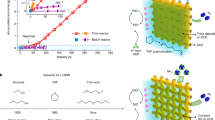
Abstract
The ammonia industry is crucial for the global supply of food through economical production of fertilizers in quantity, and it allows for the development of catalytic chemistry and technologies with ammonia as a promising carbon-free energy carrier. Although the Haber–Bosch process, where hydrogenolysis of nitrogen takes place over a promoted iron catalyst under harsh conditions, will continue to play a key role, its massive carbon footprint and energy consumption call for more sustainable production methods ideally at near ambient pressure. Here, we show a green route for the synthesis of ammonia using a nitrogen permeable membrane reactor. In the absence of an external pressure, our membrane reactor delivers a nitrogen flux of 3.1 × 10−2 ml cm−2 h−1, leading to an ammonia yield rate of 2.9 μmol cm−2 h−1 at 450 °C. The reaction of permeated N3− ions with H2 gives rise to a high ammonia concentration of 0.097 vol% in the gas phase, which is close to the limit of thermodynamic equilibrium (0.1 vol%) under the identical condition. This work not only creates a greener path for ambient-pressure ammonia synthesis but also presents a new membrane reactor design that could find applications in other areas.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the public repository: https://yunpan.360.cn/surl_yvqgghBnccC (Code: f2f2). Source data are provided with this paper.
References
Chen, J. G. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, 873 (2018).
Foster, S. L. et al. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 1, 490–500 (2018).
Sippel, D. et al. A bound reaction intermediate sheds light on the mechanism of nitrogenase. Science 359, 1484–1489 (2018).
Ashida, Y., Arashiba, K., Nakajima, K. & Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 568, 536–540 (2019).
Claus, J. H. et al. Catalyst design by interpolation in the periodic table: bimetallic ammonia synthesis catalysts. J. Am. Chem. Soc. 123, 8404–8405 (2001).
Martín, A. J., Shinagawa, T. & Pérez–Ramírez, J. Electrocatalytic reduction of nitrogen: from Haber–Bosch to ammonia artificial leaf. Chem 5, 263–283 (2019).
Buren, S., Jiménez-Vicente, E., Echavarri-Erasun, C. & Rubio, L. M. Biosynthesis of nitrogenase cofactors. Chem. Rev. 120, 4921–4968 (2020).
Vicente, E. J. & Dean, D. R. Keeping the nitrogen-fixation dream alive. Proc. Natl Acad. Sci. USA 114, 3009–3011 (2017).
Milton, R. D. et al. The in vivo potential-regulated protective protein of nitrogenase in Azotobacter vinelandii supports aerobic bioelectrochemical dinitrogen reduction in vitro. J. Am. Chem. Soc. 139, 9044–9052 (2017).
Faria, J. A. Renaissance of ammonia synthesis for sustainable production of energy and fertilizers. Curr. Opin. Green Sustain. Chem. 29, 100466 (2021).
Barboun, P. M. & Hicks, J. C. Unconventional catalytic approaches to ammonia synthesis. Annu. Rev. Chem. Biomol. Eng. 11, 503–521 (2020).
Qing, G. et al. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 120, 5437–5516 (2020).
Ye, T. N. et al. Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst. Nature 583, 391–395 (2020).
Zhao, S., Lu, X., Wang, L., Gale, J. & Amal, R. Carbon-based metal-free catalysts for electrocatalytic reduction of nitrogen for synthesis of ammonia at ambient conditions. Adv. Mater. 31, 1805367 (2019).
Cui, X., Tang, C. & Zhang, Q. A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv. Energy Mater. 8, 1800369 (2018).
Giddey, S., Badwal, S. P. S. & Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 38, 14576–14594 (2013).
Medford, A. J. & Hatzell, M. C. Photon-driven nitrogen fixation: current progress, thermodynamic considerations, and future outlook. ACS Catal. 7, 2624–2643 (2017).
Guo, C., Ran, J., Vasileff, A. & Qiao, S. Z. Rational design of electrocatalysts and photo(electro) catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci. 11, 45–56 (2018).
Chen, G., Hou, Z. & Gong, X. Structural and electronic properties of cubic HfO2 surfaces. Comp. Mater. Sci. 44, 46–52 (2008).
Lin, G., Su, Y., Duan, X. & Xie, K. High-density Lewis acid sites in porous single-crystalline monoliths to enhance propane dehydrogenation at reduced temperatures. Angew. Chem. Int. Ed. Engl. 60, 9311–9315 (2021).
Jin, L., Cheng, F., Li, H. & Xie, K. Porous tantalum nitride single crystal at two-centimeter scale with enhanced photoelectrochemical performance. Angew. Chem. Int. Ed. Engl. 59, 8891–8895 (2020).
Lin, G., Li, H. & Xie, K. Twisted surfaces in porous single crystals to deliver enhanced catalytic activity and stability. Angew. Chem. Int. Ed. Engl. 59, 16440–16444 (2020).
Hunter, S. M. et al. A study of 15N/14N isotopic exchange over cobalt molybdenum nitrides. ACS Catal. 3, 1719–1725 (2013).
Cui, H., Zhu, G., Liu, X., Liu, F. & Huang, F. Niobium nitride Nb4N5 as a new high-performance electrode material for supercapacitors. Adv. Sci. 2, 1500126 (2015).
Heyl, D. et al. Alcohol synthesis from CO2, H2, and olefins over alkali-promoted Au catalysts—a catalytic and in situ FTIR spectroscopic study. ChemSusChem 12, 651–660 (2019).
Bera, M., Prabhakar, A. & Maji, P. K. Nanotailoring of thermoplastic polyurethane by amine functionalized graphene oxide: effect of different amine modifier on final properties. Compos. B 195, 108075 (2020).
Drews, M. et al. Pathways of glutamine metabolism in Spodoptera frugiperda (Sf9) insect cells: evidence for the presence of the nitrogen assimilation system, and a metabolic switch by 1H/15N NMR. J. Biotechnol. 78, 23–37 (2000).
Ivanov, A. I. et al. Cisplatin binding sites on human albumin. J. Biol. Chem. 273, 14721–14730 (1998).
Cui, Z., Zu, C., Zhou, W., Manthiram, A. & Goodenough, J. B. Mesoporous titanium nitride-enabled highly stable lithium–sulfur batteries. Adv. Mater. 28, 6926–6931 (2016).
Yang, M., Cui, Z. & Disalvo, F. J. Mesoporous chromium nitride as a high performance non-carbon support for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 15, 7041–7044 (2013).
Ziehfreund, A., Simon, U. & Maier, W. F. Oxygen ion conductivity of platinum impregnated stabilized zirconia in bulk and microporous materials. Adv. Mater. 8, 424–427 (1996).
Ye, L. et al. Enhancing CO2 electrolysis through synergistic control of non-stoichiometry and doping to tune cathode surface structures. Nat. Commun. 8, 14785 (2017).
Wang, W. et al. Ammonia synthesis at atmospheric pressure using a reactor with thin solid electrolyte BaCe0.85Y0.15O3−δ membrane. J. Membr. Sci. 360, 397–403 (2010).
Li, Z. et al. Preparation of double-doped BaCeO3 and its application in the synthesis of ammonia at atmospheric pressure. Sci. Technol. Adv. Mat. 8, 566–570 (2007).
Hattori, M., Iijima, S., Nakao, T., Hosono, H. & Hara, M. Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C. Nat. Commun. 11, 2001 (2020).
Ogawa, T. et al. High electron density on Ru in intermetallic YRu2: the application to catalyst for ammonia synthesis. J. Phys. Chem. C. 122, 10468–10475 (2018).
Dawson, R. D., Elwell, D. & Brice, J. C. Top seeded solution growth of sodium niobate. J. Cryst. Growth 23, 65–70 (1974).
Fukuda, T. & Uematsu, Y. Preparation of KNbO3 single crystal for optical applications. Jpn. J. Appl. Phys. 11, 163–169 (2014).
Ventruti, G., Della Ventura, G., Scordari, F., Susta, U. & Gualtieri, A. F. In situ high-temperature XRD and FTIR investigation of hohmannite, a water-rich Fe-sulfate, and its decomposition products. J. Therm. Anal. Calorim. 119, 1793–1802 (2015).
Bell, V. A., Feeley, J. S., Deeba, M. & Farrauto, R. J. In situ high temperature FTIR studies of NOx reduction with propylene over Cu/ZSM–5 catalysts. Catal. Lett. 29, 15–26 (1994).
Chen, G. et al. Roadmap on sustainable mixed ionic–electronic conducting membranes. Adv. Funct. Mater. 32, 2105702 (2022).
Zhu, X. & Yang, W. Microstructural and interfacial designs of oxygen-permeable membranes for oxygen separation and reaction–separation coupling. Adv. Mater. 31, 1902547 (2019).
Zhang, Y. et al. Thermal-expansion offset for high-performance fuel cell cathodes. Nature 591, 246–251 (2021).
Duan, C. et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 557, 217–222 (2018).
Kresse, G. & Furthmüller, J. Efficiency of ab–initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Honkala, K. et al. Ammonia synthesis from first-principles calculations. Science 307, 555–558 (2005).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Abghoui, Y., Garden, A. L., Howalt, J. G., Vegge, T. & Skúlason, E. Electroreduction of N2 to ammonia at ambient conditions on mononitrides of Zr, Nb, Cr, and V: a DFT guide for experiments. ACS Catal. 6, 635–646 (2015).
Azofra, L. M., Sun, C., Cavallo, L. & MacFarlane, D. R. Feasibility of N2 binding and reduction to ammonia on Fe-deposited MoS2 2D sheets: a DFT study. Chem. Eur. J. 23, 8275–8279 (2017).
Acknowledgements
K.X. acknowledges funding from National Key Research and Development Program of China (2017YFA0700102) and Natural Science Foundation of China (91845202). L.Y. acknowledges funding from Natural Science Foundation of China (22002167).
Author information
Authors and Affiliations
Contributions
L.Y. conducted the experiments. H.L. conducted the theoretical calculations. K.X. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Jiazhen Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1 and Figs. 1–32.
Source data
Source Data Fig. 1
Picture.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
About this article
Cite this article
Ye, L., Li, H. & Xie, K. Sustainable ammonia production enabled by membrane reactor. Nat Sustain 5, 787–794 (2022). https://doi.org/10.1038/s41893-022-00908-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-022-00908-6
This article is cited by
-
Multiple reaction pathway on alkaline earth imide supported catalysts for efficient ammonia synthesis
Nature Communications (2023)
-
Atomically dispersed bimetallic Fe–Co electrocatalysts for green production of ammonia
Nature Sustainability (2022)