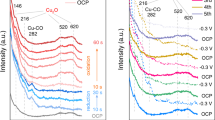
Abstract
Understanding metal surface reconstruction is of the utmost importance in electrocatalysis, as this phenomenon directly affects the nature of available active sites. However, its dynamic nature renders surface reconstruction notoriously difficult to study. Here, we report on the intermediates that drive the rearrangement of copper catalysts for the electrochemical CO2 reduction reaction (CO2RR). Online MS and UV–vis absorption spectroscopy data are consistent with a dissolution–redeposition process, as previously demonstrated by in situ electron microscopy. The data indicate that the soluble transient species contain copper in the +1 oxidation state. Density functional theory identifies copper–adsorbate complexes that can exist in solution under operating conditions. Copper carbonyls and oxalates are suggested as the major reaction-specific species driving copper reconstruction during CO2RR. This work motivates future methodological studies to enable the direct detection of these compounds and strategies that specifically target them to improve the catalyst operational stability.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated though DFT and analysed during the current study are available from the ioChem-BD database65 at https://doi.org/10.19061/iochem-bd-1-231. Experimental data are openly available in Zenodo at https://doi.org/10.5281/zenodo.6724739.
References
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Hahn, C. et al. Engineering Cu surfaces for the electrocatalytic conversion of CO2: controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl Acad. Sci. USA 114, 5918–5923 (2017).
Kim, Y. G., Baricuatro, J. H., Javier, A., Gregoire, J. M. & Soriaga, M. P. The evolution of the polycrystalline copper surface, first to Cu(111) and then to Cu(100), at a fixed CO2RR potential: a study by operando EC-STM. Langmuir 30, 15053–15056 (2014).
Manthiram, K., Beberwyck, B. J. & Alivisatos, A. P. Enhanced electrochemical methanation of carbon dioxide with a dispersible nanoscale copper catalyst. J. Am. Chem. Soc. 136, 13319–13325 (2014).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C 121, 12337–12344 (2017).
Kim, D., Kley, C. S., Li, Y. & Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl Acad. Sci. USA 114, 10560–10565 (2017).
Grosse, P. et al. Dynamic changes in the structure, chemical state and catalytic selectivity of Cu Nanocubes during CO2 electroreduction: size and support effects. Angew. Chem. 130, 6300–6305 (2018).
Huang, J. et al. Potential-induced nanoclustering of metallic catalysts during electrochemical CO2 reduction. Nat. Commun. 9, 3117–3126 (2018).
Osowiecki, W. T. et al. Factors and dynamics of Cu nanocrystal reconstruction under CO2 reduction. ACS Appl. Energy Mater. 2, 7744–7749 (2019).
Jung, H. et al. Electrochemical fragmentation of Cu2O nanoparticles enhancing selective C-C coupling from CO2 reduction reaction. J. Am. Chem. Soc. 141, 4624–4633 (2019).
Grosse, P. et al. Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nat. Commun. 12, 6736 (2021).
Vavra, J., Shen, T. H., Stoian, D., Tileli, V. & Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem. Int. Ed. 60, 1347–1354 (2021).
Hong Lee, S. et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ X-ray characterization. J. Am. Chem. Soc. 143, 588–592 (2021).
Phan, T. H. et al. Emergence of potential-controlled Cu-nanocuboids and graphene-covered Cu-nanocuboids under operando CO2 electroreduction. Nano Lett. 21, 2059–2065 (2021).
Speck, F. D. & Cherevko, S. Electrochemical copper dissolution: a benchmark for stable CO2 reduction on copper electrocatalysts. Electrochem. Commun. 115, 106739 (2020).
Simon, G. H., Kley, C. S. & Roldan Cuenya, B. Potential-dependent morphology of copper catalysts during CO2 electroreduction revealed by in situ atomic force microscopy. Angew. Chem. Int. Ed. 60, 2561–2568 (2021).
Raaijman, S. J., Arulmozhi, N. & Koper, M. T. M. Morphological stability of copper surfaces under reducing conditions. ACS Appl. Mater. Interfaces 13, 48730–48744 (2021).
Hochfilzer, D. et al. The importance of potential control for accurate studies of electrochemical CO reduction. ACS Energy Lett. 6, 1879–1885 (2021).
Li, Y. et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 17, 1312–1317 (2017).
Popovic, S., Bele, M. & Hodnik, N. Reconstruction of copper nanoparticles at electrochemical CO2 reduction reaction conditions occurs via two-step dissolution/redeposition mechanism. ChemElectroChem 8, 2634–2639 (2021).
McCafferty, E. in Introduction to Corrosion Science 95–117 (Springer, 2010).
Eren, B., Weatherup, R. S., Liakakos, N., Somorjai, G. A. & Salmeron, M. Dissociative carbon dioxide adsorption and morphological changes on Cu(100) and Cu(111) at ambient pressures. J. Am. Chem. Soc. 138, 8207–8211 (2016).
Eren, B. et al. Activation of Cu(111) surface by decomposition into nanoclusters driven by CO adsorption. Science 351, 475–478 (2016).
Xu, L. et al. Formation of active sites on transition metals through reaction-driven migration of surface atoms. Science 380, 70–76 (2023).
Amirbeigiarab, R. et al. Atomic-scale surface restructuring of copper electrodes under CO2 electroreduction conditions. Nat. Catal. 6, 837–846 (2023).
Li, Y. et al. Electrochemically scrambled nanocrystals are catalytically active for CO2-to-multicarbons. Proc. Natl Acad. Sci. USA 117, 9194–9201 (2020).
Yang, Y. et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023).
Kasian, O., Geiger, S., Mayrhofer, K. J. J. & Cherevko, S. Electrochemical on-line ICP-MS in electrocatalysis research. Chem. Rec. 19, 2130–2142 (2019).
Cherevko, S. Electrochemical dissolution of noble metals native oxides. J. Electroanal. Chem. 787, 11–13 (2017).
Armaroli, N. Photoactive mono- and polynuclear Cu(I)-phenanthrolines. A viable alternative to Ru(II)-polypyridines? Chem. Soc. Rev. 30, 113–124 (2001).
Wang, J. et al. A water-soluble Cu complex as molecular catalyst for electrocatalytic CO2 reduction on graphene-based electrodes. Adv. Energy Mater. 9, 1803151 (2019).
Soo, J. L., Doo, R. B., Won, S. H., Shim, S. L. & Jong, H. J. Different morphological organic-inorganic hybrid nanomaterials as fluorescent chemosensors and adsorbents for CuII ions. Eur. J. Inorg. Chem. 2008, 1559–1564 (2008).
Taki, M., Iyoshi, S., Ojida, A., Hamachi, I. & Yamamoto, Y. Development of highly sensitive fluorescent probes for detection of intracellular copper(I) in living systems. J. Am. Chem. Soc. 132, 5938–5939 (2010).
Smith, G. F. & McCurdy, W. H. Jr. 2,9-Dimethyl-1,10-phenanthroline. Anal. Chem. 24, 371–373 (1952).
Fishman, M., Zhuang, H. L., Mathew, K., Dirschka, W. & Hennig, R. G. Accuracy of exchange-correlation functionals and effect of solvation on the surface energy of copper. Phys. Rev. B Condens. Matter Mater. Phys. 87, 245402 (2013).
Velasco-Vélez, J. J. et al. The role of the copper oxidation state in the electrocatalytic reduction of CO2 into valuable hydrocarbons. ACS Sustain. Chem. Eng. 7, 1485–1492 (2019).
Dattila, F., Garcia-Muelas, R. & López, N. Active and selective ensembles in oxide-derived copper catalysts for CO2 reduction. ACS Energy Lett. 5, 3176–3184 (2020).
McCrum, I. T., Bondue, C. J. & Koper, M. T. M. Hydrogen-induced step-edge roughening of platinum electrode surfaces. J. Phys. Chem. Lett. 10, 6842–6849 (2019).
Hersbach, T. J. P. & Koper, M. T. M. Cathodic corrosion: 21st century insights into a 19th century phenomenon. Curr. Opin. Electrochem. 26, 100653 (2021).
Kreizer, I. V., Tutukina, N. M., Zartsyn, I. D. & Marshakov, I. K. The dissolution of a copper cathode in acidic chloride solutions. Prot. Met. 38, 226–232 (2002).
Kreizer, I. V., Marshakov, I. K., Tutukina, N. M. & Zartsyn, I. D. Partial reactions of copper dissolution under cathodic polarization in acidic media. Prot. Met. 40, 23–25 (2004).
Kreizer, V., Marshakov, I. K., Tutukina, N. M. & Zartsyn, I. D. The effect of oxygen on copper dissolution during cathodic polarization. Prot. Met. 39, 30–33 (2003).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Moradzaman, M. & Mul, G. In situ Raman study of potential-dependent surface adsorbed carbonate, CO, OH and C species on Cu electrodes during electrochemical reduction of CO2. ChemElectroChem 8, 1478–1485 (2021).
Calle-Vallejo, F., Martínez, J. I., García-Lastra, J. M., Sautet, P. & Loffreda, D. Fast prediction of adsorption properties for platinum nanocatalysts with generalized coordination numbers. Angew. Chem. Int. Ed. 53, 8316–8319 (2014).
Pasquali, M., Floriani, C. & Gaetani-Manfredotti, A. Carbon monoxide absorption by copper(I) halides in organic solvents: isolation and structure of µ-halogeno-dicopper(I) carbonyl complexes. Inorg. Chem. 20, 3382–3388 (1981).
Pike, R. D. Structure and bonding in copper(I) carbonyl and cyanide complexes. Organometallics 31, 7647–7660 (2012).
Wuttig, A. et al. Tracking a common surface-bound intermediate during CO2-to-fuels catalysis. ACS Cent. Sci. 2, 522–528 (2016).
Clark, E. L. & Bell, A. T. Direct observation of the local reaction environment during the electrochemical reduction of CO2. J. Am. Chem. Soc. 140, 7012–7020 (2018).
Wilde, P. et al. Is Cu instability during the CO2 reduction reaction governed by the applied potential or the local CO concentration? Chem. Sci. 12, 4028–4033 (2021).
Zhang, X. Y. et al. Selective methane electrosynthesis enabled by a hydrophobic carbon coated copper core-shell architecture. Energy Environ. Sci. 15, 234–243 (2022).
Zhang, L. et al. A polymer solution to prevent nanoclustering and improve the selectivity of metal nanoparticles for electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 58, 15834–15840 (2019).
Timoshenko, J. et al. Steering the structure and selectivity of CO2 electroreduction catalysts by potential pulses. Nat. Catal. 5, 259–267 (2022).
Hung, L.-I., Tsung, C.-K., Huang, W. & Yang, P. Room-temperature formation of hollow Cu2O nanoparticles. Adv. Mater. 22, 1910–1914 (2010).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Bučko, T., Hafner, J., Lebègue, S. & Ángyán, J. G. Improved description of the structure of molecular and layered crystals: ab initio DFT calculations with van der Waals corrections. J. Phys. Chem. A 114, 11814–11824 (2010).
Almora-Barrios, N., Carchini, G., Błoński, P. & López, N. Costless derivation of dispersion coefficients for metal surfaces. J. Chem. Theory Comput. 10, 5002–5009 (2014).
Chen, L. D., Urushihara, M., Chan, K. & Nørskov, J. K. Electric field effects in electrochemical CO2 reduction. ACS Catal. 6, 7133–7139 (2016).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kim, Y. G. et al. Surface reconstruction of pure-Cu single-crystal electrodes under CO-reduction potentials in alkaline solutions: a study by seriatim ECSTM-DEMS. J. Electroanal. Chem. 780, 290–295 (2016).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Makov, G. & Payne, M. C. Periodic boundary conditions in ab initio calculations. Phys. Rev. B 51, 4014–4022 (1995).
Granda-Marulanda, L. P. et al. A semiempirical method to detect and correct DFT-based gas-phase errors and its application in electrocatalysis. ACS Catal. 10, 6900–6907 (2020).
Álvarez-Moreno, M. et al. Managing the computational chemistry big data problem: the ioChem-BD platform. J. Chem. Inf. Model. 55, 95–103 (2015).
Acknowledgements
G.P.L.R. acknowledges financial support from the Ecole Normale Supérieure Paris Saclay. P.P.A. thanks NCCR Catalysis (grant no. 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation, for financial support. F.D. and N.L. acknowledge financial support by the Spanish Ministry of Science and Innovation (RTI2018-101394-B-I00, Severo Ochoa CEX2019-000925-S) and thank the Barcelona Supercomputing Center (BSC-RES) for providing generous computational resources. F.D. and N.L. thank M. A. Ortuño and S. J. Raaijman for fruitful scientific discussions. We thank M. Newton for assistance in the operando X-ray absorption measurements and analysis of the corresponding data reported in the Supplementary Information. O. Wenger is acknowledged for discussions on the phenanthroline ligands. The Swiss Norwegian beamlines (SNBL) at ESRF are acknowledged for the provision of beamtime and its staff are thanked for invaluable support. The BM31 set-up was funded by the Swiss National Science Foundation (grant no. 206021_189629) and the Research Council of Norway (grant no. 296087). N. Gasilova is acknowledged for assistance with ex situ ICP-MS measurements.
Author information
Authors and Affiliations
Contributions
J.V. carried out structural analysis of the catalyst and part of the electrocatalytic measurements, performed initial experiments with the phenanthroline ligand and wrote the first version of the manuscript. G.P.L.R. performed the UV–vis absorption, the ex situ ICP-MS, part of the electrocatalytic measurements and analysed the corresponding data. F.D. and N.L. carried out the computational simulations. A.K., T.P. and S.C. performed and analysed the data from the online ICP-MS measurements. P.P.A. contributed with electrocatalytic measurements and microscopy analysis. A.L. collected microscopy data and provided daily guidance to G.P.L.R. R.B. coordinated the entire work. All authors discussed, read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Youngku Sohn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs 1–18, Tables 1–6 and Notes 1-5
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vavra, J., Ramona, G.P.L., Dattila, F. et al. Solution-based Cu+ transient species mediate the reconstruction of copper electrocatalysts for CO2 reduction. Nat Catal 7, 89–97 (2024). https://doi.org/10.1038/s41929-023-01070-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01070-8
This article is cited by
-
Complementary probes for the electrochemical interface
Nature Reviews Chemistry (2024)
-
Reverse water gas-shift reaction product driven dynamic activation of molybdenum nitride catalyst surface
Nature Communications (2024)