no association was found between fracture risk and a long duration of sleep14. In contrast, in another population-based cohort study that included 8101 women, a long duration of sleep (but not short sleep time) was associated with an increased risk of nonspinal fractures (HR: 1.26, 95% CI: 1.00–1.58 for sleeping ≥10 h)15. In our study, the evidence from observational study suggested a U-shape association between sleep duration and fracture risk. Participants with a sleep duration of 7–8 h per day had the lowest fracture risk, suggesting that sleeping 7–8 h per day might be the appropriate sleep duration for preventing fracture. However, findings from two-sample MR supported a linear adverse effect of sleep duration (continuous trait as exposure) on the fracture risk. Due to the limited data21, we did not conduct the MR analyses while taking long/short duration (binary trait) as exposures. We found that, daytime sleepiness was associated with risk of fracture, but being morning preference was a protective factor for fracture risk in observational study. However, our MR analyses found no significant association between genetically determined chronotype and the incidence of fracture, but we cannot preclude weak genetic associations because of the limited statistical power in this study (i.e., 15.4%).
Furthermore, for snoring, a cohort study including 3220 women and 2699 men found no significant association between snoring with a high fracture risk. However, female participants in the severe-snoring group (6–7 nights per week or sleep disturbance by snoring in the next room) had an increased risk of fracture (HR: 1.682, 95% CI: 1.164–2.430, P = 0.006)22. Similarly, a prospective study that included 55,264 women in the Nurses’ Health Study (NHS) also found that participants with a history of obstructive sleep apnea, a sleep disorder associated with snoring23, were at a higher risk of vertebral fracture (HR: 1.88, 95% CI: 1.21–2.93)24. In our observational study, snoring participants rarely had insomnia and abnormal sleep duration. After excluding participants with insomnia or short/sleep duration, we did not find a significant association between snoring and the risk of any fracture. MR analysis results also did not support a causal role of snoring. Taken together, these data suggested that snoring may not be a significant modifiable risk factor for fracture. However, due to the limited data on snoring (e.g., the frequency of snoring), we could not assess the association between severe snoring and fracture risk.
Because sleep behaviors might affect each other25, for example, late chronotype and insomnia might result in shorter sleep duration and excessive daytime sleepiness, these sleep behaviors might individually act through different mechanisms, but could work synergistically to increase the risk of fracture. For instance, insomnia and abnormal sleep duration were relevant to the metabolic disruption (e.g., decreased melatonin secretion)26, and activated inflammation27, which might increase the risk of fracture28,29. Late chronotype had been linked to disrupted circadian rhythm30,31, and the deficiency of clock gene (e.g., BMAL1) in mice could result in a low bone mass32. Additionally, circadian disruption could also increase sleepiness and possibly adversely affect vigilance to environmental hazards33, which result in the increased risks of falls and fractures15. Therefore, we constructed a SRS to assess the overall relationship between the combination of sleep behaviors and fracture risk. We found that poor sleep quality was associated with an increased risk of fracture, and 17.4% of the fracture cases in this population would be removed if all participants exhibited a healthy sleep pattern. These data suggested the importance of careful consideration of sleep patterns, particularly insomnia, in fracture-risk assessment and prevention in clinical practice.
Given the associations of sleep disturbance with low bone mineral density (BMD)34 and the loss of postural control35, the potential relationship between sleep behavior and fracture risk might be caused by the mediating effect of either BMD-related or fall-related factors. In our adjusted models, when including BMD and falls as additional covariables, the magnitude of the associations between sleep behaviors and fracture risk would attenuate, with wider confidence intervals. We observed that a larger proportion of the effect of sleep on fracture risk was mediated by falls than by BMD (e.g., 24.6% vs 10.7% for insomnia). Also, estimates from MR analyses supported the causal relationship between several sleep behaviors (e.g., insomnia or sleep duration) and the risk of falls. Taken together, these results suggested the stronger mediating role of falls in the association between sleep and fracture risk.
Despite the comprehensive methodologies used in the analyses, this study had a few limitations. The population included in this study was exclusively European and of a modest age range (37–73 years), which may limit the generalizability of our findings to other races (e.g., Asian) and younger populations. Additionally, due to the sample overlap between the datasets used in the one-sample and two-sample MR analyses (e.g., snoring and sleep duration), one-sample MR results could not considered as independent replication of the two-sample MR results.
In summary, our observational and Mendelian randomization findings supported an association between poor sleep quality and an increased risk of fracture. The sleep risk score defined by our study (evening preference, sometimes or usually insomnia, abnormal sleep duration, and often or all-the-time daytime sleepiness) provided a frame of reference for identifying individuals at high risk of fracture. Among the five sleep behaviors, insomnia had a causal effect on fracture risk. Furthermore, fall prevention for reducing fracture risk in individuals with poor sleep quality could be more efficient than improving BMD.
Methods
Study design and data sources
Individual-level data
The overall study design is presented in Fig. 4. Briefly, we employed the individual-level data from the UK Biobank (Application 41376) as we used before36,37. In the UK Biobank dataset, information on more than 2000 traits, including sleep behaviors, fractures, and relevant confounding factors, was recorded through questionnaires and physical measurements. Participants were genotyped with UK Biobank Axiom Array, and genotype imputation was performed using the 1000 Genomes Project (Phase 3) reference panel38. Ethics approval for the UK Biobank research was obtained from the North West Multicentre Research Ethical Committee, and all participants provided informed consent. This study was performed under generic ethical approval obtained by UK Biobank from the National Health Service National Research Ethics Service (approval letter ref 12/NW/0382, 17 June 2011).
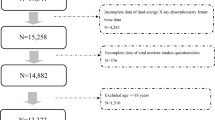
We excluded 30,486 non-European participants to minimize the population-stratification bias. Then, we excluded participants with potential comorbid diseases (i.e., rheumatoid arthritis, ulcerative colitis, multiple sclerosis, Crohn’s disease, hyperthyroidism, or lupus erythematosus) (N = 28,657). Additionally, since this study is a prospective design, we also excluded participants who had suffered a fracture before baseline, and participants with secondary fractures (i.e., participants with follow-up care involving removal of the fracture plate and other internal fixation devices, pathological fractures, and the fracture of the bone in neoplastic disease), leaving 421,761 participants in the observational study. In the one-sample MR analysis, we further excluded 139,599 participants (Field ID 22021) who had a kinship with other participants. Supplementary Data 1 listed the field ID and code for participants who were excluded in quality control.
Summary-level data and selection of instrumental variables
Single-nucleotide polymorphisms (SNPs) associated with the respective sleep behavior (chronotype39, insomnia40, sleep duration21, or snoring41) at a genome-wide significant (P < 5 × 10−8) level were selected from the largest genome-wide association studies (n = 697,828 for chronotype; n = 1,331,010 for insomnia; n = 446,118 for sleep duration; n = 408,317 for snoring) (Supplementary Table 12). To reduce the influence of linkage disequilibrium (LD) on MR analysis42, we extracted SNPs with the lowest P-value for the associated trait after clumping for LD at r2 < 0.01 in 250-kb regions. As a result, 268 SNPs were retained as instrumental variables for chronotype, 184 SNPs for insomnia, 72 SNPs for sleep duration, and 30 SNPs for snoring (Supplementary Data 2). By dividing the square of the SNP-exposure association estimate by the square of the corresponding SNP-exposure standard error43, we calculated the F-statistic for the SNP set used as instrumental variables for sleep-associated traits. We found that all instrumental variables used greatly exceed the criteria of strong instruments (F-statistic > 10), ranging from 26.9 to 220.8. Summary-level data for fracture (the outcome) were available from the GWAS that included 53,184 cases and 373,611 controls7. Additionally, genetic association estimates for BMD were obtained from a GWAS of 426,824 individuals7. We further used summary statistics for falls from a GWAS, including 361,194 participants of European ancestry (http://www.nealelab.is/uk-biobank/). For SNPs that were not available in the outcome datasets, we used proxy SNPs where available (i.e., genetic variants in LD with the corresponding SNPs at the threshold of r2 > 0.9) (Supplementary Data 2). In the bidirectional MR analyses, we had a total of 14 genetic variants as instruments for fracture, after clumping for linkage disequilibrium at r2 < 0.01 in 250-kb regions that reached genome-wide significance (P < 5 × 10−8)7.
Assessment of sleep behaviors in UK biobank and definition of sleep risk score
In the UK Biobank, participants reported five sleep behaviors: insomnia, sleep duration, snoring, daytime sleepiness, and chronotype (Supplementary Data 3). Insomnia symptoms were assessed in the question, “Do you have trouble falling asleep at night or do you wake up in the middle of the night?” with the three possible answers being “never/rarely”, “sometimes”, or “usually” or “prefer not to answer”, which we coded as 0, 1, and 2, respectively. Sleep duration was coded based on the number of reported hours using the following question: “About how many hours of sleep do you get in every 24 h? (including naps)”. Information on snoring was obtained by asking, “Does your partner or a close relative or friend complain about your snoring?” with responses being either “no” or “yes”. We derived a binary variable for snoring where “no self-reported snoring” and “having self-reported snoring” were coded as 0 and 1, respectively. To assess subjective daytime sleepiness, participants were asked, “How likely are you to doze off or fall asleep during the daytime when you don’t mean to? (e.g., when working, reading, or driving)” with responses being “never/rarely”, “sometimes”, “often”, or “all the time”. We derived an ordinal variable for subjective daytime sleepiness where “never/rarely”, “sometimes”, “often”, and “all the time” were coded as 0, 1, 2, and 3, respectively. Information on chronotype preference was collected by asking, “Do you consider yourself to be (i) definitely a ‘morning’ person, (ii) more of a ‘morning’ than an ‘evening’ person, (iii) more of an ‘evening’ than a ‘morning’ person, (iv) definitely an ‘evening’ person, (v) do not know, or (vi) prefer not to answer”? We derived a four-level ordinal variable for chronotype preference where “an ‘evening’ person”, “more of an ‘evening’ than a ‘morning’ person”, “more of a ‘morning’ than an ‘evening’ person”, and “a ‘morning’ person” were coded as 0, 1, 2, 3, respectively. For the above questions, participants who responded “Prefer not to answer” or “Do not know” were set to missing. We included the potential fracture-risk sleep factors to generate a sleep risk score (SRS). For each high-risk sleep behavior, the participant received a score of 1 if he or she met the criterion for high risk (see Results). If the participant did not meet the criterion, he or she was classified at low risk for that behavior, and received a score of 0. SRS was calculated as the sum of all these component scores, with higher scores indicating poor sleep quality.
Assessment of fracture in UK biobank
In this prospective study, the endpoint was the first diagnosis of any fracture (except secondary fracture). The fracture could be located at the skull and face, neck, vertebrae, ribs, sternum, and thoracic spine, shoulder and upper arm, spine, forearm, wrist, and hand, pelvic and thigh, femur, lower leg, foot except for the ankle, and other unspecified body regions. These cases were included from either questionnaire-based self-reported fractures or hospital-based fractures using the ICD-9 or ICD-10 codes (Supplementary Data 3).
Statistics and reproducibility
Observational study
In the prospective study of fracture risk, follow-up time was calculated from the date of attending the UK Biobank to the diagnosis of fracture, death, or the censoring date (31 March 2017). Five sleep behaviors (chronotype, insomnia, snoring, sleep duration, and excessive daytime sleepiness) and sleep risk scores were assessed in relation to the fracture risk using Cox regression. In the multivariable Cox regression, the basic model was adjusted for confounders, including age, sex, body mass index, education, smoking, alcohol consumption, physical activity, cognitive impairment, depression, and the use of glucocorticoid medication, benzodiazepines, and antidepressants (model 0), additional covariables such as BMD and falls were also included to set different models (mode1 1 = model 0 + BMD, model 2 = model 0 + falls, and model 3 = model 0 + BMD + falls). Detailed information on the corresponding covariates was provided in Supplementary Data 4.
Restricted cubic spline with five knots at 5th, 35th, 50th, 65th, and 95th centiles was used to model the potential nonlinear association of sleep duration with fracture risk. Assuming a causal relationship, we calculated proportional population-attributable risk (PAR%) to estimate the proportion of the fracture incidence in this population that would be eliminated if the exposure were eliminated. We then conducted mediation analyses to estimate the dimensionless proportion of the effect of sleep behaviors on fracture risk mediated by BMD and falls.
To account for multiple comparisons, a Bonferroni-corrected threshold of P < 0.01 (0.05/5 sleep behaviors) was considered to be statistically significant. Findings with P-values less than 0.05 but above the threshold of Bonferroni-corrected significance were considered to be suggestive evidence. All these statistical analyses were conducted in R version 4.0.2 (http://www.r-project.org/), and the PLINK2.0 software44.
One-sample MR study
In one-sample MR analyses, after extracting SNPs from the UK Biobank imputation dataset (Supplementary Data 2), weighted genetic risk score (wGRS) for four sleep behaviors (chronotype, insomnia, snoring, and sleep duration) was generated using the following equation (Eq. 1):
where SNPn was the dosage of the effective allele, βn was the effect estimate of SNPn for each sleep behavior obtained from the previous GWAS, and n was the number of instrumental variables (n = 268 for chronotype, 184 for insomnia, 72 for sleep duration, and 31 for snoring) (Supplementary Data 2). The wGRS method was performed using the PLNK2.0 software with the command of -score sum44.
After obtaining the sleep-behavior wGRS, we performed a multivariable Cox regression to obtain the population average causal hazard ratio using the prospective study of fracture risk, based on model 0.
Two-sample MR study
After extracting the genetic association estimates of the outcomes from summary-level data, ratio estimates for individual SNPs were calculated using the Wald estimator and Delta method45. Due to the presence of heterogeneity for each outcome (all P values for Cochran’s Q test < 0.05), the inverse-variance-weighted (IVW) method (under random-effect models) was further used to combine these estimates, thus obtaining the primary causal estimates45. In sensitivity analyses, to assess the influence of potential pleiotropy on the causal effect estimates, the weighted-median method, MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) test, and MR-Egger regression were conducted. Specifically, the weighted median method can provide valid estimates if more than 50% of the weights come from valid instrumental variables46. Additionally, the MR-PRESSO test can detect and correct for the influence of outliers on MR estimates47, and in MR-Egger regression, the P-value of the intercept term can be used as an indicator of directional pleiotropy (P-values less than 0.05 were considered statistically significant)48. To address the direction of causality, we further performed the bidirectional, after extracting the genetic association estimates for the sleep behaviors and fractures from GWASs of the corresponding phenotype7,21,39,40,41.
Multivariable MR analysis
Using the MendelianRandomization package, we conducted the multivariable MR analyses to find the independent effect of sleep behaviors on fracture risk. Briefly, we constructed instrumental variables by the combination of SNPs and their genetic association estimates from the GWASs for the sleep behaviors21,39,40,41. The genetic association estimates of these instrumental variables with other exposures (i.e., other sleep-related factors, except for the originally associated sleep behavior) and outcome (i.e., fracture) were obtained from the summary statistics from GWAS in the UK Biobank7,40.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The source values for Figs. 1 and 3 have been provided in Supplementary Data 5. The genetic summary statistics for BMD and fracture can be obtained from http://www.gefos.org/?q=content/data-release-2018, and the summary-level data for falls can be downloaded from www.nealelab.is/uk-biobank/. The individual-level genetic and phenotype data require the permission from the UK Biobank.
Code availability
The code is available from the corresponding author upon reasonable request.
References
Cauley, J. A. Public health impact of osteoporosis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 68, 1243–1251 (2013).
Johnell, O. & Kanis, J. A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17, 1726–1733 (2006).
Cooper, C., Campion, G. & Melton, L. J. 3rd Hip fractures in the elderly: a world-wide projection. Osteoporos. Int. 2, 285–289 (1992).
Hernlund, E. et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 8, 136 (2013).
Zhu, X., Bai, W. & Zheng, H. Twelve years of GWAS discoveries for osteoporosis and related traits: advances, challenges and applications. Bone Res. 9, 23 (2021).
Trajanoska, K. et al. Assessment of the genetic and clinical determinants of fracture risk: genome wide association and mendelian randomisation study. BMJ 362, k3225 (2018).
Morris, J. A. et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 51, 258–266 (2019).
Estrada, K. et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 44, 491–501 (2012).
Kanis, J. A. et al. Smoking and fracture risk: a meta-analysis. Osteoporos. Int. 16, 155–162 (2005).
Sadeghi, O., Saneei, P., Nasiri, M., Larijani, B. & Esmaillzadeh, A. Abdominal obesity and risk of hip fracture: a systematic review and meta-analysis of prospective studies. Adv. Nutr. 8, 728–738, https://doi.org/10.3945/an.117.015545 (2017).
Swayambunathan, J. et al. Incidence of hip fracture over 4 decades in the Framingham heart study. JAMA Intern. Med. https://doi.org/10.1001/jamainternmed.2020.2975 (2020).
Xu, C. et al. Circadian clock regulates bone resorption in mice. J. Bone Min. Res. 31, 1344–1355 (2016).
Fu, L., Patel, M. S., Bradley, A., Wagner, E. F. & Karsenty, G. The molecular clock mediates leptin-regulated bone formation. Cell 122, 803–815 (2005).
Cauley, J. A. et al. Characteristics of self-reported sleep and the risk of falls and fractures: the Women’s Health Initiative (WHI). J. Bone Miner. Res. 34, 464–474 (2019).
Stone, K. L. et al. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J. Am. Geriatrics Soc. 54, 1177–1183 (2006).
Cauley, J. A. et al. Hypoxia during sleep and the risk of falls and fractures in older men: the osteoporotic fractures in men sleep study. J. Am. Geriatr. Soc. 62, 1853–1859 (2014).
Holmberg, A. H. et al. Risk factors for hip fractures in a middle-aged population: a study of 33,000 men and women. Osteoporos. Int 16, 2185–2194 (2005).
Avidan, A. Y. et al. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J. Am. Geriatrics Soc. 53, 955–962 (2005).
Smith, G. D. & Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J. Epidemiol. 32, 1–22 (2003).
Martin, J. L. & Alessi, C. A. Limited validity of minimum data set items on sleep and hypnotic use in predicting falls and hip fracture in nursing home residents. J. Am. Geriatr. Soc. 54, 1150–1151; author reply 1152–1153, https://doi.org/10.1111/j.1532-5415.2006.00777.x (2006).
Dashti, H. S. et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat. Commun. 10, 1100 (2019).
Choi, S. B., Lyu, I. S., Lee, W. & Kim, D. W. Increased fragility fracture risk in Korean women who snore: a 10-year population-based prospective cohort study. BMC Musculoskelet. Disord. 18, 236 (2017).
Mattei, A., Tabbia, G. & Baldi, S. Diagnosis of sleep apnea. Minerva Med. 95, 213–231 (2004).
Huang, T., Tworoger, S. S., Redline, S., Curhan, G. C. & Paik, J. M. Obstructive sleep apnea and risk for incident vertebral and hip fracture in women. J. Bone Min. Res. 35, 2143–2150 (2020).
Fan, M. et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur. Heart J. 41, 1182–1189 (2020).
Blask, D. E. Melatonin, sleep disturbance and cancer risk. Sleep. Med. Rev. 13, 257–264 (2009).
Grandner, M. A. et al. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep 36, 769–779E (2013).
Frisher, M., Gibbons, N., Bashford, J., Chapman, S. & Weich, S. Melatonin, hypnotics and their association with fracture: a matched cohort study. Age Ageing 45, 801–806 (2016).
Ishii, S. et al. C-reactive protein, bone strength, and nine-year fracture risk: data from the Study of Women’s Health Across the Nation (SWAN). J. Bone Miner. Res. 28, 1688–1698 (2013).
Horne, J. A. & Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J. Chronobiol. 4, 97–110 (1976).
Yu, J. H. et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J. Clin. Endocrinol. Metab. 100, 1494–1502 (2015).
Samsa, W. E., Vasanji, A., Midura, R. J. & Kondratov, R. V. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone 84, 194–203 (2016).
Garbarino, S. et al. Professional shift-work drivers who adopt prophylactic naps can reduce the risk of car accidents during night work. Sleep 27, 1295–1302 (2004).
Ochs-Balcom, H. M. et al. Short sleep is associated with low bone mineral density and osteoporosis in the women’s health initiative. J. Bone Miner. Res. 35, 261–268 (2020).
Robillard, R., Prince, F., Boissonneault, M., Filipini, D. & Carrier, J. Effects of increased homeostatic sleep pressure on postural control and their modulation by attentional resources. Clin. Neurophysiol. 122, 1771–1778 (2011).
Bai, W. Y. et al. Identification of PIEZO1 polymorphisms for human bone mineral density. Bone 133, 115247 (2020).
Xia, J. et al. Systemic evaluation of the relationship between psoriasis, psoriatic arthritis and osteoporosis: observational and Mendelian randomisation study. Ann. Rheum. Dis. 79, 1460–1467 (2020).
Bycroft, C. et al. The UK biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Jones, S. E. et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat. Commun. 10, 343 (2019).
Jansen, P. R. et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat. Genet. 51, 394–403 (2019).
Campos, A. I. et al. Insights into the aetiology of snoring from observational and genetic investigations in the UK Biobank. Nat. Commun. 11, 817 (2020).
VanderWeele, T. J., Tchetgen Tchetgen, E. J., Cornelis, M. & Kraft, P. Methodological challenges in mendelian randomization. Epidemiology 25, 427–435 (2014).
Bowden, J. et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol. 45, 1961–1974 (2016).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J. Epidemiol. 44, 512–525 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos: 81871831 and 32061143019). We thank the GEnetic Factors for OSteoporosis (GEFOS) Consortium and the UK Biobank (Application 41376). We also thank the High-Performance Computing Center at Westlake University for the facility support and technical assistance.
Author information
Authors and Affiliations
Contributions
H.-F.Z. conceptualized and designed the study. Y.Q. and J.X. conducted analysis. Y.Q. drafted the paper, P.-K.C., G.-B.C., K.-Q.L., L.X., S.-Y.X. and S.K. reviewed and edited paper. All authors contributed, discussed and approved paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks Denis Baird and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary handling editors: Martina Rauner and George Inglis. Peer-reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qian, Y., Xia, J., Liu, KQ. et al. Observational and genetic evidence highlight the association of human sleep behaviors with the incidence of fracture. Commun Biol 4, 1339 (2021). https://doi.org/10.1038/s42003-021-02861-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02861-0
This article is cited by
-
Transcriptome-wide association study identifies new susceptibility genes and pathways for spondyloarthritis
Journal of Orthopaedic Surgery and Research (2023)
-
Both indirect maternal and direct fetal genetic effects reflect the observational relationship between higher birth weight and lower adult bone mass
BMC Medicine (2022)
-
Frequently reported adverse events of rebamipide compared to other drugs for peptic ulcer and gastroesophageal reflux disease
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.