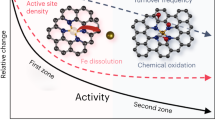
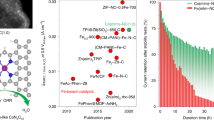
Abstract
Advances in the development of precious-group metal-free (PGM-free) catalysts for the oxygen reduction reaction (ORR) in fuel cell cathodes have produced active catalysts that reduce the performance gap to the incumbent Pt-based materials. However, utilization of state-of-the-art PGM-free catalysts for commercial applications is currently impeded by their relatively low durability. Methods designed to study catalyst degradation in the operation of fuel cells are therefore critical for understanding durability issues and, ultimately, their solutions. Here we report the use of Fourier-transform alternating current voltammetry as an electrochemical method for accurate quantification of the electrochemically active site density of PGM-free cathode catalysts, and to follow their degradation in situ during the operation of polymer electrolyte fuel cells. Using this method, we were able to quantify the electrochemical active site density, which will enable the elucidation of degradation mechanisms of PGM-free ORR catalysts in situ in fuel cells.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw data that support the findings of this study are available from the corresponding author on request.
Code availability
All simulations in this work were conducted using the MECSim simulation package obtained from the following website free of charge: http://www.garethkennedy.net/MECSim.html.
References
Yurko, Y. & Elbaz, L. The effect of membrane electrode assembly methods on the performance in fuel cells. Electrochim. Acta 389, 138676 (2021).
Cano, Z. P. et al. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3, 279–289 (2018).
He, Y., Liu, S., Priest, C., Shi, Q. & Wu, G. Atomically dispersed metal–nitrogen–carbon catalysts for fuel cells: advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 49, 3484–3524 (2020).
Martinez, U. et al. Experimental and theoretical trends of PGM-free electrocatalysts for the oxygen reduction reaction with different transition metals. J. Electrochem. Soc. 166, F3136–F3142 (2019).
Thompson, S. T. et al. ElectroCat: DOE’s approach to PGM-free catalyst and electrode R&D. Solid State Ion. 319, 68–76 (2018).
Thompson, S. T. & Papageorgopoulos, D. Platinum group metal-free catalysts boost cost competitiveness of fuel cell vehicles. Nat. Catal. 2, 558–561 (2019).
Shao, Y., Dodelet, J. P., Wu, G. & Zelenay, P. PGM‐free cathode catalysts for PEM fuel cells: a mini‐review on stability challenges. Adv. Mater. 31, 1807615 (2019).
Banham, D. et al. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 285, 334–348 (2015).
Martinez, U., Babu, S. K., Holby, E. F. & Zelenay, P. Durability challenges and perspective in the development of PGM-free electrocatalysts for the oxygen reduction reaction. Curr. Opin. Electrochem. https://doi.org/10.1016/j.coelec.2018.04.010 (2018).
Zagal, J. H. & Bedioui, F. Electrochemistry of N4 Macrocyclic Metal Vomplexes Vol. 2 (Springer, 2016).
Banham, D. & Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: an industrial perspective. ACS Energy Lett. 2, 629–638 (2017).
Meier, J. C. et al. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 5, 44–67 (2014).
Mayrhofer, K. J. J. et al. Measurement of oxygen reduction activities via the rotating disc electrode method: from Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 53, 3181–3188 (2008).
Jia, Q., Liu, E., Jiao, L., Pann, S. & Mukerjee, S. X‐ray absorption spectroscopy characterizations on PGM‐free electrocatalysts: justification, advantages, and limitations. Adv. Mater. 31, 1805157 (2019).
Jia, Q. et al. Spectroscopic insights into the nature of active sites in iron–nitrogen–carbon electrocatalysts for oxygen reduction in acid. Nano Energy 29, 65–82 (2016).
Malko, D., Kucernak, A. & Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. https://doi.org/10.1038/ncomms13285 (2016).
Osmieri, L. et al. Elucidation of Fe-NC electrocatalyst active site functionality via in-situ X-ray absorption and operando determination of oxygen reduction reaction kinetics in a PEFC. Appl. Catal. B 257, 117929 (2019).
Li, J. et al. Identification of durable and non-durable FeNx sites in Fe–N–C materials for proton exchange membrane fuel cells. Nat. Catal. 4, 10–19 (2021).
Kramm, U. I., Ni, L. & Wagner, S. 57Fe Mössbauer spectroscopy characterization of electrocatalysts. Adv. Mater. 31, 1805623 (2019).
Luo, F. et al. Surface site density and utilization of platinum group metal (PGM)-free Fe–NC and FeNi–NC electrocatalysts for the oxygen reduction reaction. Chem. Sci. 12, 384–396 (2021).
Tylus, U. et al. Elucidating oxygen reduction active sites in pyrolyzed metal–nitrogen coordinated non-precious-metal electrocatalyst systems. J. Phys. Chem. C 118, 8999–9008 (2014).
Yin, X. & Zelenay, P. Kinetic models for the degradation mechanisms of PGM-free ORR catalysts. ECS Trans. 85, 1239–1250 (2018).
Jiao, L. et al. Chemical vapour deposition of Fe–N–C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 20, 1385–1391 (2021).
Bae, G. et al. Quantification of active site density and turnover frequency: from single-atom metal to nanoparticle electrocatalysts. JACS Au 1, 586–597 (2021).
Malko, D., Kucernak, A. & Lopes, T. Performance of Fe–N/C oxygen reduction electrocatalysts toward NO2–, NO, and NH2OH electroreduction: from fundamental insights into the active center to a new method for environmental nitrite destruction. J. Am. Chem. Soc. 138, 16056–16068 (2016).
Luo, F. et al. Accurate evaluation of active-site density (SD) and turnover frequency (TOF) of PGM-free metal–nitrogen-doped carbon (MNC) electrocatalysts using CO cryo adsorption. ACS Catal. 9, 4841–4852 (2019).
Leonard, N. D. et al. Deconvolution of utilization, site density, and turnover frequency of Fe–nitrogen–carbon oxygen reduction reaction catalysts prepared with secondary N-precursors. ACS Catal. 8, 1640–1647 (2018).
Sahraie, N. R. et al. Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat. Commun. 6, 8618 (2015).
Jaouen, F. et al. Toward platinum group metal-free catalysts for hydrogen/air proton-exchange membrane fuel cells. Johnson Matthey Technol. Rev. 62, 231–255 (2018).
Boldrin, P. et al. Deactivation, reactivation and super-activation of Fe-N/C oxygen reduction electrocatalysts: gas sorption, physical and electrochemical investigation using NO and O2. Appl. Catal. B 292, 120169 (2021).
Primbs, M. et al. Establishing reactivity descriptors for platinum group metal (PGM)-free Fe–N–C catalysts for PEM fuel cells. Energy Environ. Sci. 13, 2480–2500 (2020).
Kozhushner, A., Zion, N. & Elbaz, L. Methods for assessment and measurement of the active site density in platinum group metal–free oxygen reduction reaction catalysts. Curr. Opin. Electrochem. 25, 100620 (2021).
Bond, A. M. et al. An integrated instrumental and theoretical approach to quantitative electrode kinetic studies based on large amplitude Fourier transformed AC voltammetry: a mini review. Electrochem. Commun. 57, 78–83 (2015).
Zhang, Y., Simonov, A. N., Zhang, J. & Bond, A. M. Fourier transformed alternating current voltammetry in electromaterials research: direct visualisation of important underlying electron transfer processes. Curr. Opin. Electrochem. 10, 72–81 (2018).
Ma, H. Mechanistic Electrochemistry: Investigations of Electrocatalytic Mechanisms for H2S Detection Applications. PhD thesis, Univ. Cambridge (2017); https://www.repository.cam.ac.uk/handle/1810/269396
Zhang, Y. et al. Direct detection of electron transfer reactions underpinning the tin-catalyzed electrochemical reduction of CO2 using Fourier-transformed AC voltammetry. ACS Catal. 7, 4846–4853 (2017).
Stevenson, G. P. et al. Theoretical analysis of the two-electron transfer reaction and experimental studies with surface-confined cytochrome c peroxidase using large-amplitude Fourier transformed AC voltammetry. Langmuir 28, 9864–9877 (2012).
Adamson, H. et al. Analysis of HypD disulfide redox chemistry via optimization of Fourier transformed AC voltammetric data. Anal. Chem. 89, 1565–1573 (2017).
Engblom, S. O., Myland, J. C. & Oldham, K. B. Must AC voltammetry employ small signals? J. Electroanal. Chem. 480, 120–132 (2000).
Gavaghan, D. J. & Bond, A. M. A complete numerical simulation of the techniques of alternating current linear sweep and cyclic voltammetry: analysis of a reversible process by conventional and fast Fourier transform methods. J. Electroanal. Chem. 480, 133–149 (2000).
Lee, C.-Y. et al. Theoretical and experimental investigation of surface-confined two-center metalloproteins by large-amplitude Fourier transformed AC voltammetry. J. Electroanal. Chem. 656, 293–303 (2011).
Lloyd-Laney, H., Robinson, M., Bond, A., Parkin, A. & Gavaghan, D. A spotter’s guide to dispersion in surface-confined voltammetry experiments. J. Electroanal. Chem. 894, 115204 (2021).
Lloyd-Laney, H. et al. Using purely sinusoidal voltammetry for rapid parameterization of surface-confined electrochemistry. Anal. Chem. 93, 2062–2071 (2021).
Kennedy, G. F., Bond, A. M. & Simonov, A. N. Modelling ac voltammetry with MECSim: facilitating simulation–experiment comparisons. Curr. Opin. Electrochem. 1, 140–147 (2017).
Nardis, S., Mandoj, F., Stefanelli, M. & Paolesse, R. Metal complexes of corrole. Coord. Chem. Rev. 388, 360–405 (2019).
Robinson, M., Ounnunkad, K., Zhang, J., Gavaghan, D. & Bond, A. Integration of heuristic and automated parametrization of three unresolved two‐electron surface‐confined polyoxometalate reduction processes by AC voltammetry. ChemElectroChem 5, 3771–3785 (2018).
Adamson, H., Bond, A. M. & Parkin, A. Probing biological redox chemistry with large amplitude Fourier transformed AC voltammetry. Chem. Commun. 53, 9519–9533 (2017).
Adamson, H. et al. Electrochemical evidence that pyranopterin redox chemistry controls the catalysis of YedY, a mononuclear Mo enzyme. Proc. Natl Acad. Sci. USA 112, 14506–14511 (2015).
Chen, L. et al. Electrochemical reduction of CO2 with an oxide‐derived lead nano‐coralline electrode in dimcarb. ChemElectroChem 4, 1402–1410 (2017).
Fleming, B. D., Zhang, J., Elton, D. & Bond, A. M. Detailed analysis of the electron-transfer properties of azurin adsorbed on graphite electrodes using DC and large-amplitude Fourier transformed AC voltammetry. Anal. Chem. 79, 6515–6526 (2007).
Guo, S.-X. et al. Facile electrochemical co-deposition of a graphene–cobalt nanocomposite for highly efficient water oxidation in alkaline media: direct detection of underlying electron transfer reactions under catalytic turnover conditions. Phys. Chem. Chem. Phys. 16, 19035–19045 (2014).
Lee, C.-Y. & Bond, A. M. Evaluation of levels of defect sites present in highly ordered pyrolytic graphite electrodes using capacitive and faradaic current components derived simultaneously from large-amplitude fourier transformed AC voltammetric experiments. Anal. Chem. 81, 584–594 (2008).
Li, J., Bond, A. M. & Zhang, J. Probing electrolyte cation effects on the electron transfer kinetics of the [α-SiW12O40] 4−/5− and [α-SiW12O40] 5−/6− processes using a boron-doped diamond electrode. Electrochim. Acta 178, 631–637 (2015).
Zhang, J., Guo, S.-X., Bond, A. M. & Marken, F. Large-amplitude Fourier transformed high-harmonic alternating current cyclic voltammetry: kinetic discrimination of interfering faradaic processes at glassy carbon and at boron-doped diamond electrodes. Anal. Chem. 76, 3619–3629 (2004).
Mashkina, E. A., Simonov, A. N. & Bond, A. M. Optimisation of windowing for harmonic recovery in large-amplitude Fourier transformed a.c. voltammetry. J. Electroanal. Chem. 732, 86–92 (2014).
Guo, S.-X., Bond, A. M. & Zhang, J. Fourier transformed large amplitude alternating current voltammetry: principles and applications. Rev. Polarogr. 61, 21–32 (2015).
Tan, S.-y. Advanced electrochemical techniques for investigating electron transfer kinetics. PhD thesis, Univ. Warwick (2017); http://wrap.warwick.ac.uk/93622/
Song, P. et al. Fourier transform large amplitude alternating current voltammetry investigations of the split wave phenomenon in electrocatalytic mechanisms. Phys. Chem. Chem. Phys. 19, 24304–24315 (2017).
Zhang, J. & Bond, A. M. Theoretical studies of large amplitude alternating current voltammetry for a reversible surface-confined electron transfer process coupled to a pseudo first-order electrocatalytic process. J. Electroanal. Chem. 600, 23–34 (2007).
Honeychurch, M. J. & Bond, A. M. Numerical simulation of Fourier transform alternating current linear sweep voltammetry of surface bound molecules. J. Electroanal. Chem. 529, 3–11 (2002).
Gundry, L. et al. Recent advances and future perspectives for automated parameterisation, Bayesian inference and machine learning in voltammetry. Chem. Commun. 57, 1855–1870 (2021).
Faulkner, L. R. & Bard, A. J. Electrochemical Methods: Fundamentals and Applications (Wiley, 2002).
Kishi, H. et al. Structure of active sites of Fe-NC nano-catalysts for alkaline exchange membrane fuel cells. Nanomaterials 8, 965 (2018).
Serov, A. et al. Highly stable precious metal-free cathode catalyst for fuel cell application. J. Power Sources 327, 557–564 (2016).
Vecchio, C. L. et al. Commercial platinum group metal-free cathodic electrocatalysts for highly performed direct methanol fuel cell applications. J. Power Sources 437, 226948 (2019).
Osmieri, L., Mauger, S., Ulsh, M., Neyerlin, K. C. & Bender, G. Use of a segmented cell for the combinatorial development of platinum group metal-free electrodes for polymer electrolyte fuel cells. J. Power Sources 452, 227829 (2020).
Osmieri, L. et al. Utilizing ink composition to tune bulk-electrode gas transport, performance, and operational robustness for a Fe–N–C catalyst in polymer electrolyte fuel cell. Nano Energy 75, 104943 (2020).
Khandavalli, S. et al. Effect of dispersion medium composition and ionomer concentration on the microstructure and rheology of Fe–N–C platinum group metal-free catalyst inks for polymer electrolyte membrane fuel cells. Langmuir 36, 12247–12260 (2020).
Choi, C. H. et al. The Achilles’ heel of iron-based catalysts during oxygen reduction in an acidic medium. Energy Environ. Sci. 11, 3176–3182 (2018).
Morris, G. P. et al. Theoretical analysis of the relative significance of thermodynamic and kinetic dispersion in the DC and AC voltammetry of surface-confined molecules. Langmuir 31, 4996–5004 (2015).
Acknowledgements
R.Z.S.-S., A.F. and H.C.H. thank the Israeli Ministry of Energy for their fellowships. Part of this work was conducted within the framework of the Israeli Fuel Cells Consortium. L.E. thanks the Israeli Ministry of Energy for funding this project (no. 219-11-132). This research was supported in part by the US Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office. Electron microscopy was conducted at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility.
Author information
Authors and Affiliations
Contributions
R.Z.S.-S. conceived, analysed, simulated and conducted fuel cell and electrochemical measurements and prepared the manuscript. A.F. and Y.Y. conducted some of the preliminary experiments. A.K. conducted XRD measurements and Raman spectroscopy. H.C.H. conducted XPS measurements and analysis. M.J.Z. conducted TEM and STEM measurements and analysis. A.M.B. gave advice, discussed the results and edited the manuscript. P.Z. discussed the results and edited the manuscript. L.E. conceived and analysed all experiments and discussed the results, supervised the work and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Frédéric Jaouen and Jianglan Shui for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21, Tables 1 and 2, Methods, Discussion and Notes 1–3.
Rights and permissions
About this article
Cite this article
Snitkoff-Sol, R.Z., Friedman, A., Honig, H.C. et al. Quantifying the electrochemical active site density of precious metal-free catalysts in situ in fuel cells. Nat Catal 5, 163–170 (2022). https://doi.org/10.1038/s41929-022-00748-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00748-9
This article is cited by
-
Direct measurement of the oxygen reduction reaction kinetics on iron phthalocyanine using advanced transient voltammetry
Nature Catalysis (2024)
-
Modifying Fe–N interaction to boost catalytic performance
Nature Catalysis (2023)
-
Selective CO2 electrolysis to CO using isolated antimony alloyed copper
Nature Communications (2023)
-
Unravelling the complex causality behind Fe–N–C degradation in fuel cells
Nature Catalysis (2023)
-
Microenvironment regulation of M-N-C single-atom catalysts towards oxygen reduction reaction
Nano Research (2023)