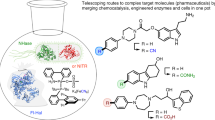
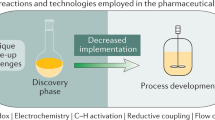
Abstract
The past decade has seen a substantial increase in successful examples of the combination of chemo- and biocatalysis for multistep syntheses. This is driven by obvious advantages such as higher yields, decreased costs, environmental benefits and high selectivity. On the downside, efforts must be undertaken to combine the divergent reaction conditions, reagent tolerance and solvent systems of these ‘different worlds of catalysis’. Owing to progress in enzyme discovery and engineering, as well as in the development of milder and more compatible conditions for operating with various chemocatalysts, many historical limitations can already be overcome. This Review highlights the opportunities available in the chemical space of combined syntheses using prominent examples, but also discusses the current challenges and emerging solutions, keeping in mind the fast progress in transition metal-, organo-, photo-, electro-, hetero- and biocatalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
07 March 2018
In this Review Article originally published, the ORCID number for the author Radka Snajdrova was incorrect; it should have been 0000-0002-4809-1066. This has now been corrected in all versions of the Review Article.
References
Muschiol, J. et al. Cascade catalysis — strategies and challenges en route to preparative synthetic biology. Chem. Commun. 51, 5798–5811 (2015).
France, S. P., Hepworth, L. J., Turner, N. J. & Flitsch, S. L. Constructing biocatalytic cascades: in vitro and in vivo approaches to de novo multi-enzyme pathways. ACS Catal. 7, 710–724 (2017).
Gröger, H. & Hummel, W. Combining the ‘two worlds’ of chemocatalysis and biocatalysis towards multi-step one-pot processes in aqueous media. Curr. Opin. Chem. Biol. 19, 171–179 (2014).
Denard, C. A., Hartwig, J. 0F. & Zhao, H. M. Multistep one-pot reactions combining biocatalysts and chemical catalysts for asymmetric synthesis. ACS Catal. 3, 2856–2864 (2013).
Ricca, E., Brucher, B. & Schrittwieser, J. H. Multi-enzymatic cascade reactions: overview and perspectives. Adv. Synth. Catal. 353, 2239–2262 (2011).
Oroz-Guinea, I. & Garcia-Junceda, E. Enzyme catalysed tandem reactions. Curr. Opin. Chem. Biol. 17, 236–249 (2013).
Bruggink, A., Schoevaart, R. & Kieboom, T. Concepts of nature in organic synthesis: cascade catalysis and multistep conversions in concert. Org. Proc. Res. Dev. 7, 622–640 (2003).
Kourist, R., Schmidt, S. & Castiglione, K. Overcoming the incompatibility challenge in chemo-enzymatic and multi-catalytic cascade reactions. Chem. Eur. J. 23, 1–15 (2017).
Makkee, M., Kieboom, A. P. G. & van Bekkum, H. Combined action of enzyme and metal catalyst, applied to the preparation of d-mannitol. J. Chem. Soc. Chem. Commun. 930–931 (1980).
Allen, J. V. & Williams, J. M. J. Dynamic kinetic resolution with enzyme and palladium combinations. Tetrahedron Lett. 37, 1859–1862 (1996).
Dinh, P. M., Howarth, J. A., Hudnott, A. R., Williams, J. M. J. & Harris, W. Catalytic racemisation of alcohols: applications to enzymatic resolution reactions. Tetrahedron Lett. 37, 7623–7626 (1996).
Kim, M. J., Ahn, Y. & Park, J. Dynamic kinetic resolutions and asymmetric transformations by enzymes coupled with metalcatalysis. Curr. Opin. Biotechnol. 13, 578–587 (2002).
Larsson, A. L. E., Persson, B. A. & Bäckvall, J.-E. Enzymic resolution of alcohols coupled with ruthenium-catalyzed racemization of the substrate alcohol. Angew. Chem. Int. Ed. 36, 1211–1212 (1997).
Reetz, M. T. & Schimossek, K. Lipase-catalyzed dynamic kinetic resolution of chiral amines: use of palladium as the racemization catalyst. Chimia 50, 668–669 (1996).
Carr, R. et al. Directed evolution of an amine oxidase for the preparative deracemisation of cyclic secondary amines. ChemBioChem 6, 637–639 (2005).
de Souza, R., Miranda, L. S. M. & Bornscheuer, U. T. A retrosynthesis approach for biocatalysis in organic synthesis. Chem. Eur. J. 23, 12040–12063 (2017). An in-depth review providing many examples for planning retrosynthesis reactions with enzymes.
Hönig, M., Sondermann, P., Turner, N. J. & Carreira, E. M. Enantioselective chemo- and biocatalysis: partners in retrosynthesis. Angew. Chem. Int. Ed. 56, 8942–8973 (2017). An in-depth review providing many examples for planning retrosynthesis reactions with enzymes.
Turner, N. J. & O’Reilly, E. Biocatalytic retrosynthesis. Nat. Chem. Biol. 9, 285–288 (2013).
Pamies, O. & Bäckvall, J. E. Combination of enzymes and metal catalysts. A powerful approach in asymmetric catalysis. Chem. Rev. 103, 3247–3262 (2003).
Persson, B. A., Larsson, A. L. E., Le Ray, M. & Bäckvall, J. E. Ruthenium- and enzyme-catalyzed dynamic kinetic resolution of secondary alcohols. J. Am. Chem. Soc. 121, 1645–1650 (1999).
Choi, J. H. et al. Aminocyclopentadienyl ruthenium chloride: catalytic racemization and dynamic kinetic resolution of alcohols at ambient temperature. Angew. Chem. Int. Ed. 41, 2373–2376 (2002).
Berkessel, A., Sebastian-Ibarz, M. L. & Müller, T. N. Lipase/aluminum-catalyzed dynamic kinetic resolution of secondary alcohols. Angew. Chem. Int. Ed. 45, 6567–6570 (2006).
Egi, M. et al. A mesoporous-silica-immobilized oxovanadium cocatalyst for the lipase-catalyzed dynamic kinetic resolution of racemic alcohols. Angew. Chem. Int. Ed. 52, 3654–3658 (2013).
Simons, C., Hanefeld, U., Arends, I. W. C. E., Maschmeyer, T. & Sheldon, R. A. Towards catalytic cascade reactions: asymmetric synthesis using combined chemo-enzymatic catalysts. Top. Catal. 40, 35–44 (2006).
Burda, E., Hummel, W. & Gröger, H. Modular chemoenzymatic one-pot syntheses in aqueous media: combination of a palladium-catalyzed cross-coupling with an asymmetric biotransformation. Angew. Chem. Int. Ed. 47, 9551–9554 (2008).
Rios-Lombardia, N. et al. From a sequential to a concurrent reaction in aqueous medium: ruthenium-catalyzed allylic alcohol isomerization and asymmetric bioreduction. Angew. Chem. Int. Ed. 55, 8691–8695 (2016). The presented study is one of the rare cases where a concurrent cascade was developed for the synthesis of chiral alcohols without applying any special engineering techniques.
Gröger, H. Metals and Metal Complexes in Cooperative Catalysis with Enzymes Within Organic-Synthetic One-Pot Processes, in Cooperative Catalysis: Designing Efficient Catalysts for Synthesis (Wiley-VCH, Weinheim, Germany, 2015).
Huo, Y., Zeng, H. & Zhang, Y. Integrating metabolic engineering and heterogeneous chemocatalysis: new opportunities for biomass to chemicals. ChemSusChem 9, 1078–1080 (2016).
Yuryev, R., Strompen, S. & Liese, A. Coupled chemo(enzymatic) reactions in continuous flow. Beilstein J. Org. Chem. 7, 1449–1467 (2011).
Fink, M. J., Schön, M., Rudroff, F., Schnürch, M. & Mihovilovic, M. D. Single operation stereoselective synthesis of aerangis lactones: combining continuous flow hydrogenation and biocatalysts in a chemoenzymatic sequence. ChemCatChem 5, 724–727 (2013).
Vennestrøm, P. N. R. et al. Chemoenzymatic combination of glucose oxidase with titanium silicalite-1. ChemCatChem 2, 943–945 (2010).
Sirasani, G., Tong, L. & Balskus, E. P. A biocompatible alkene hydrogenation merges organic synthesis with microbial metabolism. Angew. Chem. Int. Ed. 53, 7785–7788 (2014).
Suastegui, M. et al. Combining metabolic engineering and electrocatalysis: application to the production of polyamides from sugar. Angew. Chem. Int. Ed. 55, 2368–2373 (2016).
Baer, K. et al. Sequential and modular synthesis of chiral 1,3-diols with two stereogenic centers: access to all four stereoisomers by combination of organo- and biocatalysis. Angew. Chem. Int. Ed. 48, 9355–9358 (2009).
Rulli, G. et al. Direction of kinetically versus thermodynamically controlled organocatalysis and its application in chemoenzymatic synthesis. Angew. Chem. Int. Ed. 50, 7944–7947 (2011).
Rulli, G., Duangdee, N., Hummel, W., Berkessel, A. & Gröger, H. First tandem-type one-pot process combining asymmetric organo- and biocatalytic reactions in aqueous media exemplified for the enantioselective and diastereoselective synthesis of 1,3-diols. Eur. J. Org. Chem. 2017, 812–817 (2017).
Simon, R. C. et al. Stereoselective synthesis of gamma-hydroxynorvaline through combination of organo- and biocatalysis. Chem. Commun. 50, 15669–15672 (2014).
Suljic, S., Pietruszka, J. & Worgull, D. Asymmetric bio- and organocatalytic cascade reaction — laccase and secondary amine-catalyzed alpha-arylation of aldehydes. Adv. Synth. Catal. 357, 1822–1830 (2015).
Chen, S. T., Huang, W. H. & Wang, K. T. Resolution of amino-acids in a mixture of 2-methyl-2-propanol water (19/1) catalyzed by alcalase via in-situ racemization of one antipode mediated by pyridoxal 5-phosphate. J. Org. Chem. 59, 7580–7581 (1994).
Zimmermann, V., Beller, M. & Kragl, U. Modelling the reaction course of a dynamic kinetic resolution of amino acid derivatives: identifying and overcoming bottlenecks. Org. Proc. Res. Dev. 10, 622–627 (2006).
Berkessel, A., Jurkiewicz, I. & Mohan, R. Enzymatic dynamic kinetic resolution of oxazinones: a new approach to enantiopure beta(2)-amino acids. ChemCatChem 3, 319–330 (2011).
Hoyos, P., Pace, V. & Alcantara, A. R. Dynamic kinetic resolution via hydrolase-metal combo catalysis in stereoselective synthesis of bioactive compounds. Adv. Synth. Catal. 354, 2585–2611 (2012).
Lee, S. H., Kim, J. H. & Park, C. B. Coupling photocatalysis and redox biocatalysis toward biocatalyzed artificial photosynthesis. Chem. Eur. J. 19, 4392–4406 (2013).
Ji, X., Su, Z., Wang, P., Ma, G. & Zhang, S. Integration of artificial photosynthesis system for enhanced electronic energy-transfer efficacy: a case study for solar-energy driven bioconversion of carbon dioxide to methanol. Small 12, 4753–4762 (2016).
Butti, S. K. et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: maneuvering towards upscaling. Renew. Sust. Energy Rev. 53, 462–476 (2016).
Kochius, S., Magnusson, A. O., Hollmann, F., Schrader, J. & Holtmann, D. Immobilized redox mediators for electrochemical NAD(P)+ regeneration. App. Microbiol. Biotechnol. 93, 2251–2264 (2012).
Hollmann, F. & Schmid, A. Electrochemical regeneration of oxidoreductases for cell-free biocatalytic redox reactions. Biocat. Biotrans. 22, 63–88 (2004).
Fisher, K. et al. Electro-enzymatic viologen-mediated substrate reduction using pentaerythritol tetranitrate reductase and a parallel, segmented fluid flow system. Catal. Sci. Technol. 3, 1505–1511 (2013).
Nerimetla, R., Walgama, C., Singh, V., Hartson, S. D. & Krishnan, S. Mechanistic insights into voltage-driven biocatalysis of a cytochrome P450 bactosomal film on a self-assembled monolayer. ACS Catal. 7, 3446–3453 (2017).
Gunther, H., Walter, K., Köhler, P. & Simon, H. On a new artificial mediator accepting NADP(H) oxidoreductase from Clostridium thermoaceticum. J. Biotechnol. 83, 253–267 (2000).
Suye, S.-i, Aramoto, Y., Nakamura, M., Tabata, I. & Sakakibara, M. Electrochemical reduction of immobilized NADP+ on a polymer modified electrode with a co-polymerized mediator. Enzyme Microb. Technol. 30, 139–144 (2002).
Venkata Mohan, S., Velvizhi, G., Vamshi Krishna, K. & Lenin Babu, M. Microbial catalyzed electrochemical systems: a bio-factory with multi-facet applications. Bioresour. Technol. 165, 355–364 (2014).
Kuk, S. K. et al. Photoelectrochemical reduction of carbon dioxide to methanol through a highly efficient enzyme cascade. Angew. Chem. Int. Ed. 56, 3827–3832 (2017). An excellent example for the combination of three enzymes.
Sorigue, D. et al. An algal photoenzyme converts fatty acids to hydrocarbons. Science 357, 903–907 (2017).
Tomasek, J. & Schatz, J. Olefin metathesis in aqueous media. Green Chem. 15, 2317–2338 (2013).
Denard, C. A. et al. Cooperative tandem catalysis by an organometallic complex and a metalloenzyme. Angew. Chem. Int. Ed. 53, 465–469 (2014).
Heidlindemann, M., Rulli, G., Berkessel, A., Hummel, W. & Gröger, H. Combination of asymmetric organo- and biocatalytic reactions in organic media using immobilized catalysts in different compartments. ACS Catal. 4, 1099–1103 (2014).
Wang, Y. & Zhao, H. Tandem reactions combining biocatalysts and chemical catalysts for asymmetric synthesis. Catalysts 6, 194 (2016).
Huang, H. et al. Tandem catalytic conversion of glucose to 5-hydroxymethylfurfural with an immobilized enzyme and a solid acid. ACS Catal. 4, 2165–2168 (2014).
Lee, Y. C., Dutta, S. & Wu, K. C. Integrated, cascading enzyme-/chemocatalytic cellulose conversion using catalysts based on mesoporous silica nanoparticles. ChemSusChem 7, 3241–3246 (2014).
Ganai, A. K., Shinde, P., Dhar, B. B., Sen Gupta, S. & Prasad, B. L. V. Development of a multifunctional catalyst for a “relay” reaction. RSC Adv. 3, 2186–2191 (2013).
Gómez Baraibar, A. et al. A one-pot cascade reaction combining an encapsulated decarboxylase with a metathesis catalyst for the synthesis of bio-based antioxidants. Angew. Chem. Int. Ed. 55, 14823–14827 (2016).
Wang, Z. J., Clary, K. N., Bergman, R. G., Raymond, K. N. & Toste, F. D. A supramolecular approach to combining enzymatic and transition metalcatalysis. Nat. Chem. 5, 100–103 (2013). This paper describes a very elegant way of spatially separating various catalysts on the microscale by applying a supramolecular approach.
Brahma, A. et al. An orthogonal biocatalytic approach for the safe generation and use of HCN in a multistep continuous preparation of chiral O-acetylcyanohydrins. Synlett 27, 262–266 (2016).
Köhler, V. et al. Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes. Nat. Chem. 5, 93–99 (2013). This is the first combination of an artifical metallo-enzyme with various enzymes in a concurrent-type cascade.
Okamoto, Y., Köhler, V. & Ward, T. R. An NAD(P)H-dependent artificial transfer hydrogenase for multienzymatic cascades. J. Am. Chem. Soc. 138, 5781–5784 (2016).
Okamoto, Y., Köhler, V., Paul, C. E., Hollmann, F. & Ward, T. R. Efficient in situ regeneration of NADH mimics by an artificial metalloenzyme. ACS Catal. 6, 3553–3557 (2016).
Sato, H., Hummel, W. & Gröger, H. Cooperative catalysis of noncompatible catalysts through compartmentalization: Wacker oxidation and enzymatic reduction in a one-pot process in aqueous media. Angew. Chem. Int. Ed. 54, 4488–4492 (2015).
Wallace, S. & Balskus, E. P. Interfacing microbial styrene production with a biocompatible cyclopropanation reaction. Angew. Chem. Int. Ed. 54, 7106–7109 (2015).
Lee, Y., Umeano, A. & Balskus, E. P. Rescuing auxotrophic microorganisms with nonenzymatic chemistry. Angew. Chem. Int. Ed. 52, 11800–11803 (2013).
Huang, P. S., Boyken, S. E. & Baker, D. The coming of age of de novo protein design. Nature 537, 320–327 (2016).
Obexer, R., Pott, M., Zeymer, C., Griffiths, A. D. & Hilvert, D. Efficient laboratory evolution of computationally designed enzymes with low starting activities using fluorescence-activated droplet sorting. Prot. Eng. Des. Sel. 29, 355–366 (2016).
Garrabou, X., Verez, R. & Hilvert, D. Enantiocomplementary synthesis of gamma-nitroketones using designed and evolved carboligases. J. Am. Chem. Soc. 139, 103–106 (2017).
Brandenberg, O. F., Fasan, R. & Arnold, F. Exploiting and engineering hemoproteins for abiological carben and nitrne transfer reactions. Curr. Opin. Biotechnol. 47, 102–111 (2017).
Prier, C. K. & Arnold, F. H. Chemomimetic biocatalysis: exploiting the synthetic potential of cofactor-dependent enzymes to create new catalysts. J. Am. Chem. Soc. 137, 13992–14006 (2015).
Key, H. M., Dydio, P., Clark, D. S. & Hartwig, J. F. Abiological catalysis by artificial haem proteins containing noble metals in place of iron. Nature 534, 534–537 (2016).
Dydio, P. et al. An artificial metalloenzyme with the kinetics of native enzymes. Science 354, 102–106 (2016). This study demonstrates the beauty of designing and optimizing unnatural metallo-enzymes, which opens access to novel chemistries.
Dydio, P., Key, H. M., Hayashi, H., Clark, D. S. & Hartwig, J. F. Chemoselective, enzymatic C–H bond amination catalyzed by a cytochrome P450 containing an Ir(Me)-PIX cofactor. J. Am. Chem. Soc. 139, 1750–1753 (2017).
Bordeaux, M., Tyagi, V. & Fasan, R. Highly diastereoselective and enantioselective olefin cyclopropanation using engineered myoglobin-based catalysts. Angew. Chem. Int. Ed. 54, 1744–1748 (2015).
Sauer, D. F. et al. A highly active biohybrid catalyst for olefin metathesis in water: impact of a hydrophobic cavity in a beta-barrel protein. ACS Catal. 5, 7519–7522 (2015).
Srivastava, P., Yang, H., Ellis-Guardiola, K. & Lewis, J. C. Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation. Nat. Commun. 6, 7789 (2015).
Heinisch, T. & Ward, T. R. Artificial metalloenzymes based on the biotin–streptavidin technology: challenges and opportunities. Acc. Chem. Res. 49, 1711–1721 (2016).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. http://pubs.acs.org/doi/abs/10.1021/acs.chemrev.7b00014 (2017).
Jeschek, M. et al. Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 537, 661–665 (2016). The first example achieving a metathesis reaction in vivo using the streptavidin concept with a Ru catalyst.
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016). Novel chemistry was achieved by enantioselective light-driven dehalogenation of racemic halolactones.
Sandoval, B. A., Meichan, A. J. & Hyster, T. K. Enantioselective hydrogen atom transfer: discovery of catalytic promiscuity in flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 139, 11313–11316 (2017).
Hummel, W., Schütte, H., Schmidt, E., Wandrey, C. & Kula, M.-R. Isolation of l-phenylalanine dehydrogenase from Rhodococcus sp. M4 and its application for the production of l-phenylalanine. App. Microbiol. Biotechnol. 26, 409–416 (1987).
Bloh, J. Z. & Marschall, R. Heterogeneous photoredox catalysis: reactions, materials, and reaction engineering. Eur. J. Org. Chem. 2017, 2085–2094 (2017).
Brown, K. A. et al. Photocatalytic regeneration of nicotinamide cofactors by quantum dot-enzyme biohybrid complexes. ACS Catal. 6, 2201–2204 (2016).
Choudhury, S., Baeg, J. O., Park, N. J. & Yadav, R. K. A solar light-driven, eco-friendly protocol for highly enantioselective synthesis of chiral alcohols via photocatalytic/biocatalytic cascades. Green Chem. 16, 4389–4400 (2014).
Lam, Q., Kato, M. & Cheruzel, L. Ru(II)-diimine functionalized metalloproteins: from electron transfer studies to light-driven biocatalysis. Biochim. Biophys. Acta Bioenerg. 1857, 589–597 (2016).
Churakova, E. et al. Specific photobiocatalytic oxyfunctionalization reactions. Angew. Chem. Int. Ed. 50, 10716–10719 (2011).
Girhard, M., Kunigk, E., Tihovsky, S., Shumyantseva, V. V. & Urlacher, V. B. Light-driven biocatalysis with cytochrome P450 peroxygenases. Biotechnol. Appl. Biochem. 60, 111–118 (2013).
Tran, N. H. et al. An efficient light-driven P450 BM3 biocatalyst. J. Am. Chem. Soc. 135, 14484–14487 (2013).
Park, J. H. et al. Cofactor-free light-driven whole-cell cytochrome P450 catalysis. Angew. Chem. Int. Ed. 54, 969–973 (2015).
Lee, S. H. et al. Cofactor-free, direct photoactivation of enoate reductases for the asymmetric reduction of C=C bonds. Angew. Chem. Int. Ed. 56, 8681–8685 (2017).
Cannella, D. et al. Light-driven oxidation of polysaccharides by photosynthetic pigments and a metalloenzyme. Nat. Commun. 7, 11134 (2016).
Thomas, B. et al. Application of biocatalysis to on-DNA carbohydrate library synthesis. ChemBioChem 18, 858–863 (2017).
Milczek, E. M. Commercial applications for enzyme-mediated protein conjugation: new developments in enzymatic processes to deliver functionalized proteins on the commercial scale. Chem. Rev. http://pubs.acs.org/doi/abs/10.1021/acs.chemrev.6b00832 (2017).
Bornscheuer, U. T. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Kazlauskas, R. J. & Bornscheuer, U. T. Finding better protein engineering strategies. Nat. Chem. Biol. 5, 526–529 (2009).
Anastas, P. T. & Warner, J. C. Green Chemistry: Theory and Practice (Oxford Univ. Press, Oxford, 1998).
Börner, A. & Franke, R. E. Hydroformylation (Wiley-VCH, 2016).
Gabriel, C. M. et al. Effects of co-solvents on reactions run under micellar catalysis conditions. Org. Lett. 19, 194–197 (2017).
Armenise, N., Malferrari, D., Ricciardulli, S., Galletti, P. & Tagliavini, E. Multicomponent cascade synthesis of biaryl-based chalcones in pure water and in an aqueous micellar environment. Eur. J. Org. Chem. 2016, 3177–3185 (2016).
Gallou, F., Isley, N. A., Ganic, A., Onken, U. & Parmentier, M. Surfactant technology applied toward an active pharmaceutical ingredient: more than a simple green chemistry advance. Green Chem. 18, 14–19 (2016).
Choluj, A., Zielinski, A., Grela, K. & Chmielewski, M. J. Metathesis@MOF: simple and robust immobilization of olefin metathesis catalysts inside (Al)MIL-101-NH2. ACS Catal. 6, 6343–6349 (2016).
Yang, H., Fu, L., Wei, L., Liang, J. & Binks, B. P. Compartmentalization of incompatible reagents within Pickering emulsion droplets for one-pot cascade reactions. J. Am. Chem. Soc. 137, 1362–1371 (2015).
Palivan, C. G. et al. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 45, 377–411 (2016).
Worsdorfer, B., Woycechowsky, K. J. & Hilvert, D. Directed evolution of a protein container. Science 331, 589–592 (2011).
Wells, A. S., Finch, G. L., Michels, P. C. & Wong, J. W. Use of enzymes in the manufacture of active pharmaceutical ingredients — a science and safety-based approach to ensure patient safety and drug quality. Org. Proc. Res. Dev. 16, 1986–1993 (2012).
Wells, A. S. et al. Case studies illustrating a science and risk-based approach to ensuring drug quality when using enzymes in the manufacture of active pharmaceuticals ingredients for oral dosage form. Org. Proc. Res. Dev. 20, 594–601 (2016).
Lohr, T. L. & Marks, T. J. Orthogonal tandem catalysis. Nat. Chem. 7, 477–482 (2015).
Filice, M. & Palomo, J. M. Cascade reactions catalyzed by bionanostructures. ACS Catal. 4, 1588–1598 (2014).
Kroutil, W. & Rueping, M. Introduction to ACS Catalysis virtual special issue on cascade catalysis. ACS Catal. 4, 2086–2087 (2014).
Acknowledgements
We are grateful to R. J. Carroll and M. Höhne for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.S. is an employee of Novartis Pharma AG and H.I. is an employee of F. Hoffmann-La Roche Ltd.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A correction to this article is available online at https://doi.org/10.1038/s41929-018-0042-4.
Rights and permissions
About this article
Cite this article
Rudroff, F., Mihovilovic, M.D., Gröger, H. et al. Opportunities and challenges for combining chemo- and biocatalysis. Nat Catal 1, 12–22 (2018). https://doi.org/10.1038/s41929-017-0010-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-017-0010-4
This article is cited by
-
A straightforward chemobiocatalytic route for one-pot valorization of glucose into 2,5-bis(hydroxymethyl)furan
Bioresources and Bioprocessing (2024)
-
Pickering emulsion droplets and solid microspheres acting synergistically for continuous-flow cascade reactions
Nature Catalysis (2024)
-
Engineered cytochrome P450 for direct arylalkene-to-ketone oxidation via highly reactive carbocation intermediates
Nature Catalysis (2023)
-
Multi-compartmental MOF microreactors derived from Pickering double emulsions for chemo-enzymatic cascade catalysis
Nature Communications (2023)
-
Adaptable graphitic C6N6-based copper single-atom catalyst for intelligent biosensing
Nature Communications (2023)