Abstract
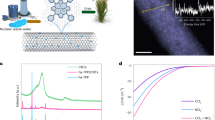
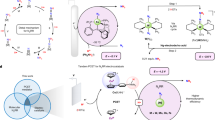
The nitrogen cycle needed for scaled agriculture relies on energy- and carbon-intensive processes and generates nitrate-containing wastewater. Here we focus on an alternative approach—the electrified co-electrolysis of nitrate and CO2 to synthesize urea. When this is applied to industrial wastewater or agricultural runoff, the approach has the potential to enable low-carbon-intensity urea production while simultaneously providing wastewater denitrification. We report a strategy that increases selectivity to urea using a hybrid catalyst: two classes of site independently stabilize the key intermediates needed in urea formation, *CO2NO2 and *COOHNH2, via a relay catalysis mechanism. A Faradaic efficiency of 75% at wastewater-level nitrate concentrations (1,000 ppm NO3− [N]) is achieved on Zn/Cu catalysts. The resultant catalysts show a urea production rate of 16 µmol h−1 cm−2. Life-cycle assessment indicates greenhouse gas emissions of 0.28 kg CO2e per kg urea for the electrochemical route, compared to 1.8 kg CO2e kg−1 for the present-day route.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and Supplementary Information. Source data are provided with this paper.
References
Fowler, D. et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B 368, 20130164 (2013).
Hattori, M., Iijima, S., Nakao, T., Hosono, H. & Hara, M. Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C. Nat. Commun. 11, 2001 (2020).
Molecule of the Week Archive: Ammonia (ACS); www.acs.org/content/acs/en/molecule-of-the-week/archive/a/ammonia.html
Molecule of the Week Archive: Urea (ACS); www.acs.org/content/acs/en/molecule-of-the-week/archive/u/urea.html
Lv, C. et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide. Nat. Sustain. 4, 868–876 (2021).
Ammonia Technology Roadmap (International Energy Agency, 2021).
Cogert, K. I., Ziels, R. M. & Winkler, M. K. H. Reducing cost and environmental impact of wastewater treatment with denitrifying methanotrophs, anammox and mainstream anaerobic treatment. Environ. Sci. Technol. 53, 12935–12944 (2019).
Damma, D., Ettireddy, P., Reddy, B. & Smirniotis, P. A review of low temperature NH3-SCR for removal of NOx. Catalysts 9, 349 (2019).
Wu, Y., Jiang, Z., Lin, Z., Liang, Y. & Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 4, 725–730 (2021).
Saravanakumar, D., Song, J., Lee, S., Hur, N. H. & Shin, W. Electrocatalytic conversion of carbon dioxide and nitrate ions to urea by a titania–Nafion composite electrode. ChemSusChem 10, 3999–4003 (2017).
Lv, C. et al. A defect engineered electrocatalyst that promotes high-efficiency urea synthesis under ambient conditions. ACS Nano 16, 8213–8222 (2022).
Li, J., Zhang, Y., Kuruvinashetti, K. & Kornienko, N. Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis. Nat. Rev. Chem. 6, 303–319 (2022).
Jouny, M. et al. Formation of carbon–nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 11, 846–851 (2019).
Tao, Z., Rooney, C. L., Liang, Y. & Wang, H. Accessing organonitrogen compounds via C−N coupling in electrocatalytic CO2 reduction. J. Am. Chem. Soc. 143, 19630–19642 (2021).
Meng, N. et al. Oxide-derived core-shell Cu@Zn nanowires for urea electrosynthesis from carbon dioxide and nitrate in water. ACS Nano 16, 9095–9104 (2022).
Fernandez-Nava, Y., Maranon, E., Soons, J. & Castrillon, L. Denitrification of wastewater containing high nitrate and calcium concentrations. Bioresour. Technol. 99, 7976–7981 (2008).
Moloantoa, K. M., Khetsha, Z. P., van Heerden, E., Castillo, J. C. & Cason, E. D. Nitrate water contamination from industrial activities and complete denitrification as a remediation option. Water 14, 799 (2022).
Shi, L., Liu, L., Yang, B., Sheng, G. & Xu, T. Evaluation of industrial urea energy consumption (EC) based on life cycle assessment (LCA). Sustainability 12, 3793 (2020).
Bargiacchi, E., Antonelli, M. & Desideri, U. A comparative assessment of power-to-fuel production pathways. Energy 183, 1253–1265 (2019).
Shibata, M., Yoshida, K. & Furuya, N. Electrochemical synthesis of urea on reduction of carbon dioxide with nitrate and nitrite ions using Cu-loaded gas-diffusion electrode. J. Electroanal. Chem. 387, 143–145 (1995).
Shibata, M., Yoshida, K. & Furuya, N. Electrochemical synthesis of urea at gas-diffusion electrodes—IV. Simultaneous reduction of carbon dioxide and nitrate ions with various metal catalysts. J. Electrochem. Soc. 145, 2348–2353 (1998).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Keuleers, R., Desseyn, H. O., Rousseau, B. & Alsenoy, C. V. Vibrational analysis of urea. J. Phys. Chem. A 103, 4621–4630 (1999).
Manivannan, M. Investigation of inhibitive action of urea−Zn2+ system in the corrosion control of carbon steel in sea water. Int. J. Eng. Sci. Technol. 3, 8084–8060 (2011).
Bossa, J. B., Theulé, P., Duvernay, F., Borget, F. & Chiavassa, T. Carbamic acid and carbamate formation in NH3:CO2 ices—UV irradiation versus thermal processes. Astron. Astrophys. 492, 719–724 (2008).
Lam, K. L., Zlatanović, L. & van der Hoek, J. P. Life cycle assessment of nutrient recycling from wastewater: a critical review. Water Res. 173, 115519 (2020).
Gowd, S. C. et al. Life cycle assessment of comparing different nutrient recovery systems from municipal wastewater: a path towards self-reliance and sustainability. J. Clean. Prod. 410, 137331 (2023).
Xia, R., Overa, S. & Jiao, F. Emerging electrochemical processes to decarbonize the chemical industry. JACS Au 2, 1054–1070 (2022).
Net Zero by 2050 (International Energy Agency, 2021); https://www.iea.org/reports/net-zero-by-2050
Arquer, F. P. G. D. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Rahmatullah, M. & Boyde, T. R. C. Improvements in the determination of urea using diacetylmonoxime; methods with and without deproteinization. Clin. Chim. Acta 107, 3–9 (1980).
Chen, C. et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 12, 717–724 (2020).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Montoya, J. H., Shi, C., Chan, K. & Nørskov, J. K. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 6, 2032–2037 (2015).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Acknowledgements
We acknowledge the support of the Banting Postdoctoral Fellowships Program (no. 01353-000, Y.L.) and the National Science Fund of China for Distinguished Young Scholars (no. 52125309, B.L.). We thank the staff at the BL11B beamline of Shanghai Synchrotron Radiation Facility (SSRF) for their technical assistance.
Author information
Authors and Affiliations
Contributions
Y.L. and K.X. proposed the idea. Y.L. contributed to all experimental work, data analysis and the paper. K.X. contributed to the catalytic performance tests of the 2D hybrid catalysts and analysis. P.O. contributed to DFT calculations, with feedback from X.-Y.L. C.L. and J.B.D. contributed to LCA analysis with Y.L., with K.X.’s feedback. T.P. contributed to in situ IRRAS experiments. Z.Z. and B.L. contributed to in situ XAS characterization. Z.C. and Y.L. performed the in situ SERS characterization and analysis. N.W. and I.G. contributed to material synthesis. D.S. contributed to the discussion of the work. E.H.S. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Feng Jiao, Yafei Li and Heiko Keller for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–35, Tables 1–12 and Notes 1 and 2.
Supplementary Data
The atomic coordinates of the optimized models.
Source data
Source Data Fig. 2–6
Numerical data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Y., Xie, K., Ou, P. et al. Selective electrochemical synthesis of urea from nitrate and CO2 via relay catalysis on hybrid catalysts. Nat Catal 6, 939–948 (2023). https://doi.org/10.1038/s41929-023-01020-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01020-4
This article is cited by
-
Synergistic coupling of CO2 and NO3− for efficient electrosynthesis of urea using oxygen vacancy-rich Ru-doped CeO2 nanorods
Science China Materials (2024)