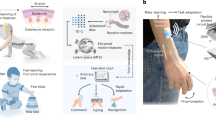
Abstract
Neuromorphic computing could be used to directly perform complex classification tasks in hardware and is of potential value in the development of wearable, implantable and point-of-care devices. Successful implementation requires low-power operation, simple sensor integration and straightforward training. Organic materials are possible building blocks for neuromorphic systems, offering low-voltage operation and excellent tunability. However, systems developed so far still rely on external training in software. Here we report a neuromorphic biosensing platform that is capable of on-chip learning and classification. The modular biosensor consists of a sensor input layer, an integrated array of organic neuromorphic devices that form the synaptic weights of a hardware neural network and an output classification layer. We use the system to classify the genetic disease cystic fibrosis from modified donor sweat using ion-selective sensors; on-chip training is done using error signal feedback to modulate the conductance of the organic neuromorphic devices. We also show that the neuromorphic biosensor can be retrained on the chip, by switching the sensor input signals and alternatively through the formation of logic gates.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data that support the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The source codes for MATLAB are available from the corresponding authors upon request.
References
van de Burgt, Y., Melianas, A., Keene, S. T., Malliaras, G. & Salleo, A. Organic electronics for neuromorphic computing. Nat. Electron. 1, 386–397 (2018).
Gkoupidenis, P., Schaefer, N., Garlan, B. & Malliaras, G. G. Neuromorphic functions in PEDOT:PSS organic electrochemical transistors. Adv. Mater. 27, 7176–7180 (2015).
van de Burgt, Y. & Gkoupidenis, P. Organic materials and devices for brain-inspired computing: from artificial implementation to biophysical realism. MRS Bull. 45, 631–640 (2020).
Fuller, E. J. et al. Parallel programming of an ionic floating-gate memory array for scalable neuromorphic computing. Science 364, 570–574 (2019).
Xia, Q. & Yang, J. J. Memristive crossbar arrays for brain-inspired computing. Nat. Mater. 18, 309–323 (2019).
van de Burgt, Y. et al. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 16, 414–418 (2017).
Keene, S. T., Melianas, A., van de Burgt, Y. & Salleo, A. Mechanisms for enhanced state retention and stability in redox-gated organic neuromorphic devices. Adv. Electron. Mater. 5, 1800686 (2019).
Kim, K.-N., Sung, M.-J., Park, H.-L. & Lee, T.-W. Organic synaptic transistors for bio-hybrid neuromorphic electronics. Adv. Electron. Mater. 8, 2100935 (2022).
Keene, S. T. et al. A biohybrid synapse with neurotransmitter-mediated plasticity. Nat. Mater. 19, 969–973 (2020).
Harikesh, P. C. et al. Organic electrochemical neurons and synapses with ion mediated spiking. Nat. Commun. 13, 901 (2022).
Rivnay, J. et al. High-performance transistors for bioelectronics through tuning of channel thickness. Sci. Adv. 1, e1400251 (2015).
Sessolo, M., Rivnay, J., Bandiello, E., Malliaras, G. G. & Bolink, H. J. Ion-selective organic electrochemical transistors. Adv. Mater. 26, 4803–4807 (2014).
Ohayon, D. et al. Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nat. Mater. 19, 456–463 (2019).
Bai, L. et al. Biological applications of organic electrochemical transistors: electrochemical biosensors and electrophysiology recording. Front. Chem. 7, 313 (2019).
Pitsalidis, C. et al. Organic bioelectronics for in vitro systems. Chem. Rev. 122, 4700–4790 (2021).
Strakosas, X., Bongo, M. & Owens, R. M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 132, 41735 (2015).
Rashid, R. B., Ji, X. & Rivnay, J. Organic electrochemical transistors in bioelectronic circuits. Biosens. Bioelectron. 190, 113461 (2021).
Ji, X. et al. Mimicking associative learning using an ion-trapping non-volatile synaptic organic electrochemical transistor. Nat. Commun. 12, 2480 (2021).
Zhou, F. & Chai, Y. Near-sensor and in-sensor computing. Nat. Electron. 3, 664–671 (2020).
Christodouleas, D. C., Kaur, B. & Chorti, P. From point-of-care testing to eHealth diagnostic devices (eDiagnostics). ACS Cent. Sci. 4, 1600–1616 (2018).
Lim, S. et al. Adaptive learning rule for hardware-based deep neural networks using electronic synapse devices. Neural Comput. Appl. 31, 8101–8116 (2018).
Krauhausen, I. et al. Organic neuromorphic electronics for sensorimotor integration and learning in robotics. Sci. Adv. 7, eabl5068 (2021).
Wright, L. G. et al. Deep physical neural networks trained with backpropagation. Nature 601, 549–555 (2022).
LeGrys, V. A. Sweat testing for the diagnosis of cystic fibrosis: practical considerations. J. Pediatr. 129, 892–897 (1996).
Gonzalo-Ruiz, J. et al. Early determination of cystic fibrosis by electrochemical chloride quantification in sweat. Biosens. Bioelectron. 24, 1788–1791 (2009).
Bandodkar, A. J. et al. Sweat-activated biocompatible batteries for epidermal electronic and microfluidic systems. Nat. Electron. 3, 554–562 (2020).
Kwon, K. et al. An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time. Nat. Electron. 4, 302–312 (2021).
Han, S., Yamamoto, S., Polyravas, A. G. & Malliaras, G. G. Microfabricated ion-selective transistors with fast and super-Nernstian response. Adv. Mater. 32, 2004790 (2020).
Pierre, A., Doris, S. E., Lujan, R. & Street, R. A. Monolithic integration of ion-selective organic electrochemical transistors with thin film transistors on flexible substrates. Adv. Mater. Technol. 4, 1800577 (2019).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Paulsen, B. D., Tybrandt, K., Stavrinidou, E. & Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 19, 13–26 (2020).
Li, Y. et al. Ion-selective organic electrochemical transistors: recent progress and challenges. Small 18, 2107413 (2022).
Battistoni, S., Erokhin, V. & Iannotta, S. Organic memristive devices for perceptron applications. J. Phys. D: Appl. Phys. 51, 284002 (2018).
Prezioso, M. et al. Training and operation of an integrated neuromorphic network based on metal-oxide memristors. Nature 521, 61–64 (2015).
Chen, J., Li, J., Li, Y. & Miao, X. Multiply accumulate operations in memristor crossbar arrays for analog computing. J. Semicond. 42, 013104 (2021).
Acknowledgements
E.R.W.v.D and Y.v.d.B. gratefully acknowledge funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant no. 802615). X.J. and J.R. gratefully acknowledge support from the Alfred P. Sloan Foundation (FG-2019-12046).
Author information
Authors and Affiliations
Contributions
Y.v.d.B and J.R. conceived the idea. E.R.W.v.D performed and analysed the experiments. X.J. fabricated the IS-OECTs. E.R.W.v.D and Y.v.d.B wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Electronics thanks Zhongrui Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Voltage output as a function of the ion concentration in KCl including donor sweat.
a, Commercially available ion selective electrodes (ISE, Mettler Toledo) were used for the detection of [K+] and [Cl−] and voltage output calibration, measured after an 11x amplification, with stock solutions. Sweat samples from 3 donors was also measured and indicated in the graph. b, Input voltage (calculated before amplification) to define the values for high/low ion concentration used for training. The negative sweat sample is obtained from the sweat of 2 healthy donors and the positive sweat sample is based on the sweat of 1 healthy donor with additional NaCl. A low concentration corresponds to −0.015V for chloride and −0.090V for potassium and a high concentration corresponds to −0.045V for chloride and −0.060V for potassium.
Extended Data Fig. 2 Offset circuit.
a, Schematic of the offset circuit for a single sensor input and output. The offset resistor is a potentiometer that can be increased or decreased to define the desired offset. b, Photo of the offset circuit PCB for two inputs and outputs that can each be offset independently.
Extended Data Fig. 3 Characteristics of the potassium IS-OECT.
a, b, Two transfer curves of the potassium IS-OECT in 10−4 M KCl and 10−1 M KCl (VDS= −0.1V). c, Steady-state current response against KCl with different concentration (VG= 0.4V, VDS= −0.1V).
Extended Data Fig. 4 Voltage output IS-OECT circuit.
a, Circuit diagram of the IS-OECT in a voltage divider configuration with Vdrain = −0.4 V. For the potassium selective sensor Vgate = 0.2V and R = 300 Ω and for the chloride selective sensor gate is grounded and R = 91 Ω. b, Output voltage (Vout) of the chloride selective sensor for 1, 20, 80 and 100 mM [NaCl] averaged over 5 measurements as well as the output voltage of the potassium selective sensor for 1, 20, 80 and 100 mM [KCl] averaged over 3 measurements.
Extended Data Fig. 5 Sensor module for scaling sensor output.
a, Circuit diagram of the IS-OECT in a voltage divider configuration together with the offset circuit. For the chloride sensor module \(R_{offset}\) = 10kΩ and for the potassium sensor module \({R}_{{offset}}\) = 24kΩ. b, The resulting voltage output for both sensor modules.
Extended Data Fig. 6 EC-RAM conductance modulation with aqueous electrolyte.
a, Conductance modulation by applying alternating positive (128x) and negative (128x) gate pulses with amplitude 5V and duration 1s (Vdrain = −0.1V), and b, zoom in of the measurement data to visualize the single conductance states.
Extended Data Fig. 7 EC-RAM conductance modulation comparing aqueous and solid electrolyte.
Conductance modulation by applying alternating positive (64x) and negative (64x) gate pulses with amplitude 5V and duration 1s (Vdrain = −0.1V), on the same device once with an aqueous and once with a solid electrolyte, showing no difference. Applying more pulses (128x) to the aqueous electrolyte gate device shows the maximum conductance range.
Extended Data Fig. 8 EC-RAM state retention.
a, State retention of multiple states in a potentiation and depression cycle measured for 5 minutes of an aqueous electrolyte gated device and b, a solid electrolyte gated device.
Extended Data Fig. 9 Visualization of the weight update during a training cycle using ion selective electrodes without offset.
a, Values of the weights corresponding to b, the inputs representing the voltage output for a high (100mM) or low (1mM) ion concentration measured with the commercially available ISEs (Extended Data Fig. 1). A low concentration (1mM) corresponds to 0.075V for chloride and −0.150V for potassium and a high concentration corresponds to −0.050V for chloride and −0.015V for potassium. c, The output for each measurement cycle, measured before the threshold activation function triggering the LEDs. d, 2-D graphical representation of the classification problem of cystic fibrosis where the dashed lines show the decision boundary on the first and every 10th iteration. The solid line corresponds to the last (60th) measurement and shows a correct classification.
Extended Data Fig. 10 Visualization of the synaptic weight update and corresponding classification line during training with IS-OECTs.
a, Values of the weights corresponding to b, the inputs representing the voltage output for a high or low ion concentration measured with the IS-OECTs with an offset. c, The output for each measurement cycle, measured before the threshold activation function triggering the LEDs. d, 2-D graphical representation of the classification problem of cystic fibrosis where the dashed lines show the decision boundary on every 5th iteration. The solid line corresponds to the last (45th) measurement and shows a correct classification.
Supplementary information
Supplementary Information
Supplementary Notes 1–4 and Figs. 1–6.
Supplementary Video 1
Recording of the training process shown in Fig. 3.
Supplementary Data 1
Source data for Supplementary Fig. 1.
Supplementary Data 2
Source data for Supplementary Fig. 4a.
Supplementary Data 3
Source data for Supplementary Fig. 4b.
Source data
Source Data Fig. 1
Measurement data for Fig. 1c,d.
Source Data Fig. 3
Measurement data Fig. 3a–d.
Source Data Fig. 4
Measurement data.
Source Data Extended Data Fig. 1
Measurement data.
Source Data Extended Data Fig. 3
Measurement data.
Source Data Extended Data Fig. 4
Measurement data for Extended Data Fig. 4b.
Source Data Extended Data Fig. 5
Measurement data for Extended Data Fig. 5b.
Source Data Extended Data Fig. 6
Measurement data.
Source Data Extended Data Fig. 7
Measurement data.
Source Data Extended Data Fig. 8
Measurement data for Extended Data Fig. 8a,b.
Source Data Extended Data Fig. 9
Measurement data for Extended Data Fig. 9a–d.
Source Data Extended Data Fig. 10
Measurement data for Extended Data Fig. 10a–d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Doremaele, E.R.W., Ji, X., Rivnay, J. et al. A retrainable neuromorphic biosensor for on-chip learning and classification. Nat Electron 6, 765–770 (2023). https://doi.org/10.1038/s41928-023-01020-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41928-023-01020-z
This article is cited by
-
Organic mixed conductors for bioinspired electronics
Nature Reviews Materials (2023)