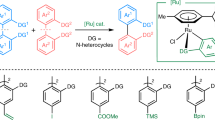
Abstract
Constructive functionalization of unstrained aryl–aryl bonds has been a fundamental challenge in organic synthesis due to the inertness of these bonds. Here we report a split cross-coupling strategy that allows twofold arylation with diverse aryl iodides through cleaving unstrained aryl–aryl bonds of common 2,2′-biphenols. The reaction is catalysed by a rhodium complex and promoted by a removable phosphinite directing group and an organic reductant such as tetrakis(dimethylamino)ethylene. The combined experimental and computational mechanistic studies reveal a turnover-limiting reductive elimination step that can be accelerated by a Lewis acid co-catalyst. The utility of this coupling method has been illustrated in the modular and simplified syntheses of unsymmetrical 2,6-diarylated phenols and skeletal insertion of phenylene units.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or Supplementary Information. Additional data are available from the corresponding authors upon request. Metrical parameters for the structure of 1i are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference number CCDC 2240576.
References
Torssell, K. G. B. Natural Product Chemistry (Wiley, 1983).
Zhu, J., Wang, J. & Dong, G. Catalytic activation of unstrained C(aryl)–C(aryl) bonds in 2,2′-biphenols. Nat. Chem. 11, 45–51 (2019).
Zhu, J. et al. Ruthenium-catalyzed reductive cleavage of unstrained aryl–aryl bonds: reaction development and mechanistic study. J. Am. Chem. Soc. 141, 18630–18640 (2019).
Sojdak, C. A. & Kozlowski, M. C. HAT catalysts to facilitate acid mediated cleavage of biaryl C–C bonds in binaphthols. Org. Lett. 24, 8326–8330 (2022).
Zhu, J., Zhang, R. & Dong, G. Orthogonal cross-coupling through intermolecular metathesis of unstrained C(aryl)–C(aryl) single bonds. Nat. Chem. 13, 836–842 (2021).
Onodera, S., Ishikawa, S., Kochi, T. & Kakiuchi, F. Direct alkenylation of allylbenzenes via chelation-assisted C–C bond cleavage. J. Am. Chem. Soc. 140, 9788–9792 (2018).
Onodera, S. et al. Catalytic, directed C–C bond functionalization of styrenes. J. Am. Chem. Soc. 142, 7345–7349 (2020).
Gozin, M., Weisman, A., Ben-David, Y. & Milstein, D. Activation of a carbon–carbon bond in solution by transition-metal insertion. Nature 364, 699–701 (1993).
Horton, D. A., Bourne, G. T. & Smythe, M. L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 103, 893–930 (2003).
Wu, J. M. & Kozlowski, C. Catalytic oxidative coupling of phenols and related compounds. ACS Catal. 12, 6532–6549 (2022).
Brown, A. G. & Edwards, P. D. Synthesis of analogues of the bafilomycin antibiotics. Tetrahedron Lett. 131, 6581–6584 (1990).
Egami, H. & Katsuki, T. Iron-catalyzed asymmetric aerobic oxidation: oxidative coupling of 2-naphthols. J. Am. Chem. Soc. 131, 6082–6083 (2009).
Guo, Q.-X. et al. Highly enantioselective oxidative couplings of 2-naphthols catalyzed by chiral bimetallic oxovanadium complexes with either oxygen or air as oxidant. J. Am. Chem. Soc. 129, 13927–13938 (2007).
Elsler, B., Schollmeyer, D., Dyballa, K. M., Franke, R. & Waldvogel, S. R. Metal‐ and reagent‐free highly selective anodic cross‐coupling reaction of phenols. Angew. Chem. Int. Ed. 53, 5210–5213 (2014).
Dermenci, A., Coe, J. W. & Dong, G. Direct activation of relatively unstrained carbon–carbon bonds in homogeneous systems. Org. Chem. Front. 1, 567–581 (2014).
Xia, Y. & Dong, G. Temporary or removable directing groups enable activation of unstrained C–C bonds. Nat. Rev. Chem. 4, 600–614 (2020).
Chen, F., Wang, T. & Jiao, N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev. 114, 8613–8661 (2014).
Song, F. et al. Catalytic activations of unstrained C–C bond involving organometallic intermediates. Chem. Soc. Rev. 47, 7078–7115 (2018).
van der Boom, M. E. & Milstein, D. Cyclometalated phosphine-based pincer complexes: mechanistic insight in catalysis, coordination, and bond activation. Chem. Rev. 103, 1759–1792 (2003).
Lewis, L. N. & Smith, J. F. Catalytic carbon–carbon bond formation via ortho-metalated complexes. J. Am. Chem. Soc. 108, 2728–2735 (1986).
Bedford, R. B. et al. The catalytic intermolecular orthoarylation of phenols. Angew. Chem. Int. Ed. 42, 112–114 (2003).
Schlosser, M., Mangano, G. & Leroux, F. The superbase-mediated pairwise substitution of the 2,2′- and 6,6′-positions in a biphenyl derivative. Eur. J. Org. Chem. 5, 1014–1017 (2004).
Wiberg, N. Tetraaminoäthylene als starke Elektronendonoren. Angew. Chem. Int. Ed. 7, 766–779 (1968).
Lyu, H. et al. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 372, 175–182 (2021).
Forrester, J. D., Zalkin, A. & Templeton, D. H. Crystal and molecular structure of indium(III) iodide (In2I6). Inorg. Chem. 3, 63–67 (1964).
Lin, D. et al. Structural derivatization strategies of natural phenols by semi-synthesis and total-synthesis. Nat. Prod. Bioprospect. 12, 8 (2022).
Youn, S. W. & Cho, C.-G. Transition-metal-catalyzed ortho-selective C–H functionalization reactions of free phenols. Org. Biomol. Chem. 19, 5028–5047 (2021).
Liu, Q.-S. et al. P(NMe2)3-promoted ortho-selective arylation of phenols with diaryliodonium triflates via rhodium catalysis. Tetrahedron 73, 3591–3595 (2017).
Gaster, E. et al. Significant enhancement in the efficiency and selectivity of iron-catalyzed oxidative cross-coupling of phenols by fluoroalcohols. Angew. Chem. Int. Ed. 54, 4198–4202 (2015).
Zhuravel, M. A. & Nguyen, S. T. Preparation of 3-aryl-substituted salicylaldehydes via Suzuki coupling. Tetrahedron Lett. 42, 7925–7928 (2001).
Bedford, R. B. et al. The catalytic ortho-arylation of tyrosine. Org. Biomol. Chem. 7, 3119–3127 (2009).
Yang, J.-F. et al. Ligand-accelerated direct C-H arylation of BINOL: a rapid one-step synthesis of racemic 3,3′-diaryl BINOLs. Angew. Chem. Int. Ed. 55, 14116–14120 (2016).
Correa, A. Metal-catalyzed C(sp2)–H functionalization processes of phenylalanine- and tyrosine-containing peptides. Eur. J. Inorg. Chem. 2928–2941 (2021).
Noisier, A. F. M. & Brimble, M. A. C–H functionalization in the synthesis of amino acids and peptides. Chem. Rev. 114, 8775–8806 (2014).
Chen, P.-H. et al. “Cut and sew” transformations via transition-metal-catalyzed carbon–carbon bond activation. ACS Catal. 7, 1340–1360 (2017).
Xue, Y. & Dong, G. Deconstructive synthesis of bridged and fused rings via transition-metal-catalyzed “cut-and-sew” reactions of benzocyclobutenones and cyclobutanones. Acc. Chem. Res. 55, 2341–2354 (2022).
Albrecht, M. & Schneider, M. Dinuclear triple-stranded helicates from rigid oligo-p-phenylene ligands: self-assembly and ligand self-recognition. Eur. J. Inorg. Chem. 1301–1306 (2002).
Acknowledgements
Financial supports from NIGMS (2R01GM109054) and the University of Chicago are acknowledged. Umicore AG & Co. KG is thanked for a generous donation of Rh salts. We are grateful for the support of the Research Computing Center at the University of Chicago for the calculation performed in this work. R. Zhang from the University of Chicago is thanked for checking the experimental procedures.
Author information
Authors and Affiliations
Contributions
C.Y. discovered the reaction. C.Y. and G.D. conceived the idea. C.Y. conducted the experimental investigation. Z.Z. conducted the DFT calculation. C.Y., Z.Z. and G.D. wrote the manuscript. G.D. directed the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, discussion, note, references, Figs. 1–14 and Tables 1–4.
Supplementary Data
.cif file of the X-ray structure of 1i.
Supplementary Data
Structure factors for the X-ray structure of 1i.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, C., Zhang, Z. & Dong, G. Split cross-coupling via Rh-catalysed activation of unstrained aryl–aryl bonds. Nat Catal 7, 432–440 (2024). https://doi.org/10.1038/s41929-024-01120-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01120-9