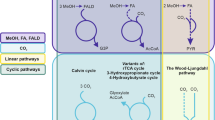
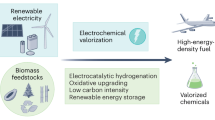
Abstract
Concerns regarding petroleum depletion and global climate change caused by greenhouse gas emissions have spurred interest in renewable alternatives to fossil fuels. Third-generation (3G) biorefineries aim to utilize microbial cell factories to convert renewable energies and atmospheric CO2 into fuels and chemicals, and hence represent a route for assessing fuels and chemicals in a carbon-neutral manner. However, to establish processes competitive with the petroleum industry, it is important to clarify/evaluate/identify the most promising CO2 fixation pathways, the most appropriate CO2 utilization models and the necessary productivity levels. Here, we discuss the latest advances in 3G biorefineries. Following an overview of applications of CO2 feedstocks, mainly from flue gas and waste gasification, we review prominent opportunities and barriers in CO2 fixation and energy capture. We then summarize reported CO2-based products and industries, and describe trends and key challenges for future advancement of 3G biorefineries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Petit, J.-R. et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 399, 429 (1999).
Earth’s CO2 Home Page. CO 2.earth https://www.co2.earth (2019).
Xu, Y., Ramanathan, V. & Victor, D. G. Global warming will happen faster than we think. Nature 564, 30–32 (2018).
Glikson, A. The lungs of the Earth: review of the carbon cycle and mass extinction of species. Energy Procedia 146, 3–11 (2018).
Hughes, T. P. et al. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (2003).
Enguídanos, M., Soria, A., Kavalov, B. & Jensen, P. Techno-Economic Analysis of Bio-alcohol Production in the EU: A Short Summary for Decision-Makers (European Commission, 2002); https://core.ac.uk/download/pdf/38614579.pdf
Musa, S. D., Zhonghua, T., Ibrahim, A. O. & Habib, M. China’s energy status: a critical look at fossils and renewable options. Renew. Sust. Energ. Rev. 81, 2281–2290 (2017).
Jones, S. W. et al. CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion. Nat. Commun. 7, 12800 (2016).
Charubin, K. & Papoutsakis, E. T. Direct cell-to-cell exchange of matter in a synthetic Clostridium syntrophy enables CO2 fixation, superior metabolite yields, and an expanded metabolic space. Metab. Eng. 52, 9–19 (2019).
Global Energy and CO 2 Status Report (IEA, 2017); http://www.iea.org/publications/freepublications/publication/GECO2017.pdf
Special Report on Carbon Dioxide Capture and Storage (IPCC, 2005); http://www.precaution.org/lib/ipcc_ccs_report.050901.pdf
Global Waste Generation Could Increase 70% by 2050 (World Bank, 2018); http://www.wastedive.com/news/world-bank-global-waste-generation-2050/533031
Vuppaladadiyam, A. K. et al. Impact of flue gas compounds on microalgae and mechanisms for carbon assimilation and utilization. ChemSusChem 11, 334–355 (2018).
Chandolias, K., Richards, T. & Taherzadeh, M. J. Waste Biorefinery (Elsevier, 2018).
Chiu, S.-Y. et al. Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. cultures. Bioresour. Technol. 102, 9135–9142 (2011).
Direct Air Capture of CO 2 with Chemicals (American Physical Society, 2016); http://www.aps.org/policy/reports/assessments/upload/dac2011.pdf
Liew, F., Koepke, M. & Simpson, S. Liquid, Gaseous and Solid Biofuels – Conversion Techniques (IntechOpen, 2013).
Hafenbradl, D. & Hein, M. Power-to-gas: a solution for energy storage. Gas. Energy 4, 26–29 (2015).
Daniels, L., Fuchs, G., Thauer, R. K. & Zeikus, J. G. Carbon monoxide oxidation by methanogenic bacteria. J. Bacteriol. 132, 118–126 (1977).
Nithiya, E. M., Tamilmani, J., Vasumathi, K. K. & Premalatha, M. Improved CO2 fixation with Oscillatoria sp. in response to various supply frequencies of CO2 supply. J. CO2 Util. 18, 198–205 (2017).
Duarte, J. H., de Morais, E. G., Radmann, E. M. & Costa, J. A. V. Biological CO2 mitigation from coal power plant by Chlorella fusca and Spirulina sp. Bioresour. Technol. 234, 472–475 (2017).
Liang, F. et al. The effects of physicochemical factors and cell density on nitrite transformation in a lipid-rich Chlorella. J. Microbiol. Biotechnol. 25, 2116–2124 (2015).
Calvin, M. & Benson, A. A. The path of carbon in photosynthesis. Science 107, 476–480 (1948).
Fuchs, G. Alternative pathways of carbon dioxide fixation: insights into the early evolution of life? Annu. Rev. Microbiol. 65, 631–658 (2011).
Ducat, D. C. & Silver, P. A. Improving carbon fixation pathways. Curr. Opin. Chem. Biol. 16, 337–344 (2012).
Kumar, M., Sundaram, S., Gnansounou, E., Larroche, C. & Thakur, I. S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: a review. Bioresour. Technol. 247, 1059–1068 (2018).
Gleizer, S. et al. Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 179, 1255–1263 (2019). This work illustrates how to transform the heterotrophic mode of a microbial cell factory into the autotrophic mode, providing guidelines for future 3G biorefineries.
Schulman, M., Parker, D., Ljungdahl, L. G. & Wood, H. G. Total synthesis of acetate from CO2 V. determination by mass analysis of the different types of acetate formed from13CO2 by heterotrophic bacteria. J. Bacteriol. Parasitol. 109, 633–644 (1972).
Figueroa, I. A. et al. Metagenomics-guided analysis of microbial chemolithoautotrophic phosphite oxidation yields evidence of a seventh natural CO2 fixation pathway. Proc. Natl Acad. Sci. USA 115, 92–101 (2018).
Kikuchi, G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol. Cell. Biochem. 1, 169–187 (1973).
Fast, A. G. & Papoutsakis, E. T. Functional expression of the Clostridium ljungdahlii acetyl-coenzyme A synthase in Clostridium acetobutylicum as demonstrated by a novel in vivo CO exchange activity en route to heterologous installation of a functional Wood-Ljungdahl pathway. Appl. Environ. Microbiol. 84, 2307–2317 (2018).
Bang, J. & Lee, S. Y. Assimilation of formic acid and CO2 by engineered Escherichia coli equipped with reconstructed one-carbon assimilation pathways. Proc. Natl Acad. Sci. USA 115, 9271–9279 (2018).
Bar-Even, A. Formate assimilation: the metabolic architecture of natural and synthetic pathways. Biochemistry 55, 3851–3863 (2016).
Döring, V., Darii, E., Yishai, O., Bar-Even, A. & Bouzon, M. Implementation of a reductive route of one-carbon assimilation in Escherichia coli through directed evolution. ACS Synth. Biol. 7, 2029–2036 (2018).
Tashiro, Y., Hirano, S., Matson, M. M., Atsumi, S. & Kondo, A. Electrical-biological hybrid system for CO2 reduction. Metab. Eng. 47, 211–218 (2018).
Yishai, O., Bouzon, M., Döring, V. & Bar-Even, A. In vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli. ACS Synth. Biol. 7, 2023–2028 (2018).
Gonzalez de la Cruz, J., Machens, F., Messerschmidt, K. & Bar-Even, A. Core catalysis of the reductive glycine pathway demonstrated in yeast. ACS Synth. Biol. 8, 911–917 (2019).
Huber, H. et al. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proc. Natl Acad. Sci. USA 105, 7851–7856 (2008).
Berg, I. A., Kockelkorn, D., Buckel, W. & Fuchs, G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science 318, 1782–1786 (2007).
Hügler, M., Huber, H., Stetter, K. O. & Fuchs, G. Autotrophic CO2 fixation pathways in archaea (Crenarchaeota). Arch. Microbiol. 179, 160–173 (2003).
Holo, H. Chloroflexus aurantiacus secretes 3-hydroxypropionate, a possible intermediate in the assimilation of CO2 and acetate. Arch. Microbiol. 151, 252–256 (1989).
Strauss, G. & Fuchs, G. Enzymes of a novel autotrophic CO2 fixation pathway in the phototrophic bacterium Chloroflexus aurantiacus, the 3‐hydroxypropionate cycle. Eur. J. Biochem. 215, 633–643 (1993).
Evans, M., Buchanan, B. B. & Arnon, D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc. Natl Acad. Sci. USA 55, 928–934 (1966).
Fuchs, G., Stupperich, E. & Eden, G. Autotrophic CO2 fixation in Chlorobium limicola. Evidence for the operation of a reductive tricarboxylic acid cycle in growing cells. Arch. Microbiol. 128, 64–71 (1980).
Ramos-Vera, W. H., Berg, I. A. & Fuchs, G. Autotrophic carbon dioxide assimilation in Thermoproteales revisited. J. Bacteriol. 191, 4286–4297 (2009).
Blochl, E. Pyrolobus fumarii, gen. and sp. nov., represents and novel group of archaea, extending the upper temperature limit for life to 113°C. Extremophiles 1, 14–21 (1997).
Keller, M. W. et al. Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide. Proc. Natl Acad. Sci. USA 110, 5840–5845 (2013).
Hügler, M., Menendez, C., Schägger, H. & Fuchs, G. Malonyl-coenzyme A reductase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation. J. Bacteriol. 184, 2404–2410 (2002).
Alber, B. E. & Fuchs, G. Propionyl-coenzyme A synthase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation. J. Biol. Chem. 277, 12137–12143 (2002).
Mattozzi, M. D., Ziesack, M., Voges, M. J., Silver, P. A. & Way, J. C. Expression of the sub-pathways of the Chloroflexus aurantiacus 3-hydroxypropionate carbon fixation bicycle in E. coli: Toward horizontal transfer of autotrophic growth. Metab. Eng. 16, 130–139 (2013).
Fast, A. G. & Papoutsakis, E. T. Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals. Curr. Opin. Chem. Eng. 1, 380–395 (2012). A comprehensive review comparing energetic efficiencies of four non-photosynthetic carbon fixation pathways for cell growth and production of ethanol, acetate, 2,3-butanediol and butyrate.
Ivanovsky, R., Sintsov, N. & Kondratieva, E. ATP-linked citrate lyase activity in the green sulfur bacterium Chlorobium limicola forma thiosulfatophilum. Arch. Microbiol. 128, 239–241 (1980).
Hügler, M., Huber, H., Molyneaux, S. J., Vetriani, C. & Sievert, S. M. Autotrophic CO2 fixation via the reductive tricarboxylic acid cycle in different lineages within the phylum Aquificae: evidence for two ways of citrate cleavage. Environ. Microbiol. 9, 81–92 (2007).
Mall, A. et al. Reversibility of citrate synthase allows autotrophic growth of a thermophilic bacterium. Science 359, 563–567 (2018).
Nunoura, T. et al. A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile. Science 359, 559–563 (2018).
Guo, L. et al. Enhancement of malate production through engineering of the periplasmic rTCA pathway in Escherichia coli. Biotechnol. Bioeng. 115, 1571–1580 (2018).
Schwander, T. et al. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 354, 900–904 (2016). This work illustrates how the design and construction of a synthetic CO 2 fixation pathway that is in vitro much faster than the CBB cycle in cell extracts.
Gong, F. & Li, Y. Fixing carbon, unnaturally. Science 354, 830–831 (2016).
Erb, T. J., Brecht, V., Fuchs, G., Müller, M. & Alber, B. E. Carboxylation mechanism and stereochemistry of crotonyl-CoA carboxylase/reductase, a carboxylating enoyl-thioester reductase. Proc. Natl Acad. Sci. USA 106, 8871–8876 (2009).
Schwander, T. & Erb, T. J. Do it your (path) way–synthetische Wege zur CO2-Fixierung. BIOspektrum 22, 590–592 (2016).
Stoffel, G. M. M. et al. Four amino acids define the CO2 binding pocket of enoyl-CoA carboxylases/reductases. Proc. Natl Acad. Sci. USA 116, 13964–13969 (2019).
Berg, I. A. et al. Autotrophic carbon fixation in archaea. Nat. Rev. Microbiol. 8, 447–460 (2010).
Bar-Even, A., Noor, E. & Milo, R. A survey of carbon fixation pathways through a quantitative lens. J. Exp. Bot. 63, 2325–2342 (2012). This work presents a thorough technoeconomic analysis of current identified carbon fixation pathways and suggests potential metabolic structures of yet to be identified CO 2 fixation pathways.
Bar-Even, A., Noor, E., Lewis, N. E. & Milo, R. Design and analysis of synthetic carbon fixation pathways. Proc. Natl Acad. Sci. USA 107, 8889–8894 (2010).
Näser, U. et al. Synthesis of 13C-labeled γ-hydroxybutyrates for EPR studies with 4-hydroxybutyryl-CoA dehydratase. Bioorg. Chem. 33, 53–66 (2005).
Könneke, M. et al. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc. Natl Acad. Sci. USA 111, 8239–8244 (2014).
South, P. F., Cavanagh, A. P., Liu, H. W. & Ort, D. R. J. S. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363, eaat9077 (2019).
Arai, H., Kanbe, H., Ishii, M. & Igarashi, Y. Complete genome sequence of the thermophilic, obligately chemolithoautotrophic hydrogen-oxidizing bacterium Hydrogenobacter thermophilus TK-6. J. Bacteriol. 192, 2651–2652 (2010).
Ramos-Vera, W. H., Weiss, M., Strittmatter, E., Kockelkorn, D. & Fuchs, G. Identification of missing genes and enzymes for autotrophic carbon fixation in. Crenarchaeota. J. Bacteriol. 193, 1201–1211 (2011).
Emerson, D. F. & Stephanopoulos, G. Limitations in converting waste gases to fuels and chemicals. Curr. Opin. Biotechnol. 59, 39–45 (2019).
Li, F.-F. et al. Microalgae capture of CO2 from actual flue gas discharged from a combustion chamber. Ind. Eng. Chem. Res. 50, 6496–6502 (2011).
Liew, F. et al. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab. Eng. 40, 104–114 (2017).
Alberty, R. A. Thermodynamics of Biochemical Reactions (John Wiley and Sons, 2003).
Tran, Q. H. & Unden, G. Changes in the proton potential and the cellular energetics of Escherichia coli during growth by aerobic and anaerobic respiration or by fermentation. FEBS J. 251, 538–543 (1998).
Boyle, N. R. & Morgan, J. A. Computation of metabolic fluxes and efficiencies for biological carbon dioxide fixation. Metab. Eng. 13, 150–158 (2011). This study provides a quantitative study of all six native CO 2 fixation pathways for their thermodynamic efficiencies for biomass production, and suggests that, when taking into account the cost of hydrogen production, photoautotrophic pathways are more efficient than chemoautotrophic pathways.
Lahtvee, P.-J. et al. Absolute quantification of protein and mRNA abundances demonstrate variability in gene-specific translation efficiency in yeast. Cell Syst. 4, 1–10 (2017).
Roger, M., Brown, F., Gabrielli, W. & Sargent, F. Efficient hydrogen-dependent carbon dioxide reduction by Escherichia coli. Curr. Biol. 28, 140–145 (2018).
Bennett, B. D. et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 (2009).
Bar-Even, A., Noor, E., Flamholz, A., Buescher, J. M. & Milo, R. Hydrophobicity and charge shape cellular metabolite concentrations. PLoS Comput. Biol. 7, e1002166 (2011).
Bekers, K., Heijnen, J. & Van Gulik, W. Determination of the in vivo NAD: NADH ratio in Saccharomyces cerevisiae under anaerobic conditions, using alcohol dehydrogenase as sensor reaction. Yeast 32, 541–557 (2015).
Jinich, A. et al. Quantum chemistry reveals thermodynamic principles of redox biochemistry. PLoS Comput. Biol. 14, e1006471 (2018).
Perkins, C. & Weimer, A. W. Solar-thermal production of renewable hydrogen. AlChE J. 55, 286–293 (2009).
Angermayr, S. A., Rovira, A. G. & Hellingwerf, K. J. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 33, 352–361 (2015).
Bernhardsgrütter, I. et al. Awakening the sleeping carboxylase function of enzymes: engineering the natural CO2-binding potential of reductases. J. Am. Chem. Soc. 141, 9778–9782 (2019).
Sundaram, T. Physiological role of pyruvate carboxylase in a thermophilic Bacillus. J. Bacteriol. 113, 549–557 (1973).
Cotton, C. A., Edlich-Muth, C. & Bar-Even, A. Reinforcing carbon fixation: CO2 reduction replacing and supporting carboxylation. Curr. Opin. Biotechnol. 49, 49–56 (2018).
Garrastazu, C., Iniesta, M., Aranguez, M. & Ruiz, M. A. Comparative analysis of propionyl-CoA carboxylase from liver and mammary gland of mid-lactation cow. Comp. Biochem. Physiol. B 99, 613–617 (1991).
Kai, Y. et al. Three-dimensional structure of phosphoenolpyruvate carboxylase: a proposed mechanism for allosteric inhibition. Proc. Natl Acad. Sci. USA 96, 823–828 (1999).
Erb, T. J. et al. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc. Natl Acad. Sci. USA 104, 10631–10636 (2007).
Sage, R. F. Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J. Exp. Bot. 53, 609–620 (2002).
Claassens, N. J. A warm welcome for alternative CO2 fixation pathways in microbial biotechnology. Microb. Biotechnol. 10, 31–34 (2017).
Varaljay, V. et al. Functional metagenomic selection of RuBisCO from uncultivated bacteria. Environ. Microbiol. 18, 1187–1199 (2015).
Bachu, S. & Adams, J. Sequestration of CO2 in geological media in response to climate change: capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 44, 3151–3175 (2003).
Berg, I. A. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl. Environ. Microbiol. 77, 1925–1936 (2011).
Comotti, A. et al. Porous dipeptide crystals as selective CO2 adsorbents: experimental isotherms vs. grand canonical Monte Carlo simulations and MAS NMR spectroscopy. CrystEngComm 15, 1503–1507 (2013).
Jajesniak, P., Ali, H. E. M. O. & Wong, T. S. Carbon dioxide capture and utilization using biological systems: opportunities and challenges. J. Bioprocess. Biotech. 4, 3 (2014).
Mackinder, L. C. et al. A repeat protein links Rubisco to form the eukaryotic carbon-concentrating organelle. Proc. Natl Acad. Sci. USA 113, 5958–5963 (2016).
Yeates, T. O., Crowley, C. S. & Tanaka, S. Bacterial microcompartment organelles: protein shell structure and evolution. Annu. Rev. Biophys. 39, 185–205 (2010).
Rosenberg, E., DeLong, E. F., Lory, S., Stackebrandt, E. & Thompson, F. The Prokaryotes (Springer, 2006).
Claassens, N. J., Sousa, D. Z., dos Santos, V. A. M., de Vos, W. M. & van der Oost, J. Harnessing the power of microbial autotrophy. Nat. Rev. Microbiol. 14, 692–706 (2016). A comprehensive review discussing advances and bottlenecks for engineering autotrophic microbial cell factories, focusing on the energy harvesting perspective.
Liu, C., Colón, B. C., Ziesack, M., Silver, P. A. & Nocera, D. G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352, 1210–1213 (2016).
Yu, J. Bio-based products from solar energy and carbon dioxide. Trends Biotechnol. 32, 5–10 (2014).
Mohan, S. V., Modestra, J. A., Amulya, K., Butti, S. K. & Velvizhi, G. A circular bioeconomy with biobased products from CO2 sequestration. Trends Biotechnol. 34, 506–519 (2016). This review provides a comprehensive summary of different energy harvesting techniques for 3G biorefinery and proposes an integrated CO 2 biorefinery model that interlinks multiple processes and circulates resources and waste.
Tabita, F. R. Anoxygenic Photosynthetic Bacteria (Springer, 1995).
Raven, J. A. Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquat. Microb. Ecol. 56, 177–192 (2009).
Frost-Christensen, H. & Sand-Jensen, K. The quantum efficiency of photosynthesis in macroalgae and submerged angiosperms. Oecologia 91, 337–384 (1992).
Larsen, H., Yocum, C. S. & Niel, C. Bv On the energetics of the photosynthesis in green sulfur bacteria. J. Gen. Physiol. 36, 161–171 (1952).
Martinez, A., Bradley, A., Waldbauer, J., Summons, R. & DeLong, E. Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host. Proc. Natl Acad. Sci. USA 104, 5590–5595 (2007).
Guo, J. et al. Light-driven fine chemical production in yeast biohybrids. Science 362, 813–816 (2018).
Zhao, T.-T. et al. Artificial bioconversion of carbon dioxide. Chin. J. Catal. 40, 1421–1437 (2019).
Shen, Y. Carbon dioxide bio-fixation and wastewater treatment via algae photochemical synthesis for biofuels production. RSC Adv. 4, 49672–49722 (2014).
Nürnberg, D. J. et al. Photochemistry beyond the red limit in chlorophyll f–containing photosystems. Science 360, 1210–1213 (2018).
Ort, D. R. et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl Acad. Sci. USA 112, 8529–8536 (2015).
Sakimoto, K. K., Wong, A. B. & Yang, P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 351, 74–77 (2016).
Zhang, H. et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production. Nat. Nanotechnol. 13, 900–905 (2018).
Hauska, G., Schoedl, T., Remigy, H. & Tsiotis, G. The reaction center of green sulfur bacteria (1). Biochim. Biophys. Acta 1507, 260–277 (2001).
Manske, A. K., Glaeser, J., Kuypers, M. M. & Overmann, J. Physiology and phylogeny of green sulfur bacteria forming a monospecific phototrophic assemblage at a depth of 100 meters in the Black Sea. Appl. Environ. Microbiol. 71, 8049–8060 (2005).
Wang, J. et al. Field study on attached cultivation of Arthrospira (Spirulina) with carbon dioxide as carbon source. Bioresour. Technol. 283, 270–276 (2019).
Chen, J. et al. Microalgal industry in China: challenges and prospects. J. Appl. Phycol. 28, 715–725 (2016).
Bhola, V., Swalaha, F., Ranjith Kumar, R., Singh, M. & Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 11, 2103–2118 (2014).
Liu, Y. & Whitman, W. B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 1125, 171–189 (2008).
Lamont, C. M. & Sargent, F. Design and characterisation of synthetic operons for biohydrogen technology. Arch. Microbiol. 199, 495–503 (2017).
Laurinavichene, T. V. & Tsygankov, A. A. H2 consumption by Escherichia coli coupled via hydrogenase 1 or hydrogenase 2 to different terminal electron acceptors. FEMS Microbiol. Lett. 202, 121–124 (2001).
Gong, F., Zhu, H., Zhang, Y. & Li, Y. Biological carbon fixation: From natural to synthetic. J. CO 2 Util. 28, 221–227 (2018).
Claassens, N. J., Sánchez-Andrea, I., Sousa, D. Z. & Bar-Even, A. Towards sustainable feedstocks: A guide to electron donors for microbial carbon fixation. Curr. Opin. Biotechnol. 50, 195–205 (2018). This review systematically evaluates different electron donors, and suggests that formate, H 2 and CO are the most promising for growth and bioproduction.
Guzman, M. S. et al. Phototrophic extracellular electron uptake is linked to carbon dioxide fixation in the bacterium Rhodopseudomonas palustris. Nat. Commun. 10, 1355 (2019).
Nevin, K. P., Woodard, T. L., Franks, A. E., Summers, Z. M. & Lovley, D. R. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1, e00103-10 (2010).
Tremblay, P.-L., Angenent, L. T. & Zhang, T. Extracellular electron uptake: among autotrophs and mediated by surfaces. Trends Biotechnol. 35, 360–371 (2017).
Chen, X., Cao, Y., Li, F., Tian, Y. & Song, H. Enzyme-assisted microbial electrosynthesis of poly (3-hydroxybutyrate) via CO2 bioreduction by engineered Ralstonia eutropha. ACS Catal. 8, 4429–4437 (2018).
Jiang, Y. et al. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 149, 42–55 (2018).
Holmes, D. E., Bond, D. R. & Lovley, D. R. Electron transfer by Desulfobulbus propionicus to Fe (III) and graphite electrodes. Appl. Environ. Microbiol. 70, 1234–1237 (2004).
Liao, J. C., Mi, L., Pontrelli, S. & Luo, S. Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 14, 288–304 (2016). This study thoroughly discusses 2G biorefinery, 3G biorefinery and methane biorefinery on their strength on bioproduction.
Rodrigues, R. M. et al. Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricity-driven microbial CO2 reduction. Nat. Catal. 2, 407–414 (2019).
Haas, T., Krause, R., Weber, R., Demler, M. & Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 1, 32–39 (2018).
Su, L. & Ajo-Franklin, C. M. Reaching full potential: bioelectrochemical systems for storing renewable energy in chemical bonds. Curr. Opin. Biotechnol. 57, 66–72 (2019). This review comprehensively summarizes state-of-the-art technologies of bioelectrochemical systems and biohybrid systems.
Woo, H. M. Solar-to-chemical and solar-to-fuel production from CO2 by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 45, 1–7 (2017).
Cornejo, J. A., Sheng, H., Edri, E., Ajo-Franklin, C. & Frei, H. Nanoscale membranes that chemically isolate and electronically wire up the abiotic/biotic interface. Nat. Commun. 9, 2263 (2018).
Lai, M. J. & Lan, E. I. Photoautotrophic synthesis of butyrate by metabolically engineered cyanobacteria. Biotechnol. Bioeng. 116, 893–903 (2019).
Tran, M., Zhou, B., Pettersson, P. L., Gonzalez, M. J. & Mayfield, S. P. Synthesis and assembly of a full‐length human monoclonal antibody in algal chloroplasts. Biotechnol. Bioeng. 104, 663–673 (2009).
Ni, J., Liu, H.-Y., Tao, F., Wu, Y.-T. & Xu, P. Remodeling of the photosynthetic chain promotes direct CO2 conversion to valuable aromatics. Angew. Chem. 57, 15990–15994 (2018).
Ferreira, G., Pinto, L. R., Maciel Filho, R. & Fregolente, L. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sust. Energ. Rev. 109, 448–466 (2019).
Yunus, I. S. et al. Synthetic metabolic pathways for photobiological conversion of CO2 into hydrocarbon fuel. Metab. Eng. 49, 201–211 (2018).
Humphreys, C. M. & Minton, N. P. Advances in metabolic engineering in the microbial production of fuels and chemicals from C1 gas. Curr. Opin. Biotechnol. 50, 174–181 (2018).
Ishizaki, A., Tanaka, K. & Taga, N. Microbial production of poly-d-3-hydroxybutyrate from CO2. Appl. Microbiol. Biotechnol. 57, 6–12 (2001).
Ammam, F., Tremblay, P.-L., Lizak, D. M. & Zhang, T. Effect of tungstate on acetate and ethanol production by the electrosynthetic bacterium Sporomusa ovata. Biotechnol. Biofuels 9, 163 (2016).
Bajracharya, S., Vanbroekhoven, K., Buisman, C. J. N., Strik, D. & Pant, D. Bioelectrochemical conversion of CO2 to chemicals: CO2 as a next generation feedstock for electricity-driven bioproduction in batch and continuous modes. Faraday Discuss. 202, 433–449 (2017).
Vassilev, I. et al. Microbial electrosynthesis of isobutyric, butyric, caproic acids, and corresponding alcohols from carbon dioxide. ACS Sustain. Chem. Eng. 6, 8485–8493 (2018).
LaBelle, E. V. & May, H. D. Energy efficiency and productivity enhancement of microbial electrosynthesis of acetate. Front. Microbiol. 8, 756 (2017).
Ganigué, R., Puig, S., Batlle-Vilanova, P., Balaguer, M. D. & Colprim, J. Microbial electrosynthesis of butyrate from carbon dioxide. Chem. Commun. 51, 3235–3238 (2015).
Jourdin, L., Raes, S. M., Buisman, C. J. & Strik, D. P. Critical biofilm growth throughout unmodified carbon felts allows continuous bioelectrochemical chain elongation from CO2 up to caproate at high current density. Front. Energy Res. 6, 7 (2018).
Krieg, T., Sydow, A., Faust, S., Huth, I. & Holtmann, D. CO2 to terpenes: autotrophic and electroautotrophic α‐humulene production with Cupriavidus necator. Angew. Chem. Int. Ed. 57, 1879–1882 (2018).
Full Final Report Section Synopsis (National Alliance for Advanced Biofuels and Bioproducts, 2017); https://energy.gov/sites/prod/files/2014/07/f18/naabb_full_final_report_section_I.pdf
Campbell, P. K., Beer, T. & Batten, D. Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour. Technol. 102, 50–56 (2011).
May, H. D., Evans, P. J. & LaBelle, E. V. The bioelectrosynthesis of acetate. Curr. Opin. Biotechnol. 42, 225–233 (2016).
Christodoulou, X. & Velasquez-Orta, S. B. Microbial electrosynthesis and anaerobic fermentation: an economic evaluation for acetic acid production from CO2 and CO. Environ. Sci. Technol. 50, 11234–11242 (2016).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Khan, N. E., Myers, J. A., Tuerk, A. L. & Curtis, W. R. A process economic assessment of hydrocarbon biofuels production using chemoautotrophic organisms. Bioresour. Technol. 172, 201–211 (2014).
Renewable Power: Climate-Safe Eneryg Competes on Cost Alone (IRENA, 2018); https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2018/Dec/IRENA_COP24_costs_update_2018.pdf
Karamanev, D. et al. Biological conversion of hydrogen to electricity for energy storage. Energy 129, 237–245 (2017).
Pricing Carbon Emissions through Taxes and Emissions Trading (OECD, 2018); http://www.oecd.org/tax/effective-carbon-rates-2018-9789264305304-en.htm
Junne, S. & Kabisch, J. Fueling the future with biomass: processes and pathways for a sustainable supply of hydrocarbon fuels and biogas. Eng. Life Sci. 17, 14–26 (2016).
Gross, M. Counting carbon costs. Curr. Biol. 28, 1221–1224 (2018).
Ricke, K., Drouet, L., Caldeira, K. & Tavoni, M. Country-level social cost of carbon. Nat. Clim. Change 8, 895–900 (2018).
Coma, M. et al. Organic waste as a sustainable feedstock for platform chemicals. Faraday Discuss. 202, 175–195 (2017).
Du, K. et al. Integrated lipid production, CO2 fixation, and removal of SO2 and NO from simulated flue gas by oleaginous Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 26, 16195–16209 (2019).
Yu, J. et al. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 5, 8132 (2015).
Charubin, K., Bennett, R. K., Fast, A. G. & Papoutsakis, E. T. Engineering Clostridium organisms as microbial cell-factories: challenges & opportunities. Metab. Eng. 50, 173–191 (2018).
Liu, C. et al. Nanowire–bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals. Nano Lett. 15, 3634–3639 (2015).
Subhash, G. V. & Mohan, S. V. Deoiled algal cake as feedstock for dark fermentative biohydrogen production: an integrated biorefinery approach. Int. J. Hydrog. Energ. 39, 9573–9579 (2014).
ElMekawy, A. et al. Food and agricultural wastes as substrates for bioelectrochemical system (BES): the synchronized recovery of sustainable energy and waste treatment. Food Res. Int. 73, 213–225 (2015).
Hermida-Carrera, C., Kapralov, M. V. & Galmés, J. Rubisco catalytic properties and temperature response in crops. Plant Physiol. 171, 2549–2561 (2016).
Altaş, N. et al. Heterologous production of extreme alkaline thermostable NAD+-dependent formate dehydrogenase with wide-range pH activity from Myceliophthora thermophila. Process Biochem. 61, 110–118 (2017).
Wilcoxen, J., Snider, S. & Hille, R. Substitution of silver for copper in the binuclear Mo/Cu center of carbon monoxide dehydrogenase from Oligotropha carboxidovorans. J. Am. Chem. Soc. 133, 12934–12936 (2011).
Hawkins, A. B., Adams, M. W. W. & Kelly, R. M. Conversion of 4-hydroxybutyrate to acetyl coenzyme A and its anapleurosis in the Metallosphaera sedula 3-hydroxypropionate/4-hydroxybutyrate carbon fixation pathway. Appl. Environ. Microbiol. 80, 2536–2545 (2014).
Liu, C., Wang, Q., Xian, M., Ding, Y. & Zhao, G. Dissection of malonyl-coenzyme A reductase of Chloroflexus aurantiacus results in enzyme activity improvement. PLoS ONE 8, e75554 (2013).
Fan, F. et al. On the catalytic mechanism of human ATP citrate lyase. Biochemistry 51, 5198–211 (2012).
Yoo, H. G. et al. Characterization of 2-octenoyl-CoA carboxylase/reductase utilizing pteB from Streptomyce avermitilis. Biosci. Biotechnol. Biochem. 75, 1191–1193 (2011).
ElMekawy, A. et al. Technological advances in CO2 conversion electro-biorefinery: a step toward commercialization. Bioresour. Technol. 215, 357–370 (2016).
Acknowledgements
This work was supported by the Beijing Advanced Innovation Center for Soft Matter Science and Engineering, National Natural Science Foundation of China (21811530003), National Key Research and Development Program (2018YFA0903000 and 2018YFA0900100), the Double First-Rate Program (ylkxj03), the Novo Nordisk Foundation (NNF10CC1016517) and the Knut and Alice Wallenberg Foundation.
Author information
Authors and Affiliations
Contributions
Z.L., T.T. and J.N. drafted the outline. Z.L., K.W., Y.C., T.T. and J.N. wrote the manuscript. T.T. and J.N. supervised the research. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1, Supplementary References
Rights and permissions
About this article
Cite this article
Liu, Z., Wang, K., Chen, Y. et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2. Nat Catal 3, 274–288 (2020). https://doi.org/10.1038/s41929-019-0421-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0421-5
This article is cited by
-
Solar-driven sugar production directly from CO2 via a customizable electrocatalytic–biocatalytic flow system
Nature Communications (2024)
-
Increased CO2 fixation enables high carbon-yield production of 3-hydroxypropionic acid in yeast
Nature Communications (2024)
-
Biochemical conversion of CO2 in fuels and chemicals: status, innovation, and industrial aspects
Biomass Conversion and Biorefinery (2024)
-
Deep subsurface igneous rocks from the Deccan traps harbor H2 and CO2 utilizing chemolithoautotrophic bacteria
Biologia (2024)
-
Transcriptome analysis of Kluyveromyces marxianus under succinic acid stress and development of robust strains
Applied Microbiology and Biotechnology (2024)