Abstract
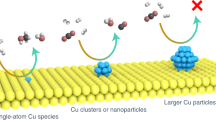
Selective conversion of CO2 to ethanol is of great interest but presents a significant challenge in forming a C–C bond while keeping a C–O bond intact throughout the process. Here, we report cooperative CuI sites on a Zr12 cluster of a metal–organic framework (MOF) for selective hydrogenation of CO2 to ethanol. With the assistance of an alkali cation, the spatially proximate Zr12-supported CuI centres activate hydrogen via bimetallic oxidative addition and promote C–C coupling to produce ethanol. The Cs+-modified MOF catalyst, in 10 hours, produces ethanol with >99% selectivity and a turnover number (based on all Cu atoms) of 4,080 in supercritical CO2, with 30 MPa of CO2 and 5 MPa of H2 at 85 °C, or a turnover number of 490 at 2 MPa of CO2/H2 (1/3) and 100 °C. Our work highlights the potential of using MOFs as a tunable platform to design earth-abundant metal catalysts for CO2 conversion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data that support the findings of this study are available within the paper and its Supplementary Information files, or from the corresponding author on reasonable request.
References
Kargari, A. & Ravanchi, M. in Greenhouse Gases: Capturing, Utilization and Reduction 1–30 (IntechOpen, 2012).
Álvarez, A. et al. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 117, 9804–9838 (2017).
Gao, P. et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024 (2017).
Cabrero-Antonino, J. R., Adam, O. R. & Beller, M. Catalytic reductive N-alkylations using CO2 and carboxylic acid derivatives: recent progress and developments. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.201810121 (2018).
Olah, G. A., Goeppert, A. & Prakash, G. K. S. in Beyond Oil and Gas: The Methanol Economy 233–278 (Wiley, 2009).
Langeslay, R. R. et al. Catalytic applications of vanadium: a mechanistic perspective. Chem. Rev. 119, 2128–2191 (2019).
Goldemberg, J. Ethanol for a sustainable energy future. Science 315, 808–810 (2007).
Farrell, A. E. et al. Ethanol can contribute to energy and environmental goals. Science 311, 506–508 (2006).
Prieto, G. et al. Design and synthesis of copper–cobalt catalysts for the selective conversion of synthesis gas to ethanol and higher alcohols. Angew. Chem. Int. Ed. 53, 6397–6401 (2014).
Sun, J. et al. Promotional effects of cesium promoter on higher alcohol synthesis from syngas over cesium-promoted Cu/ZnO/Al2O3 catalysts. ACS Catal. 6, 5771–5785 (2016).
Zhang, R., Wang, G. & Wang, B. Insights into the mechanism of ethanol formation from syngas on Cu and an expanded prediction of improved Cu-based catalyst. J. Catal. 305, 238–255 (2013).
Kauffman, D. R. & Alfonso, D. Directing CO2 conversion with copper nanoneedles. Nat. Catal. 1, 99 (2018).
Cao, A. et al. Growing layered double hydroxides on CNTs and their catalytic performance for higher alcohol synthesis from syngas. J. Mater. Sci. 51, 5216–5231 (2016).
Liu, Y., Murata, K., Inaba, M., Takahara, I. & Okabe, K. Synthesis of mixed alcohols from syngas over Cs-modified Cu/Ce1 - xZrxO2 catalysts. J. Jpn Pet. Inst. 53, 153–159 (2010).
Pan, X. et al. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 6, 507–511 (2007).
He, Z. et al. Water-enhanced synthesis of higher alcohols from CO2 hydrogenation over a Pt/Co3O4 catalyst under milder conditions. Angew. Chem. Int. Ed. 55, 737–741 (2016).
Bai, S. et al. Highly active and selective hydrogenation of CO2 to ethanol by ordered Pd–Cu nanoparticles. J. Am. Chem. Soc. 139, 6827–6830 (2017).
Gong, J. et al. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0–Cu+ sites. J. Am. Chem. Soc. 134, 13922–13925 (2012).
Anton, J. et al. The effect of sodium on the structure–activity relationships of cobalt-modified Cu/ZnO/Al2O3 catalysts applied in the hydrogenation of carbon monoxide to higher alcohols. J. Catal. 335, 175–186 (2016).
Lopez, L. et al. Syngas conversion to ethanol over a mesoporous Cu/MCM-41 catalyst: effect of K and Fe promoters. Appl. Catal. A Gen. 526, 77–83 (2016).
Angamuthu, R., Byers, P., Lutz, M., Spek, A. L. & Bouwman, E. Electrocatalytic CO2 conversion to oxalate by a copper complex. Science 327, 313–315 (2010).
Gnanamani, M. K. et al. Hydrogenation of carbon dioxide over K-promoted FeCo bimetallic catalysts prepared from mixed metal oxalates. ChemCatChem 9, 1303–1312 (2017).
Xu, M. & Iglesia, E. Carbon–carbon bond formation pathways in Co hydrogenation to higher alcohols. J. Catal. 188, 125–131 (1999).
Burcham, M. M., Herman, R. G. & Klier, K. Higher alcohol synthesis over double bed Cs−Cu/ZnO/Cr2O3 catalysts: optimizing the yields of 2-methyl-1-propanol (isobutanol). Ind. Eng. Chem. Res. 37, 4657–4668 (1998).
Zhao, M. et al. Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nature 539, 76–80 (2016).
Diercks, C. S., Liu, Y., Cordova, K. E. & Yaghi, O. M. The role of reticular chemistry in the design of CO2 reduction catalysts. Nat. Mater. 17, 301–307 (2018).
Fei, H. et al. Reusable oxidation catalysis using metal-monocatecholato species in a robust metal–organic framework. J. Am. Chem. Soc. 136, 4965–4973 (2014).
Metzger, E. D., Brozek, C. K., Comito, R. J. & Dincă, M. Selective dimerization of ethylene to 1-butene with a porous catalyst. ACS Cent. Sci. 2, 148–153 (2016).
Valvekens, P., Vermoortele, F. & De Vos, D. Metal–organic frameworks as catalysts: the role of metal active sites. Catal. Sci. Technol. 3, 1435–1445 (2013).
Wu, C.-D. & Lin, W. Highly porous, homochiral metal–organic frameworks: solvent-exchange-induced single-crystal to single-crystal transformations. Angew. Chem. Int. Ed. 44, 1958–1961 (2005).
Chambers, M. B. et al. Photocatalytic carbon dioxide reduction with rhodium-based catalysts in solution and heterogenized within metal–organic frameworks. ChemSusChem 8, 603–608 (2015).
Yang, D. et al. Metal–organic framework nodes as nearly ideal supports for molecular catalysts: NU-1000- and UiO-66-supported iridium complexes. J. Am. Chem. Soc. 137, 7391–7396 (2015).
Ji, P. et al. Single-site cobalt catalysts at new Zr12(µ3-O)8(µ3-OH)8(µ2-OH)6 metal–organic framework nodes for highly active hydrogenation of nitroarenes, nitriles and isocyanides. J. Am. Chem. Soc. 139, 7004–7011 (2017).
Manna, K. et al. Chemoselective single-site earth-abundant metal catalysts at metal–organic framework nodes. Nat. Commun. 7, 12610 (2016).
Ji, P. et al. Single-site cobalt catalysts at new Zr8(µ2-O)8(µ2-OH)4 metal–organic framework nodes for highly active hydrogenation of alkenes, imines, carbonyls and heterocycles. J. Am. Chem. Soc. 138, 12234–12242 (2016).
Guo, Z. et al. A metal–organic framework with optimized open metal sites and pore spaces for high methane storage at room temperature. Angew. Chem. Int. Ed. 50, 3178–3181 (2011).
Dai, R. et al. Electron crystallography reveals atomic structures of metal–organic nanoplates with M12(µ3-O)8(µ3-OH)8(µ2-OH)6 (M = Zr, Hf) secondary building units. Inorg. Chem. 56, 8128–8134 (2017).
Cavka, J. H. et al. A new zirconium inorganic building brick forming metal–organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13850–13851 (2008).
Ikuno, T. et al. Methane oxidation to methanol catalyzed by Cu-Oxo clusters stabilized in NU-1000 metal–organic framework. J. Am. Chem. Soc. 139, 10294–10301 (2017).
An, B. et al. Confinement of ultrasmall Cu/ZnOx nanoparticles in metal–organic frameworks for selective methanol synthesis from catalytic hydrogenation of CO2. J. Am. Chem. Soc. 139, 3834–3840 (2017).
Ji, P. et al. Cerium-hydride secondary building units in a porous metal–organic framework for catalytic hydroboration and hydrophosphination. J. Am. Chem. Soc. 138, 14860–14863 (2016).
Ji, P. et al. Transformation of metal–organic framework secondary building units into hexanuclear Zr-alkyl catalysts for ethylene polymerization. J. Am. Chem. Soc. 139, 11325–11328 (2017).
Texter, J., Strome, D., Herman, R. & Klier, K. Chemical and spectroscopic properties of copper containing zeolites. J. Phys. Chem. 81, 333–338 (1977).
Chen, Y.-Z. et al. Multifunctional PdAg@MIL-101 for one-pot cascade reactions: combination of host–guest cooperation and bimetallic synergy in catalysis. ACS Catal. 5, 2062–2069 (2015).
Nunan, J. G. et al. Methanol and C2 oxygenate synthesis over cesium doped CuZnO and Cu/ZnO/Al2O3 catalysts: a study of selectivity and 13C incorporation patterns. J. Catal. 113, 410–433 (1988).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 (2005).
Rehr, J. J. & Albers, R. C. Theoretical approaches to X-ray absorption fine structure. Rev. Mod. Phys. 72, 621–654 (2000).
Frisch, M. J. et al. Gaussian 09, Revision E.01 (Gaussian, 2016).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Lee, C. T., Yang, W. T. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Acknowledgements
The authors thank C. Wang and Z. Lin for advice on isotopic mass-spectrometric analysis, K. Cheng and J. Lei (from Xiamen HanDe Engineering Co.) for experimental and equipment help, P. Ji for XAFS data collection and Q. Li for 3Li NMR tests. The authors acknowledge funding support from the Ministry of Science and Technology of China (2016YFA0200702) and the National Natural Science Foundation of China (21671162, 21471126 and 21721001). XAS analysis was performed at Beamline 10-BM of the Advanced Photon Source (APS), Argonne National Laboratory (ANL). Use of the APS, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
B.A., C.W. and W.L. conceived and designed the project. B.A., Z.L., Y.S. and J.Z. carried out the synthesis of the materials, characterized the materials and analysed the data. B.A. performed the high-throughput catalysis with the help of L.Z., while Z.L. performed the theoretical calculations. B.A. and Z.L. performed the experiments investigating the catalytic cycle, under the supervision of C.W. All authors discussed the results and commented on the manuscript. B.A., Z.L., C.W. and W.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information
Supplementary methods, Supplementary Figs. 1–58, Supplementary Tables 1–11, Supplementary Notes 1-3, Supplementary references
Rights and permissions
About this article
Cite this article
An, B., Li, Z., Song, Y. et al. Cooperative copper centres in a metal–organic framework for selective conversion of CO2 to ethanol. Nat Catal 2, 709–717 (2019). https://doi.org/10.1038/s41929-019-0308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0308-5
This article is cited by
-
Recent Advances on the MOFs-Based Materials for the Elimination or Utilization of Typical Gaseous Pollutants
Topics in Catalysis (2024)
-
Photocatalytic CO2 reduction using La-Ni bimetallic sites within a covalent organic framework
Nature Communications (2023)
-
Synergistic binding sites in a metal-organic framework for the optical sensing of nitrogen dioxide
Nature Communications (2023)
-
Comparative theoretical study of CO2 activation on clean and potassium-preadsorbed low index surfaces of transition metals
Journal of Molecular Modeling (2023)
-
The role of CO2 dissociation in CO2 hydrogenation to ethanol on CoCu/silica catalysts
Nano Research (2023)