Abstract
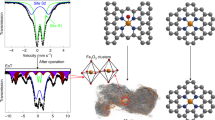
Fe–N–C catalysts are the most promising platinum group metal-free oxygen-reduction catalysts, but they suffer from a low density of active metal sites and the so-called activity–stability trade-off. Here we report an Fe–N–C catalyst prepared by adding an optimal amount of H2 to the traditional inert atmosphere during the thermal activation. The presence of H2 significantly increases the total density of FeN4 sites, suppressing the unstable pyrrolic-N-coordinated S1 sites and favouring the stable pyridinic-N-coordinated S2 sites with shortened Fe–N bond lengths. We propose that the intrinsically stable S2 sites are probably arranged in well-graphitized carbon layers, and the S1 sites exist in less-graphitized carbon. H2 could remove unstable S1 sites and retain stable S2 sites during the pyrolysis to break the challenging activity–stability trade-off. The Fe–N–C catalyst in membrane electrode assemblies maintains a current density of 67 mA cm−2 at 0.8 V (H2–air) after 30,000 voltage cycles (0.60 to 0.95 V under H2–air), achieving encouraging durability and performance simultaneously.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and Supplementary Information, or from the authors upon request. Source data are provided with this paper.
References
Wang, X. X., Swihart, M. T. & Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2, 578–589 (2019).
He, Y. & Wu, G. PGM-free oxygen-reduction catalyst development for proton-exchange membrane fuel cells: challenges, solutions and promises. Acc. Mater. Res. 3, 224–236 (2022).
Wang, W., Jia, Q., Mukerjee, S. & Chen, S. Recent insights into the oxygen-reduction electrocatalysis of Fe/N/C materials. ACS Catal. 9, 10126–10141 (2019).
Martinez, U. et al. Progress in the development of Fe-based PGM-free electrocatalysts for the oxygen reduction reaction. Adv. Mater. 31, 1806545 (2019).
Zhang, H. et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J. Am. Chem. Soc. 139, 14143–14149 (2017).
Liu, S. et al. Atomically dispersed iron sites with a nitrogen–carbon coating as highly active and durable oxygen reduction catalysts for fuel cells. Nat. Energy 7, 652–663 (2022).
Asset, T., Maillard, F. & Jaouen, F. in Supported Metal Single Atom Catalysis (eds Serp, P. & Minh, D. P.) 531–582 (Wiley, 2022).
Glibin, V. P., Dodelet, J.-P. & Zhang, G. Energetics and thermodynamic stability of potential Fe(II)-hexa-aza-active sites for O2 reduction in PEM fuel cells. SusMat 2, 731–748 (2022).
Liu, S., Shi, Q. & Wu, G. Solving the activity–stability trade-off riddle. Nat. Catal. 4, 6–7 (2021).
Liu, K., Wu, G. & Wang, G. Role of local carbon structure surrounding FeN4 sites in boosting the catalytic activity for oxygen reduction. J. Phys. Chem. C 121, 11319–11324 (2017).
Mao, K., Yang, L., Wang, X., Wu, Q. & Hu, Z. Identifying iron-nitrogen/carbon active structures for oxygen reduction reaction under the effect of electrode potential. J. Phys. Chem. Lett. 11, 2896–2901 (2020).
Shao, Y., Dodelet, J.-P., Wu, G. & Zelenay, P. PGM-free cathode catalysts for PEM fuel cells: a mini-review on stability challenges. Adv. Mater. 31, 1807615 (2019).
Kumar, K. et al. On the influence of oxygen on the degradation of Fe‐N‐C catalysts. Angew. Chem. Int. Ed. 59, 3235–3243 (2020).
Zhu, Y. et al. Engineering local coordination environments of atomically dispersed and heteroatom-coordinated single metal site electrocatalysts for clean energy-conversion. Adv. Energy Mater. 10, 1902844 (2020).
Li, J. et al. Identification of durable and non-durable FeNx sites in Fe–N–C materials for proton exchange membrane fuel cells. Nat. Catal. 4, 10–19 (2021).
Marshall-Roth, T. et al. A pyridinic Fe-N4 macrocycle models the active sites in Fe/N-doped carbon electrocatalysts. Nat. Commun. 11, 5283 (2020).
Menga, D. et al. Resolving the dilemma of Fe–N–C catalysts by the selective synthesis of tetrapyrrolic active sites via an imprinting strategy. J. Am. Chem. Soc. 143, 18010–18019 (2021).
Muñoz-Becerra, K., Venegas, R., Duque, L., Zagal, J. H. & Recio, F. J. Recent advances of Fe–N–C pyrolyzed catalysts for the oxygen reduction reaction. Curr. Opin. Electrochem. 23, 154–161 (2020).
Lefèvre, M., Dodelet, J. P. & Bertrand, P. O2 reduction in PEM fuel cells: activity and active site structural information for catalysts obtained by the pyrolysis at high temperature of Fe precursors. J. Phys. Chem. B 104, 11238–11247 (2000).
Wu, G., More, K. L., Johnston, C. M. & Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron and cobalt. Science 332, 443–447 (2011).
Proietti, E. et al. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2, 416 (2011).
Lefèvre, M., Proietti, E., Jaouen, F. & Dodelet, J.-P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009).
Kramm, U. I. et al. On an easy way to prepare metal–nitrogen doped carbon with exclusive presence of MeN4-type sites active for the ORR. J. Am. Chem. Soc. 138, 635–640 (2016).
Jaouen, F. & Dodelet, J.-P. O2 reduction mechanism on non-noble metal catalysts for PEM fuel cells. Part I: experimental rates of O2 electroreduction, H2O2 electroreduction and H2O2 disproportionation. J. Phys. Chem. C 113, 15422–15432 (2009).
Tabassum, H. et al. Hydrogen generation via ammonia decomposition on highly efficient and stable Ru-free catalysts: approaching complete conversion at 450 °C. Energy Environ. Sci. 15, 4190–4200 (2022).
Kumar, K. et al. Fe–N–C electrocatalysts’ durability: effects of single atoms’ mobility and clustering. ACS Catal. 11, 484–494 (2021).
Workman, M. J., Serov, A., Tsui, L., Atanassov, P. & Artyushkova, K. Fe–N–C catalyst graphitic layer structure and fuel cell performance. ACS Energy Lett. 2, 1489–1493 (2017).
Malko, D., Kucernak, A. & Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. 7, 13285 (2016).
Mehmood, A. et al. High loading of single atomic iron sites in Fe–NC oxygen reduction catalysts for proton exchange membrane fuel cells. Nat. Catal. 5, 311–323 (2022).
Zhang, H. et al. High-performance fuel cell cathodes exclusively containing atomically dispersed iron active sites. Energy Environ. Sci. 12, 2548–2558 (2019).
Laviron, E. Surface linear potential sweep voltammetry: equation of the peaks for a reversible reaction when interactions between the adsorbed molecules are taken into account. J. Electroanal. Chem. Interf. Electrochem. 52, 395–402 (1974).
Zhang, H. et al. Standardized protocols for evaluating platinum group metal-free oxygen reduction reaction electrocatalysts in polymer electrolyte fuel cells. Nat. Catal. 5, 455–462 (2022).
Osmieri, L., Cullen, D. A., Chung, H. T., Ahluwalia, R. K. & Neyerlin, K. C. Durability evaluation of a Fe–N–C catalyst in polymer electrolyte fuel cell environment via accelerated stress tests. Nano Energy 78, 105209 (2020).
Qiao, Z. et al. Atomically dispersed single iron sites for promoting Pt and Pt3Co fuel cell catalysts: performance and durability improvements. Energy Environ. Sci. 14, 4948–4960 (2021).
Shi, Q. et al. Methanol tolerance of atomically dispersed single metal site catalysts: mechanistic understanding and high-performance direct methanol fuel cells. Energy Environ. Sci. 13, 3544–3555 (2020).
Zitolo, A. et al. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 14, 937–942 (2015).
Kramm, U. I., Ni, L. & Wagner, S. 57Fe Mössbauer spectroscopy characterization of electrocatalysts. Adv. Mater. 31, 1805623 (2019).
Ni, L. et al. Identification of the catalytically dominant iron environment in iron- and nitrogen-doped carbon catalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 144, 16827–16840 (2022).
Mineva, T. et al. Understanding active sites in pyrolyzed Fe–N–C catalysts for fuel cell cathodes by bridging density functional theory calculations and 57Fe Mössbauer spectroscopy. ACS Catal. 9, 9359–9371 (2019).
Thompson, E., Danks, A. E., Bourgeois, L. & Schnepp, Z. Iron-catalyzed graphitization of biomass. Green Chem. 17, 551–556 (2015).
Jaouen, F., Lefèvre, M., Dodelet, J.-P. & Cai, M. Heat-treated Fe/N/C catalysts for O2 electroreduction: are active sites hosted in micropores? J. Phys. Chem. B 110, 5553–5558 (2006).
Zhao, X. et al. Single-iron site catalysts with self-assembled dual-size architecture and hierarchical porosity for proton-exchange membrane fuel cells. Appl. Catal. B 279, 119400 (2020).
Uddin, A. et al. High power density platinum group metal-free cathodes for polymer electrolyte fuel cells. ACS Appl. Mater. Interfaces 12, 2216–2224 (2020).
Liu, Y. et al. Facile synthesis of MOF-derived concave cube nanocomposite by self-templated toward lightweight and wideband microwave absorption. Carbon 186, 574–588 (2022).
Zheng, L., Zhao, Y., Zhang, H., Xia, W. & Tang, J. Space-confined anchoring of Fe–Nx on concave N-doped carbon cubes for catalyzing oxygen reduction. ChemSusChem 15, e202102642 (2022).
Ferrari, A. C. et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97, 187401–187405 (2006).
Wan, X. et al. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268 (2019).
Wu, G. & Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 46, 1878–1889 (2013).
Wang, M. & Feng, Z. Pitfalls in X-ray absorption spectroscopy analysis and interpretation: a practical guide for general users. Curr. Opin. Electrochem. 30, 100803 (2021).
Mohd Adli, N. et al. Engineering atomically dispersed FeN4 active sites for CO2 electroreduction. Angew. Chem. Int. Ed. 60, 1022–1032 (2021).
Liu, S. et al. Chemical vapor deposition for atomically dispersed and nitrogen coordinated single metal site catalysts. Angew. Chem. Int. Ed. 59, 21698–21705 (2020).
Saveleva, V. A. et al. Fe–N–C electrocatalyst and its electrode: are we talking about the same material? ACS Appl. Energy Mater. 6, 611–616 (2023).
Hess, W. M. & Herd, C. R. in Carbon Black: Science and Technology (eds Donnet, J.-B. et al) 89–173 (Routledge, 2018).
Yu, M. & Trinkle, D. R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 134, 064111 (2011).
Xie, X. et al. Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells. Nat. Catal. 3, 1044–1054 (2020).
Vitos, L., Ruban, A. V., Skriver, H. L. & Kollár, J. The surface energy of metals. Surf. Sci. 411, 186–202 (1998).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 136, B864–B871 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, 1133–1138 (1965).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Norskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106–084114 (2014).
Acknowledgements
We acknowledge support from the US Department of Energy (DOE), Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office. Scanning transmission electron microscopy research was supported by the Center for Nanophase Materials Sciences (CNMS), which is a US Department of Energy, Office of Science User Facility at Oak Ridge National Laboratory. This work was in part authored by Argonne National Laboratory, which is operated for the US DOE by the University of Chicago Argonne LLC under contract no. DE-AC02-06CH11357. G. Wu also thanks the New York State’s Center of Excellence in Materials Informatics (CMI) at the University at Buffalo, as well as the National Science Foundation (CBET-1604392, 1804326 and 2223467), for partial support.
Author information
Authors and Affiliations
Contributions
G. Wu, J.-P.D. and Y.Z. were the primary writers of the paper. Y.Z., J.L., B.Z., S.K. and G. Wu designed the catalyst synthesis and performed the electrochemical experiments, characterized the catalyst and analysed the data. C.L. and J.X. carried out fuel cell tests and data analysis. D.A.C. and M.J.Z. together performed the electron microscopy imaging and further characterizations. B. Li and G. Wang designed and performed DFT calculations. M.L., M.W. and Z.F. designed and performed X-ray absorption spectroscopy and data analysis. R.P.H., E.E.A., B. Lavina and D.J.M. designed and performed Mössbauer spectroscopy experiments and data analysis. G. Wu supervised the execution of the overall project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Yaqiong Su, Kavita Kumar and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23, Tables 1–16 and Notes 1–5.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, Y., Li, C., Li, B. et al. Tuning the thermal activation atmosphere breaks the activity–stability trade-off of Fe–N–C oxygen reduction fuel cell catalysts. Nat Catal 6, 1215–1227 (2023). https://doi.org/10.1038/s41929-023-01062-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01062-8
This article is cited by
-
Modifying Fe–N interaction to boost catalytic performance
Nature Catalysis (2023)