Abstract
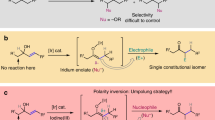
The enantioselective synthesis of chiral Z-olefins is an important but challenging topic in organic chemistry. Iridium-catalysed Z-retentive asymmetric allylic substitution reactions have recently emerged as a promising strategy for trapping thermodynamically less stable anti-π-allyliridium intermediates. However, detailed mechanistic knowledge about this process has remained elusive. Here we report the structural characterization and transformations of the previously assumed putative anti-π-allyliridium intermediates. These complexes are highly efficient catalysts that enable Z-retentive asymmetric allylic alkylation of oxindoles accommodating a wide substrate scope. An iridium catalyst with a different metal-to-ligand ratio from that of a similar catalyst reported in the literature has been found to be crucial for regioselective nucleophilic attack at the less substituted allylic terminus. This simple yet powerful approach lays a solid foundation towards a general platform for the enantioselective synthesis of chiral Z-olefins.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Detailed experimental procedures, characterizations of new compounds and computational results are provided in the Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2161348 (K1c-Cl), 2161343 (K1c-Br), 2161345 (K1c-I), 2161346 (K2a-Cl-H), 2161347 (K2a-I-H), 2161354 (K2c-Br-H), 2161350 (K2c-I-H), 2161340 (anti-K2c-Br-Me), 2161342 (anti-K2c-Br-Pr), 2161344 (anti-K2c-Br-Ph), 2161341 (exo-syn-K2c-Br-Me), 2161353 (endo-syn-K2c-Br-Pr), 2161349 (Z-3bA), 2161351 (Z-3a′A) and 2161352 (Z-3uA, structure shown in Supplementary Fig. 20). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. The output files of the theoretical calculations in this work have been deposited at Zenodo (https://doi.org/10.5281/zenodo.6817573). All other data are available from the corresponding authors upon reasonable request.
References
Siau, W.-Y., Zhang, Y. & Zhao, Y. Stereoselective synthesis of Z-alkenes. Top. Curr. Chem. 327, 33–58 (2012).
Oger, C., Balas, L., Durand, T. & Galano, J.-M. Are alkyne reductions chemo-, regio-, and stereoselective enough to provide pure (Z)-olefins in polyfunctionalized bioactive molecules? Chem. Rev. 113, 1313–1350 (2013).
Kluwer, A. M. & Elsevier, C. J. in Handbook of Homogeneous Hydrogenation (eds de Vries, J. G. & Elsevier, C. J.) 375–412 (Wiley-VCH, 2007).
Negishi, E.-i. et al. Recent advances in efficient and selective synthesis of di-, tri-, and tetrasubstituted alkenes via Pd-catalyzed alkenylation–carbonyl olefination synergy. Acc. Chem. Res. 41, 1474–1485 (2008).
Xu, S., Kamada, H., Kim, E. H., Oda, A. & Negishi, E.-i. in Metal-Catalyzed Cross-Coupling Reactions and More (eds de Meijere, A. et al.) 133–278 (Wiley-VCH, 2014).
Armstrong, M. K., Goodstein, M. B. & Lalic, G. Diastereodivergent reductive cross coupling of alkynes through tandem catalysis: Z- and E-selective hydroarylation of terminal alkynes. J. Am. Chem. Soc. 140, 10233–10241 (2018).
Ogba, O. M., Warner, N. C., O’Leary, D. J. & Grubbs, R. H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 47, 4510–4544 (2018).
Koh, M. J. et al. High-value alcohols and higher-oxidation-state compounds by catalytic Z-selective cross-metathesis. Nature 517, 181–186 (2015).
Mu, Y., Nguyen, T. T., Koh, M. J., Schrock, R. R. & Hoveyda, A. H. E- and Z-, di- and tri-substituted alkenyl nitriles through catalytic cross-metathesis. Nat. Chem. 11, 478–487 (2019).
Nevesely, T., Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E → Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2022).
Maryanoff, B. E. & Reitz, A. B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 89, 863–927 (1989).
Kazmaier, U. (ed.) Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis (Springer, 2012).
Trost, B. M. & Crawley, M. L. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 103, 2921–2944 (2003).
Lu, Z. & Ma, S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. Int. Ed. 47, 258–297 (2008).
Consiglio, G. & Waymouth, R. M. Enantioselective homogeneous catalysis involving transition-metal-allyl intermediates. Chem. Rev. 89, 257–276 (1989).
Faller, J. W., Thomsen, M. E. & Mattina, M. J. Organometallic conformational equilibriums. X. Steric factors and their mechanistic implications in π-allyl(amine)chloropalladium(II) complexes. J. Am. Chem. Soc. 93, 2642–2653 (1971).
Gibson, D. H. & Erwin, D. K. Anti to syn isomerization in π-allyliron carbonyl complexes. J. Org. Chem. 86, C31–C33 (1975).
Tobisch, S. & Taube, R. Density functional (DFT) study of the anti–syn isomerization of the butenyl group in cationic and neutral (butenyl)(butadiene)(monoligand)nickel(II) complexes. Organometallics 18, 3045–3060 (1999).
Takeuchi, R. & Kashio, M. Iridium complex-catalyzed allylic alkylation of allylic esters and allylic alcohols: unique regio- and stereoselectivity. J. Am. Chem. Soc. 120, 8647–8655 (1998).
Kazmaier, U. & Zumpe, F. L. Palladium-catalyzed allylic alkylations without isomerization—dream or reality? Angew. Chem. Int. Ed. 39, 802–804 (2000).
Lin, H.-C. et al. Nucleophile-dependent Z/E- and regioselectivity in the palladium-catalyzed asymmetric allylic C–H alkylation of 1,4-dienes. J. Am. Chem. Soc. 141, 5824–5834 (2019).
Jette, C. I., Tong, Z. J., Hadt, R. G. & Stoltz, B. M. Copper-catalyzed enantioselective allylic alkylation with a γ-butyrolactone-derived silyl ketene acetal. Angew. Chem. Int. Ed. 59, 2033–2038 (2020).
Janssen, J. P. & Helmchen, G. First enantioselective alkylations of monosubstituted allylic acetates catalyzed by chiral iridium complexes. Tetrahedron Lett. 38, 8025–8026 (1997).
Cheng, Q. et al. Iridium-catalyzed asymmetric allylic substitution reactions. Chem. Rev. 119, 1855–1969 (2019).
Rössler, S. L., Petrone, D. A. & Carreira, E. M. Iridium-catalyzed asymmetric synthesis of functionally rich molecules enabled by (phosphoramidite,olefin) ligands. Acc. Chem. Res. 52, 2657–2672 (2019).
Qu, J. & Helmchen, G. Applications of iridium-catalyzed asymmetric allylic substitution reactions in target-oriented synthesis. Acc. Chem. Res. 50, 2539–2555 (2017).
Bartels, B. & Helmchen, G. Ir-catalysed allylic substitution: mechanistic aspects and asymmetric synthesis with phosphorus amidites as ligands. Chem. Commun. 741–742 (1999).
Madrahimov, S. T. & Hartwig, J. F. Origins of enantioselectivity during allylic substitution reactions catalyzed by metallacyclic iridium complexes. J. Am. Chem. Soc. 134, 8136–8147 (2012).
Liu, W.-B., Zheng, C., Zhuo, C.-X., Dai, L.-X. & You, S.-L. Iridium-catalyzed allylic alkylation reaction with N-aryl phosphoramidite ligands: scope and mechanistic studies. J. Am. Chem. Soc. 134, 4812–4821 (2012).
Jiang, R., Ding, L., Zheng, C. & You, S.-L. Iridium-catalyzed Z-retentive asymmetric allylic substitution reactions. Science 371, 380–386 (2021).
Ding, L., Song, H., Zheng, C. & You, S.-L. Enantioselective synthesis of medium-sized-ring lactones via iridium-catalyzed Z-retentive asymmetric allylic substitution reaction. J. Am. Chem. Soc. 144, 4770–4775 (2022).
Rossler, S. L., Krautwald, S. & Carreira, E. M. Study of intermediates in iridium–(phosphoramidite,olefin)-catalyzed enantioselective allylic substitution. J. Am. Chem. Soc. 139, 3603–3606 (2017).
Wolf, J. & Werner, H. Basic metals. Part 61. Synthesis of [(C5H5)Rh(η3-1-MeC3H4)[P(CHMe2)3]PF6 from (C5H5)Rh(MeC≡CMe)[P(CHMe2)3]. The mechanism of conversion of an alkyne into an allyl ligand via an allene intermediate. Organometallics 6, 1164–1169 (1987).
Appleton, T. G., Clark, H. C. & Manzer, L. E. The trans-influence: its measurement and significance. Coord. Chem. Rev. 10, 335–422 (1973).
Madrahimov, S. T., Markovic, D. & Hartwig, J. F. The allyl intermediate in regioselective and enantioselective iridium-catalyzed asymmetric allylic substitution reactions. J. Am. Chem. Soc. 131, 7228–7229 (2009).
Spiess, S., Raskatov, J. A., Gnamm, C., Brodner, K. & Helmchen, G. Ir-catalyzed asymmetric allylic substitutions with (phosphoramidite)Ir complexes—resting states, synthesis, and characterization of catalytically active (π-allyl)Ir complexes. Chem. Eur. J. 15, 11087–11090 (2009).
Frisch, M. J. et al. Gaussian 16, Revision A.03 (Gaussian Inc., 2016).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Acknowledgements
We thank the National Key Research and Development Program of China (2021YFA1500100), the National Natural Science Foundation of China (21821002, 22031012, 22171282 and 91856201), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (Y2021075) and the Science and Technology Commission of Shanghai Municipality (19590750400 and 21520780100) for generous financial support. We also thank X. Leng (SIOC) and L. Li (Shanghai Jiao Tong University) for X-ray crystallographic analysis, and R. Leveson-Gower (University of Groningen) for proofreading the manuscript. S.-L.Y. thanks the support of the Tencent Foundation through an XPLORER PRIZE.
Author information
Authors and Affiliations
Contributions
S.-L.Y. conceived and supervised the project; R.J. synthesized and characterized the Ir complexes and developed the Ir-catalysed Z-retentive asymmetric allylic substitution reactions; Q.-R.Z. contributed to expanding the scope of the reaction; R.J. and C.Z. performed the electronic structure calculations; C.Z. wrote the manuscript with revisions suggested by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Liqin Qiu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–21, Tables 1–19, notes and references.
Supplementary Data 1
Cif file for K1c-I.
Supplementary Data 2
Cif file for K1c-Br.
Supplementary Data 3
Cif file for K1c-Cl.
Supplementary Data 4
Cif file for K2a-Cl-H.
Supplementary Data 5
Cif file for K2a-I-H.
Supplementary Data 6
Cif file for K2c-Br-H.
Supplementary Data 7
Cif file for K2c-I-H.
Supplementary Data 8
Cif file for anti-K2c-Br-Me.
Supplementary Data 9
Cif file for anti-K2c-Br-Pr.
Supplementary Data 10
Cif file for anti-K2c-Br-Ph.
Supplementary Data 11
Cif file for exo-syn-K2c-Br-Me.
Supplementary Data 12
Cif file for endo-syn-K2c-Br-Pr.
Supplementary Data 13
Cif file for Z-3bA.
Supplementary Data 13
Cif file for Z-3a′A.
Supplementary Data 13
Cif file for Z-3uA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, R., Zhao, QR., Zheng, C. et al. Structurally defined anti-π-allyliridium complexes catalyse Z-retentive asymmetric allylic alkylation of oxindoles. Nat Catal 5, 1089–1097 (2022). https://doi.org/10.1038/s41929-022-00879-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00879-z