Abstract
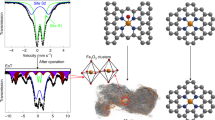
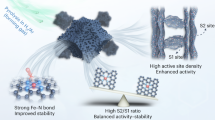
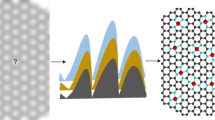
Beyond great advances in initial activity, Fe–N–C catalysts face the next challenge of stability issue in acidic medium that must be overcome to replace Pt in fuel cell cathode. However, the complex phenomena in fuel cells and consequential difficulty in understanding deactivation mechanisms of Fe–N–C cathodes impede solutions for prolonged stability. Here we show time-resolved changes in active site density and turnover frequency of Fe–N–C along with concurrent decrease in oxygen reduction reaction current in a temperature/gas controllable gas-diffusion electrode flow cell. Operando diagnosis of Fe leaching identifies a strong dependence of site density changes on operating parameters and draws a lifetime-dependent stability diagram that reveals a shift in the prime degradation mechanism during operation. A proof-of-concept strategy with site-isolated Pt ions as a non-catalytic stabilizer, supported by theoretical calculations, demonstrates enhanced fuel cell stability with reduced Fe dissolution, offering design principles for durable Fe–N–C catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data generated in this study have been deposited in the Zenodo repository database without accession code (https://doi.org/10.5281/zenodo.8287921)52. The DFT data generated in this study have been deposited in the Zenodo repository database without accession code (https://doi.org/10.5281/zenodo.8287952)53. Additional data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Yoshida, T. & Kojima, K. Toyota MIRAI fuel cell vehicle and progress toward a future hydrogen society. Electrochem. Soc. Interface 24, 45–49 (2015).
Thompson, S. T. & Papageorgopoulos, D. Platinum group metal-free catalysts boost cost competitiveness of fuel cell vehicles. Nat. Catal. 2, 558–561 (2019).
Proietti, E. et al. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2, 416 (2011).
Chung, H. T. et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 357, 479–484 (2017).
Lefèvre, M., Proietti, E., Jaouen, F. & Dodelet, J.-P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009).
Yin, X. & Zelenay, P. (Invited) Kinetic models for the degradation mechanisms of PGM-free ORR catalysts. ECS Trans. 85, 1239–1250 (2018).
Chenitz, R. et al. A specific demetalation of Fe–N4 catalytic sites in the micropores of NC_Ar + NH3 is at the origin of the initial activity loss of the highly active Fe/N/C catalyst used for the reduction of oxygen in PEM fuel cells. Energy Environ. Sci. 11, 365–382 (2018).
Zhang, G. et al. Non-PGM electrocatalysts for PEM fuel cells: effect of fluorination on the activity and stability of a highly active NC_Ar + NH3 catalyst. Energy Environ. Sci. 12, 3015–3037 (2019).
Choi, C. H. et al. Stability of Fe–N–C catalysts in acidic medium studied by operando spectroscopy. Angew. Chem. Int. Ed. 54, 12753–12757 (2015).
Choi, C. H. et al. Minimizing operando demetallation of Fe–N–C electrocatalysts in acidic medium. ACS Catal. 6, 3136–3146 (2016).
Kumar, K. et al. Fe–N–C electrocatalysts’ durability: effects of single atoms’ mobility and clustering. ACS Catal. 11, 484–494 (2021).
Kumar, K. et al. On the influence of oxygen on the degradation of Fe–N–C catalysts. Angew. Chem. Int. Ed. 59, 3235–3243 (2020).
Herranz, J. et al. Unveiling N-protonation and anion-binding effects on Fe/N/C catalysts for O2 reduction in proton-exchange-membrane fuel cells. J. Phys. Chem. C 115, 16087–16097 (2011).
Choi, C. H. et al. The Achilles’ heel of iron-based catalysts during oxygen reduction in an acidic medium. Energy Environ. Sci. 11, 3176–3182 (2018).
Ehelebe, K., Escalera-López, D. & Cherevko, S. Limitations of aqueous model systems in the stability assessment of electrocatalysts for oxygen reactions in fuel cell and electrolyzers. Curr. Opin. Electrochem. 29, 100832 (2021).
Li, J. et al. Identification of durable and non-durable FeNx sites in Fe–N–C materials for proton exchange membrane fuel cells. Nat. Catal. 4, 10–19 (2021).
Snitkoff-Sol, R. Z. et al. Quantifying the electrochemical active site density of precious metal-free catalysts in situ in fuel cells. Nat. Catal. 5, 163–170 (2022).
Xie, H. et al. Ta–TiOx nanoparticles as radical scavengers to improve the durability of Fe–N–C oxygen reduction catalysts. Nat. Energy 7, 281–289 (2022).
Liu, S. et al. Atomically dispersed iron sites with a nitrogen–carbon coating as highly active and durable oxygen reduction catalysts for fuel cells. Nat. Energy 7, 652–663 (2022).
Zitolo, A. et al. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 14, 937–942 (2015).
Kim, D. H. et al. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 12, 1856 (2021).
Goellner, V. et al. Degradation of Fe/N/C catalysts upon high polarization in acid medium. Phys. Chem. Chem. Phys. 16, 18454–18462 (2014).
Chen, Z. et al. Operando characterization of iron phthalocyanine deactivation during oxygen reduction reaction using electrochemical tip-enhanced Raman spectroscopy. J. Am. Chem. Soc. 141, 15684–15692 (2019).
Holby, E. F., Wang, G. & Zelenay, P. Acid stability and demetalation of PGM-free ORR electrocatalyst structures from density functional theory: a model for ‘single-atom catalyst’ dissolution. ACS Catal. 10, 14527–14539 (2020).
Jia, Q. et al. Spectroscopic insights into the nature of active sites in iron–nitrogen–carbon electrocatalysts for oxygen reduction in acid. Nano Energy 29, 65–82 (2016).
Beverskog, B. & Puigdomenech, I. Revised pourbaix diagrams for iron at 25–300 °C. Corros. Sci. 38, 2121–2135 (1996).
Parida, K. M. & Das, N. N. Reductive dissolution of hematite in hydrochloric acid medium by some inorganic and organic reductants: a comparative study. Indian J. Eng. Mater. Sci. 3, 243 (1996).
Bae, G. et al. Quantification of active site density and turnover frequency: from single-atom metal to nanoparticle electrocatalysts. JACS Au 1, 586–597 (2021).
Malko, D., Kucernak, A. & Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. 7, 13285 (2016).
Lei, M. et al. CeO2 nanocubes-graphene oxide as durable and highly active catalyst support for proton exchange membrane fuel cell. Sci. Rep. 4, 7415 (2014).
Wang, L., Advani, S. G. & Prasad, A. K. Degradation reduction of polymer electrolyte membranes using CeO2 as a free-radical scavenger in catalyst layer. Electrochim. Acta 109, 775–780 (2013).
Bae, G., Chung, M. W., Ji, S. G., Jaouen, F. & Choi, C. H. pH effect on the H2O2-induced deactivation of Fe–N–C catalysts. ACS Catal. 10, 8485–8495 (2020).
Mechler, A. K. et al. Stabilization of iron-based fuel cell catalysts by non-catalytic platinum. J. Electrochem. Soc. 165, F1084–F1091 (2018).
Xiao, F. et al. Atomically dispersed Pt and Fe sites and Pt–Fe nanoparticles for durable proton exchange membrane fuel cells. Nat. Catal. 5, 503–512 (2022).
Choi, C. H. et al. Hydrogen peroxide synthesis via enhanced two-electron oxygen reduction pathway on carbon-coated Pt surface. J. Phys. Chem. C 118, 30063–30070 (2014).
Ferre-Vilaplana, A., Perales-Rondón, J. V., Buso-Rogero, C., Feliu, J. M. & Herrero, E. Formic acid oxidation on platinum electrodes: a detailed mechanism supported by experiments and calculations on well-defined surfaces. J. Mater. Chem. A 5, 21773–21784 (2017).
Chung, D. Y., Lee, K.-J. & Sung, Y.-E. Methanol electro-oxidation on the Pt surface: revisiting the cyclic voltammetry interpretation. J. Phys. Chem. C 120, 9028–9035 (2016).
Neyerlin, K. C. et al. A. Study of the exchange current density for the hydrogen oxidation and evolution reactions. J. Electrochem. Soc. 154, B631–B635 (2007).
Zhang, G. et al. Is iron involved in the lack of stability of Fe/N/C electrocatalysts used to reduce oxygen at the cathode of PEM fuel cells? Nano Energy 29, 111–125 (2016).
Kida, K., Okita, M., Fujita, K., Tanaka, S. & Miyake, Y. Formation of high crystalline ZIF-8 in an aqueous solution. Cryst. Eng. Comm. 15, 1794–1801 (2013).
Sa, Y. J. et al. A general approach to preferential formation of active Fe–Nx sites in Fe–N/C electrocatalysts for efficient oxygen reduction reaction. J. Am. Chem. Soc. 138, 15046–15056 (2016).
Badam, R. et al. Sacrificial reducing agent free photo-generation of platinum nano particle over carbon/TiO2 for highly efficient oxygen reduction reaction. Sci. Rep. 6, 37006 (2016).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Methfessel, M. & Paxton, A. T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 40, 3616 (1989).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Hay, P. J. & Wadt, W. R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270–283 (1985).
Bochevarov, A. D. et al. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 113, 2110–2142 (2013).
Friedrichs, M., Zhou, R., Edinger, S. R. & Friesner, R. A. Poisson–Boltzmann analytical gradients for molecular modeling calculations. J. Phys. Chem. B 103, 3057–3061 (1999).
Bae, G. et al. Unraveling the complex causality behind Fe–N–C degradation in fuel cells. Zenodo https://doi.org/10.5281/zenodo.8287921 (2023).
Bae, G. et al. Unraveling the complex causality behind Fe–N–C degradation in fuel cells. Zenodo https://doi.org/10.5281/zenodo.8287952 (2023).
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (nos. 2019M3D1A1079309, 2021R1A5A1030054 and 2023R1A2C2005278 to C.H.C.) and by the KIST Institutional Program. We acknowledge the Pohang Accelerator Laboratory (PAL) for beamline use (8C, PLS-II). We are grateful to M. H. Seo and S. Jin for helpful discussions on this paper.
Author information
Authors and Affiliations
Contributions
C.H.C., F.J., H.K. and H.-S.O. conceived and supervised the project. G.B. conducted most of the experimental analyses. M.M.K. conducted DFT calculations. M.H.H. conducted fuel cell experiment. J.C. and D.H.K. contributed to the part of electrochemical analyses. M.-T.S. contributed to 57Fe Mössbauer spectroscopy measurement. K.-S.L contributed to XAS measurement. J.K. and S.H.J. contributed to part of the catalyst synthesis. W.A.G. contributed to DFT calculations. The paper was written through the contributions of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Catalysis thanks Lior Elbaz, Michael Bron and Zheng Wang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–8, Figs. 1–47, Tables 1–4 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bae, G., Kim, M.M., Han, M.H. et al. Unravelling the complex causality behind Fe–N–C degradation in fuel cells. Nat Catal 6, 1140–1150 (2023). https://doi.org/10.1038/s41929-023-01039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01039-7
This article is cited by
-
Modifying Fe–N interaction to boost catalytic performance
Nature Catalysis (2023)