Abstract
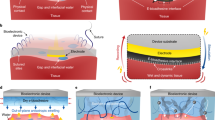
Bioadhesive devices can be used to create conformable tissue–device interfaces without suturing. However, the development of such technology faces challenges related to the need for external stimuli or long periods of time for tissue adhesion, fatigue-related breakdown of the stretchable electrodes and the use of solid substrates with non-uniform surface coverage of the tissue. Here, we report a bioelectronic patch that is capable of instantaneous and conformable tissue adhesion on a heart for precise cardiac monitoring. The patch is composed of three layers: an ionically conductive tissue adhesive, a viscoelastic networked film and a fatigue-resistant conducting composite. The system provides conformable tissue adhesion in less than 0.5 s without external stimuli, spontaneous modulus matching based on efficient strain adaptivity and small resistance changes of less than 0.2% at 50.0% tensile strain after 1,000 stretching cycles. We show that the patch can be used for the long-term measurement of electrocardiogram signals (up to four weeks of implantation) in awake rats without causing tissue damage, as well as spatiotemporal mapping in a myocardial ischaemia reperfusion model.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper. The other data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Viventi, J. et al. A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci. Transl. Med. 2, 24ra22 (2010).
Park, S. et al. Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics. Nature 561, 516–521 (2018).
Boutry, C. M. et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3, 47–57 (2019).
Kim, D.-H. et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat. Mater. 10, 316–323 (2011).
Liu, R., Zhao, S. & Liu, J. From lithographically patternable to genetically patternable electronic materials for miniaturized, scalable, and soft implantable bioelectronics to interface with nervous and cardiac systems. Appl. Electron. Mater. 3, 101–118 (2021).
Hong, Y. J. et al. Wearable and implantable devices for cardiovascular healthcare: from monitoring to therapy based on flexible and stretchable electronics. Adv. Funct. Mater. 29, 1808247 (2019).
Son, D. et al. Bioresorbable electronic stent integrated with therapeutic nanoparticles for endovascular diseases. ACS Nano 9, 5937–5946 (2015).
Choi, S. et al. Highly conductive, stretchable and biocompatible Ag-Au core-sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 13, 1048–1056 (2018).
Sim, K. et al. An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity. Nat. Electron. 3, 775–784 (2020).
Park, J. et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl. Med. 8, 344ra86 (2016).
Lee, W. et al. Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping. Sci. Adv. 4, eaau2426 (2018).
Kang, J. et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv. Mater. 30, 1706846 (2018).
Seo, H. et al. Durable and fatigue-resistant soft peripheral neuroprosthetics for in vivo bidirectional signaling. Adv. Mater. 33, 2007346 (2021).
Li, J. et al. Tough adhesives for diverse wet surfaces. Science 357, 378–381 (2017).
Motossian, C., Makari, S. & Potvin, R. Cataract surgery and methods of wound closure: a review. Clin. Ophthalmol. 9, 921–928 (2015).
Liu, J. et al. Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc. Natl Acad. Sci. USA 117, 14769–14778 (2020).
Lee, H. et al. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Yang, Q. et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissue. Nat. Mater. 20, 1559–1570 (2021).
Deng, J. et al. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 20, 229–236 (2021).
Tringides, C. M. et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 16, 1019–1029 (2021).
Liu, Y. et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 38, 1031–1036 (2020).
Song, K.-I. et al. Adaptive self-healing electronic epineurium for chronic bidirectional neural interfaces. Nat. Commun. 11, 4195 (2020).
Dickey, M. D. Stretchable and soft electronics using liquid metals. Adv. Mater. 29, 16064225 (2017).
Palleau, E., Reece, S., Desai, S. C., Smith, M. E. & Dickey, M. D. Self-healing stretchable wires for reconfigurable circuit wiring and 3D microfluidics. Adv. Mater. 25, 1589–1592 (2013).
Noskovicova, N. et al. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-β. Nat. Biomed. Eng. 5, 1437–1456 (2021).
Wang, Y. et al. Robust, self-adhesive, reinforced polymeric nanofilms enabling gas-permeable dry electrodes for long-term application. Proc. Natl Acad. Sci. USA 118, e2111904118 (2021).
Mou, L. et al. Highly stretchable and biocompatible liquid metal-elastomer conductors for self-healing electronics. Small 16, 2005336 (2020).
Kim, S. H. et al. An ultrastretchable and self-healable nanocomposite conductor enabled by autonomously percolative electrical pathways. ACS Nano 13, 6531–6539 (2019).
Rahim, Md. A. et al. Polyphenol-induced adhesive liquid metal inks for substrate-independent direct pen writing. Adv. Funct. Mater. 31, 2007336 (2021).
Shafiei, M., Hoshyargar, F., Motta, N. & O’Mullane, A. P. Utilizing p-type native oxide on liquid metal microdroplets for low temperature gas sensing. Mater. Des. 122, 288–295 (2017).
Hong, S. H. et al. STAPLE: stable alginate gel prepared by linkage exchange from ionic to covalent bonds. Adv. Healthc. Mater. 5, 75–79 (2016).
Kastrup, C. J. et al. Painting blood vessels and atherosclerotic plaques with an adhesive drug depot. Proc. Natl Acad. Sci. USA 109, 21444–21449 (2012).
Ma, Z. et al. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 20, 859–868 (2021).
Kusumoto, F. M. ECG Interpretation: From Pathophysiology to Clinical Application (Springer, 2009).
Longmore, M., Wilkinson, I. B., Baldwin, A., Wallin, E. Oxford Handbook of Clinical Medicine—Mini Edition (Oxford Univ. Press, 2014).
Temple, I. P. et al. Atrioventricular node dysfunction and ion channel transcriptome in pulmonary hypertension. Circ.: Arrhythmia Electrophysiol. 9, e003432 (2016).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant by the Korea government (MSIT) (nos. 2020R1C1C1005567, RS-2023-00208262 and 2022M3E5E9018583). This research was also supported by the Institute for Basic Science (IBS-R015-D1).
Author information
Authors and Affiliations
Contributions
This project was originally proposed by D.S. M.S. and D.S. supervised the project. H.C., Y.K. and S.K. conducted all the experiments with assistance from H.J., S.L., K.K., M.S. and D.S. H.J. analysed the strain distribution on the SAFIE patch using a mechanical simulation software. H.-S.H. and J.Y.K. provided the interpretation of the biological and histological data. H.C., Y.K., S.K., M.S. and D.S. analysed the data and prepared the manuscript with input from all the co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Xiaozhong Qiu, Tae-Woo Lee and Kyoseung Sim for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of stable ECG recording of SAFIE patch to that of conventional ECG sensors without tissue adhesion and conformability.
Optimized SAFIE patch via adhesion coating on network substrate to record stable cardiac signals for long periods.
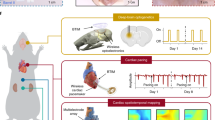
Extended Data Fig. 2 ECG signals and their spatiotemporal map data of the simplified MI model using the SAFIE.
(i), (ii), and (iii) ECG signals correspond to normal heart rhythms, ST segment evaluation, and ventricular fibrillation, respectively. Their ECG signals are shown as the spatiotemporal map data.
Extended Data Fig. 3 The ECG monitoring by the SAFIE patch in a rat MIR model.
a, Observation of raw 4-channel ECG signals from the SAFIE patch in the disease model. b, Surface ECG signals (left inset graphs) and activation map before (left) and after (right) myocardial infarction. c, Histological image of the heart tissue with infarcted region (purple) stained by Masson’s-trichrome (MT) reagents.
Extended Data Fig. 4 Spatiotemporal mapping of the ECG signals recorded by the SAFIE patch with the 8 screen-printed EGaIn/SHP composite electrodes.
a, Various screen-printed EGaIn/SHP composites on the SHP substrate with different line-shaped patterns (200 – 1000 μm). b, Electrical resistance value of printed interconnects versus line width. c, Photograph of the SAFIE patch with 8 channels attached onto rat’s heart. Data points are means ± s.e.m. (n = 3). d, Normalized ECG signals recorded from the multichannel SAFIE patch. The blue-dashed box indicates the magnified view (10 times) of the 3rd peak where the 1st peak with a specific pattern of an inverted triangle shape is detected from each channel. e, Activation map during the sinus rhythm. f, Epicardial spatiotemporal mapping achieved during the sinus rhythm every 0.5 ms. All the map data were interpolated with K-nearest neighbors interpolation algorithm.
Extended Data Fig. 5 Histological analysis.
a–c, H&E stained histological appearance of the heart tissues dissected after 3 days of (a) only surgical procedures (for example, no treatment), (b) suturing of the patch without Alg-CA layer, and (c) implantation of the SAFIE. The image was magnified ×1 (left), ×5 (middle), and ×20 (right) to show each region where epicardial devices were implanted (red boxes) or not (blue boxes).
Extended Data Fig. 6 Histological analysis at 1-week and 4-week implantation of the SAFIE patch.
a–d, Representative histological images at 1 week (a, b) and 4 weeks (c, d) for the control group without any implantation (a, c) and the SAFIE group (b, d) stained with hematoxylin and eosin (H&E) (left panels) and Masson’s trichrome (MT) agents (right panels)). The boxes indicate the region where epicardial device was either implanted (red) or not applied (blue).
Supplementary information
Supplementary Information
Supplementary Notes 1–14, Table 1, Figs. 1–39 and captions for Supplementary Videos 1–9.
Supplementary Video 1
Three-dimensional reconstructed Alg-CA-coated E-SHN patch by confocal z-stack images.
Supplementary Video 2
In vivo test for the instantaneous conformal adhesion of the E-SHN coated with Alg-CA on rat’s heart.
Supplementary Video 3
Instantaneous underwater tissue adhesion of E-SHN with Alg-CA ex vivo in small and large animals. E-SHN quickly and evenly attached without any additional pressure on both rat and porcine heart organs and maintained adhesion during rinsing with PBS (1×, pH 7.4) and blood.
Supplementary Video 4
Comparison of conformability and adhesiveness of E-SHN with Alg-CA (SAFIE), E-SHN without Alg-CA, solid film with Alg-CA and solid film without Alg-CA on rat’s heart. An uncovered area existed in the solid film with/without Alg-CA, and a slip occurred in the E-SHN and solid film without Alg-CA within 60 s. The SAFIE patch showed stable adhesion and full coverage of the rat heart.
Supplementary Video 5
In vivo stimulation with the SAFIE patch on rat’s heart. Heart rhythms were not synchronized in the subthreshold voltage stimulation (for example, 1.0 Vpp) but were synchronized over the threshold voltage stimulation (for example, 1.5 Vpp).
Supplementary Video 6
A rat-based simplified MI model prepared after implantation of the SAFIE patch on the heart.
Supplementary Video 7
Spatiotemporal mapping of ECG signals during sinus rhythm from the eight-channel SAFIE patch.
Supplementary Video 8
In vivo implantation of the SAFIE patch. Total implantation time was less than 1 min and proceeded without any damage to the heart tissue of the rat.
Supplementary Video 9
Real-time ECG signal recording of a freely moving rat from four sensing channels of the SAFIE patch three days after implantation. Real-time bpm analysis was also conducted using LabChart software.
Supplementary Data
Source data for the supplementary figures.
Source data
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Extended Data Figs. 1–6
Source data for Extended Data Figs. 1–6.
Source Data Fig. 3
Original CDX file for Fig. 3e(iii),(iv).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, H., Kim, Y., Kim, S. et al. Adhesive bioelectronics for sutureless epicardial interfacing. Nat Electron 6, 779–789 (2023). https://doi.org/10.1038/s41928-023-01023-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-023-01023-w
This article is cited by
-
Anti-friction gold-based stretchable electronics enabled by interfacial diffusion-induced cohesion
Nature Communications (2024)
-
Motion artefact management for soft bioelectronics
Nature Reviews Bioengineering (2024)
-
A sutureless bioelectronic patch for electrocardiography
Nature Electronics (2023)