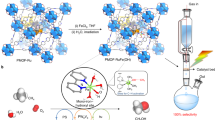
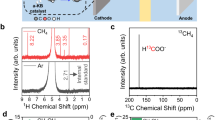
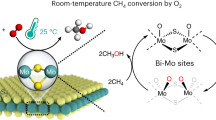
Abstract
The selective partial oxidation of methane to methanol using molecular oxygen (O2) represents a long-standing challenge, inspiring extensive study for many decades. However, considerable challenges still prevent low-temperature methane activation via the aerobic route. Here we report a precipitated Pd-containing phosphomolybdate, which, after activation by molecular hydrogen (H2), converts methane and O2 almost exclusively to methanol at room temperature. The highest activity reaches 67.4 μmol gcat−1 h−1. Pd enables rapid H2 activation and H spillover to phosphomolybdate for Mo reduction, while facile O2 activation and subsequent methane activation occur on the reduced phosphomolybdate sites. Continuous production of methanol from methane was also achieved by concurrently introducing H2, O2 and methane into the system, where H2 assists in maintaining a moderately reduced state of phosphomolybdate. This work reveals the underexplored potential of such a Mo-based catalyst for aerobic methane oxidation and highlights the importance of regulating the chemical valence state to construct methane active sites.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available from the authors upon reasonable request. Source data are provided with this paper.
References
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & Bokhoven, J. A. V. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017).
Shan, J., Li, M., Allard, L. F., Lee, S. & Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 551, 605–608 (2017).
Schwach, P., Pan, X. & Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev. 117, 8497–8520 (2017).
Wang, V. C. et al. Alkane oxidation: methane monooxygenases, related enzymes, and their biomimetics. Chem. Rev. 117, 8574–8621 (2017).
Meng, X. et al. Direct methane conversion under mild condition by thermo-, electro-, or photocatalysis. Chem 5, 2296–2325 (2019).
Sher Shah, M. S. A. et al. Catalytic oxidation of methane to oxygenated products: recent advancements and prospects for electrocatalytic and photocatalytic conversion at low temperatures. Adv. Sci. 7, 2001946 (2020).
Resources to Reserves 2013 (IEA, 2013).
Koo, C. W. & Rosenzweig, A. C. Biochemistry of aerobic biological methane oxidation. Chem. Soc. Rev. 50, 3424–3436 (2021).
Hammond, C. et al. Direct catalytic conversion of methane to methanol in an aqueous medium by using copper-promoted Fe-ZSM-5. Angew. Chem. Int. Ed. 51, 5129–5133 (2012).
Qi, G. et al. Au-ZSM-5 catalyses the selective oxidation of CH4 to CH3OH and CH3COOH using O2. Nat. Catal. 5, 45–54 (2022).
Baek, J. et al. Bioinspired metal–organic framework catalysts for selective methane oxidation to methanol. J. Am. Chem. Soc. 140, 18208–18216 (2018).
Ikuno, T. et al. Methane oxidation to methanol catalyzed by Cu-oxo clusters stabilized in NU-1000 metal–organic framework. J. Am. Chem. Soc. 139, 10294–10301 (2017).
Zheng, J. et al. Selective methane oxidation to methanol on Cu-oxo dimers stabilized by zirconia nodes of an NU-1000 metal–organic framework. J. Am. Chem. Soc. 141, 9292–9304 (2019).
Osadchii, D. Y. et al. Isolated Fe sites in metal organic frameworks catalyze the direct conversion of methane to methanol. ACS Catal. 8, 5542–5548 (2018).
Huang, W. et al. Low-temperature transformation of methane to methanol on Pd1O4 single sites anchored on the internal surface of microporous silicate. Angew. Chem. Int. Ed. 55, 13441–13445 (2016).
Kwon, Y., Kim, T. Y., Kwon, G., Yi, J. & Lee, H. Selective activation of methane on single-atom catalyst of rhodium dispersed on zirconia for direct conversion. J. Am. Chem. Soc. 139, 17694–17699 (2017).
Cui, X. et al. Room-temperature methane conversion by graphene-confined single iron atoms. Chem 4, 1902–1910 (2018).
Shen, Q. et al. Single chromium atoms supported on titanium dioxide nanoparticles for synergic catalytic methane conversion under mild conditions. Angew. Chem. Int. Ed. 59, 1216–1219 (2020).
Bai, S. et al. High-efficiency direct methane conversion to oxygenates on a cerium dioxide nanowires supported rhodium single-atom catalyst. Nat. Commun. 11, 954 (2020).
Tang, X. et al. Direct oxidation of methane to oxygenates on supported single Cu atom catalyst. Appl. Catal. B 285, 119827 (2021).
Ab Rahim, M. H. et al. Oxidation of methane to methanol with hydrogen peroxide using supported gold–palladium alloy nanoparticles. Angew. Chem. Int. Ed. 52, 1280–1284 (2013).
Agarwal, N. et al. Aqueous Au–Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 358, 223–227 (2017).
Chen, J. et al. Oxidation of methane to methanol over Pd@Pt nanoparticles under mild conditions in water. Catal. Sci. Technol. 11, 3493–3500 (2021).
He, Y. et al. Low-temperature direct conversion of methane to methanol over carbon materials supported Pd–Au nanoparticles. Catal. Today 339, 48–53 (2020).
Bai, S., Xu, Y., Wang, P., Shao, Q. & Huang, X. Activating and converting CH4 to CH3OH via the CuPdO2/CuO nanointerface. ACS Catal. 9, 6938–6944 (2019).
Wu, B. et al. Cu single-atoms embedded in porous carbon nitride for selective oxidation of methane to oxygenates. Chem. Commun. 56, 14677–14680 (2020).
Xie, J. et al. Highly selective oxidation of methane to methanol at ambient conditions by titanium dioxide-supported iron species. Nat. Catal. 1, 889–896 (2018).
McVicker, R. et al. Low temperature selective oxidation of methane using gold–palladium colloids. Catal. Today 342, 32–38 (2020).
Jin, Z. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367, 193–197 (2020).
Kang, J. & Park, E. D. Selective oxidation of methane over Fe-zeolites by in situ generated H2O2. Catalysts 10, 299 (2020).
Kang, J., Puthiaraj, P., Ahn, W.-s. & Park, E. D. Direct synthesis of oxygenates via partial oxidation of methane in the presence of O2 and H2 over a combination of Fe-ZSM-5 and Pd supported on an acid-functionalized porous polymer. Appl. Catal. A 602, 117711 (2020).
Luo, L. et al. Synergy of Pd atoms and oxygen vacancies on In2O3 for methane conversion under visible light. Nat. Commun. 13, 2930 (2022).
An, B. et al. Direct photo-oxidation of methane to methanol over a mono-iron hydroxyl site. Nat. Mater. 21, 932–938 (2022).
Fan, Y. et al. Selective photocatalytic oxidation of methane by quantum-sized bismuth vanadate. Nat. Sustain. 4, 509–515 (2021).
Srivastava, R. K., Sarangi, P. K., Bhatia, L., Singh, A. K. & Shadangi, K. P. Conversion of methane to methanol: technologies and future challenges. Biomass. Convers. Biorefin. 12, 1851–1875 (2021).
Lopez, X., Carbo, J. J., Bo, C. & Poblet, J. M. Structure, properties and reactivity of polyoxometalates: a theoretical perspective. Chem. Soc. Rev. 41, 7537–7571 (2012).
Wang, S.-S. & Yang, G.-Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 115, 4893–4962 (2015).
Zhang, B. et al. Stabilizing a platinum1 single-atom catalyst on supported phosphomolybdic acid without compromising hydrogenation activity. Angew. Chem. Int. Ed. 55, 8319–8323 (2016).
Zhang, B. et al. Atomically dispersed Pt1-polyoxometalate catalysts: how does metal–support interaction affect stability and hydrogenation activity? J. Am. Chem. Soc. 141, 8185–8197 (2019).
Hülsey, M. J., Fung, V., Hou, X., Wu, J. & Yan, N. Hydrogen spillover and its relation to hydrogenation: observations on structurally defined single-atom sites. Angew. Chem. Int. Ed. 61, e202208237 (2022).
Hülsey, M. J. et al. Identifying key descriptors for the single-atom catalyzed CO oxidation. CCS Chem. 4, 3296–3308 (2022).
Geng, Y. & Li, H. Hydrogen spillover-enhanced heterogeneously catalyzed hydrodeoxygenation for biomass upgrading. ChemSusChem 15, e202102495 (2022).
Xiong, M., Gao, Z. & Qin, Y. Spillover in heterogeneous catalysis: new insights and opportunities. ACS Catal. 11, 3159–3172 (2021).
Xi, Q. et al. In-situ fabrication of MoO3 nanobelts decorated with MoO2 nanoparticles and their enhanced photocatalytic performance. Appl. Surf. Sci. 480, 427–437 (2019).
Dieterle, M., Weinberg, G. & Mestl, G. Raman spectroscopy of molybdenum oxides. Phys. Chem. Chem. Phys. 4, 812–821 (2002).
Ravi, M. et al. Misconceptions and challenges in methane-to-methanol over transition-metal-exchanged zeolites. Nat. Catal. 2, 485–494 (2019).
Richards, T. et al. A residue-free approach to water disinfection using catalytic in situ generation of reactive oxygen species. Nat. Catal. 4, 575–585 (2021).
Fontmorin, J. M., Burgos Castillo, R. C., Tang, W. Z. & Sillanpaa, M. Stability of 5,5-dimethyl-1-pyrroline-N-oxide as a spin-trap for quantification of hydroxyl radicals in processes based on Fenton reaction. Water Res. 99, 24–32 (2016).
Conte, M. et al. Insights into the reaction mechanism of cyclohexane oxidation catalysed by molybdenum blue nanorings. Catal. Lett. 146, 126–135 (2016).
Liu, X. et al. Molybdenum blue nano-rings: an effective catalyst for the partial oxidation of cyclohexane. Catal. Sci. Technol. 5, 217–227 (2015).
Cozar, O., Magdas, D. A. & Ardelean, I. EPR study of molybdenum-lead-phosphate glasses. J. Non-Cryst. Solids 354, 1032–1035 (2008).
Almidani, A. H. et al. The reaction of HV(CO)4dppe with MoO3: a well-defined model of hydrogen spillover. Catal. Sci. Technol. 11, 7540–7544 (2021).
Timmiati, S. N., Jalil, A. A., Triwahyono, S., Setiabudi, H. D. & Annuar, N. H. R. Formation of acidic Brönsted (MoOx)−(Hy)+ evidenced by XRD and 2,6-lutidine FTIR spectroscopy for cumene cracking. Appl. Catal. A 459, 8–16 (2013).
Triwahyono, S., Jalil, A. A., Ruslan, N. N., Setiabudi, H. D. & Kamarudin, N. H. N. C5–C7 linear alkane hydroisomerization over MoO3–ZrO2 and Pt/MoO3–ZrO2 catalysts. J. Catal. 303, 50–59 (2013).
Spencer, J., Folli, A., Richards, E. & Murphy, D. M. in Electron Paramagnetic Resonance, Vol. 26 (eds Chechik, V. & Murphy, D. M.) 130–170 (Royal Society of Chemistry, 2018).
Selvaraj, U. & Rao, K. J. ESR and optical studies of Mo5+ and W5+ ions in phosphomolybdate and phosphotungstate glasses. Chem. Phys. 123, 141–150 (1988).
Kivelson, D. & Lee, S. K. ESR studies and the electronic structure of vanadyl ion complexes. J. Chem. Phys. 41, 1896–1903 (2004).
Abragam, A., Pryce, M. H. L. & Simon, F. E. Theory of the nuclear hyperfine structure of paramagnetic resonance spectra in crystals. Proc. R. Soc. Lond. A Math. Phys. Sci. 205, 135–153 (1951).
Abragam, A., Pryce, M. H. L. & Bleaney, B. The theory of the nuclear hyperfine structure of paramagnetic resonance spectra in the copper Tutton salts. Proc. R. Soc. Lond. A Math. Phys. Sci. 206, 164–172 (1951).
Łabanowska, M. EPR monitoring of redox processes in transition metal oxide catalysts. ChemPhysChem 2, 712–731 (2001).
Łabanowska, M. Paramagnetic defects in MoO3—revisited. Phys. Chem. Chem. Phys. 1, 5385–5392 (1999).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
Kresse, G., & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A. Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
We thank the National Natural Science Foundation of China (92061109) for supporting the project. N.Y. and Q.H. sincerely acknowledge the support of the National Research Foundation (NRF) Singapore, under its NRF Investigatorship (NRF-NRFI07–2021–0006) and NRF Fellowship (NRF-NRFF11-2019-0002), respectively. R.J.L. and G.J.H. gratefully acknowledge Cardiff University and the Max Planck Centre for Fundamental Heterogeneous Catalysis (FUNCAT) for financial support. Z.Y. acknowledges support from the National Natural Science Foundation of China (52222102, 22272024 and 51871058) and the Eyas Program of Fujian Province. DFT simulations were conducted at the Center for Nanophase Materials Sciences (CNMS), which is a US Department of Energy, Office of Science User Facility at Oak Ridge National Laboratory.
Author information
Authors and Affiliations
Contributions
N.Y. conceived and supervised the project. Q.H. and G.J.H. co-supervised the project. S.W. conducted most experiments including synthesis, characterization and testing, as well as data analysis. V.F. carried out DFT calculations and wrote the related section. M.J.H. and J.C. carried out the catalyst synthesis and characterization. X.L. and Z.Y. conducted TEM analysis. A.F. and R.J.L. contributed to data analysis of the EPR spectra and H2O2 detection. S.W., Q.H. and N.Y. wrote the paper. G.J.H., A.F. and R.J.L. revised the paper. All authors discussed the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Lars Grabow, Gunnar Jeschke and Liang Wang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32, Notes 1 and 2, Tables 1–7 and Refs. 1–11.
Source data
Source Data Fig. 4
INCAR and POSCAR files for the structures shown in Fig. 4 for DFT calculations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Fung, V., Hülsey, M.J. et al. H2-reduced phosphomolybdate promotes room-temperature aerobic oxidation of methane to methanol. Nat Catal 6, 895–905 (2023). https://doi.org/10.1038/s41929-023-01011-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01011-5
This article is cited by
-
Methane oxidation by catalyst reduction
Nature Catalysis (2023)