Abstract
ACCEPT-AI is a framework of recommendations for the safe inclusion of pediatric data in artificial intelligence and machine learning (AI/ML) research. It has been built on fundamental ethical principles of pediatric and AI research and incorporates age, consent, assent, communication, equity, protection of data, and technological considerations. ACCEPT-AI has been designed to guide researchers, clinicians, regulators, and policymakers and can be utilized as an independent tool, or adjunctively to existing AI/ML guidelines.
Similar content being viewed by others
The interests of children must be protected in artificial intelligence research
While a number of medical devices have been formally licensed for usage in children, there remains to date no guidance on the ethical use of pediatric data in artificial intelligence or machine learning (AI/ML) research1. To ensure fundamental ethical principles are prioritized through the ideation, development, iteration, deployment, and evaluation of AI/ML studies, researchers have highlighted the importance of formal ethics review and reporting procedures to improve safety and promote equity, and these efforts must be inclusive of the pediatric community2,3.
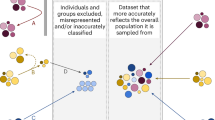
One definition of “algorithmic bias” refers to systematic inaccuracies in an AI/ML algorithm, causing potentially erroneous outputs that can result in the misclassification of select patients or subgroups and lead to actual harm4. Children and young people (CYP) under the age of eighteen are underrepresented in research, with pediatric studies presenting age-specific challenges that span ethical, legislative, financial, and relational domains5. Further, concerns about racial and gender disparities in pediatric research have been expressed, with calls to improve demographic reporting. It is important these considerations are accounted for in the development of pediatric AI/ML technology6,7,8,9.
If best practice standards are not established for CYP, the rapid expansion of AI/ML research has the potential to widen existing gaps. It is, therefore, crucial to define age-specific safety measures to prevent algorithmic bias and attain equitable, ethical, and appropriate representation of CYP.
Age as a source of algorithmic bias
Sources of algorithmic bias that affect CYP may arise from a lack of transparency in participant age reporting, a lack of clinically and developmentally appropriate representation of children, and inappropriate generalizations made to the pediatric population from adult data, and vice versa. We describe the effect of age as a source of algorithmic bias using the term “age-related algorithmic bias”.
ACCEPT-AI is a framework of ethical principles and key recommendations for pediatric data utilization and the assessment of age-related algorithmic bias in AI/ML research for researchers, regulators, policymakers, and clinicians. ACCEPT-AI has been designed for independent usage to uphold ethical standards, and/or for adjunctive integration into existing guidelines such as CONSORT-AI10 and SPIRIT-AI11, and emerging guidelines such as TRIPOD-AI, PROBAST-AI12, STARD-AI13, and QUADAS-AI14 as well as future guidelines (Fig. 1).
Pediatric data has age-specific considerations and should be viewed distinctly from adults
Pediatric health is heterogeneous, encompassing a range of developmental stages from birth to adolescence. Disease incidence, prevalence, presentation, outcome, and prognosis vary significantly between adult and pediatric populations, with vast physiologic and anatomic differences. Clinical needs, assessments, and treatment approaches are therefore distinct in both populations15.
Pediatric data presents unique ethical and practical challenges in conducting research. Such research must be grounded in ethical principles of autonomy and respect for persons, beneficence, non-maleficence, and justice16. Approaches to study enrollment, conduct, and implementation differ significantly from adult patients, with consenting, parental or guardian roles, and data protection necessitating tailored considerations for safe and age-appropriate care5. The U.S. Department of Health and Human Services requires special protections for CYP involved in research, defining standards that are more stringent than those of adults17. Researchers have emphasized the importance of including protected groups in research such that they are represented equitably5.
Typical developmental stages in the pediatric population present complexities and inherent differences in approach to study conception, design, data collection, usage, application, interpretability, and translation of research18. This variation is particularly important in the context of AI/ML research as the combination of pediatric and adult data and inclusion of different developmental stages of pediatric data, without training the technology on those differences, may result in algorithmic outputs that are not valid, applicable, effective or generalizable across age subgroups.
Recent AI/ML studies in pediatrics have spanned a variety of body systems19. One study on the use of deep learning for the estimation of left ventricular ejection fraction suggests that the algorithm trained on pediatric data generalized better than an adult model when testing on pediatric cases20. Such findings highlight a critical need to recognize differences between pediatric and adult data in AI/ML research. While the explicit inclusion of pediatric data is important to allow for representation in age diversity across datasets, it must be done so safely and equitably (Fig. 2).
Contents and usability of ACCEPT-AI
Background
The generation of formalized guidelines, such as SPIRIT-AI and CONSORT-AI, have laid the foundation for safe design, conduct, reporting, and early-stage evaluation of AI/ML studies10. However, the lack of defined best practice standards, frameworks, or guidelines incorporating age-specific considerations for AI/ML research that involves pediatric data warrants attention. The ACCEPT-AI framework highlights fundamental ethical considerations for pediatric data use in AI/ML research. At each stage of the AI life cycle (problem selection, data collection, outcome definition, algorithm development, and post-deployment considerations), the framework promotes the evaluation and maximization of principled and ethical AI use by incorporating respect for persons, beneficence, non-maleficence, justice, transparency, and explainability in its recommendations16.
Structure
ACCEPT-AI (Table 1) contains six key sections: age, communication, consent and assent, equity, protection of data, and technological considerations including transparency of techniques, training, and testing. Accompanying each section are key recommendations, which allow these ethical principles to be translated into actionable tasks by researchers, regulators, and clinicians who use or evaluate AI studies to mitigate age-related algorithmic bias.
Vision
The usage of ACCEPT-AI pertains to studies designed for CYP as the primary target population for which an algorithm is applied, for adult research that incorporates pediatric data as a secondary measure, and in the utilization of public AI/ML datasets that may contain labeled or unlabeled pediatric data. While ACCEPT-AI can currently be used as an independent framework, it has also been designed to integrate into existing formalized guidelines such as CONSORT-AI and SPIRIT-AI, emerging guidelines such as TRIPOD-AI, PROBAST-AI, STARD-AI, and QUADAS-AI, and future guidelines with focus on pediatric considerations (Fig. 3)9,10,11,12,13.
Limitations
ACCEPT-AI is a proposal that aims to provide broad guidance to meet the time-sensitive demands of rapidly emerging pediatric AI/ML studies. However, the framework has not yet undergone an E-Delphi process to strengthen expert consensus in this area, although there are plans to pursue this direction. Some sections in the ACCEPT-AI framework such as ‘Equity’ and ‘Technological considerations’ are relevant to studies across age ranges and have therefore been included for completeness despite some overlap with, for example, CONSORT-AI and SPIRIT-AI10,11, to allow for incorporation into current and emerging guidelines. Further, although ACCEPT-AI has been designed for a broad range of AI/ML research studies involving pediatric data, not all elements will apply to every study.
Applications of ACCEPT-AI—illustrated examples
Case 1: Parental consent and subject assent
A tertiary academic center enrolls pediatric patients in a study that involves the creation of an AI/ML algorithm for assessing vascular malformations of the face. This study utilizes identifiable images of the face in its training data.
Because CYP cannot legally consent for themselves, federal regulations include special protections for pediatric study subjects, including parental consent and assent of older pediatric subjects. It is essential that the consent process accounts for both chronological and developmental ages. Communication with the parent and subjects should include the risks, benefits, and alternatives, both at the time of enrollment and in the future, and these discussions must be documented21. It is important that social circumstances of the child are accounted for in the consent process, particularly if there are any sources of complexity in parent-child relationships. For children in state custody, researchers must determine if subjects may be included in the study, and if so, who is legally responsible to provide consent. Researchers must explicitly discuss relevant and important reasons to include identifiable pediatric data, such as a condition or presentation that uniquely impacts children, or where symptoms are distinct from adults. Further, whether and how subjects will be able to remove their images from a dataset in the future should be disclosed during the consent process. In instances where data has been utilized to train and test an algorithm, and cannot be removed, the likelihood that this will occur must be disclosed at the time of enrollment to key stakeholders. The ACCEPT-AI framework highlights these key considerations.
Case 2: Communication and equity
An AI/ML researcher plans to develop an AI algorithm for assessing pneumonia on chest X-rays in the emergency room and include adolescents. Both parental consent and participant permissions are required. The researchers wish to ensure the pediatric study population understands the risks and benefits of enrolling in this study. In addition to the study participants, they also wish to communicate their research study to the broader pediatric community to seek feedback.
It has been acknowledged that engaging CYP in AI research is important22. A recent qualitative exploration of twenty-one CYP showed that they wished to contribute insights to the safe development of AI research22. Age-appropriate communication is the cornerstone of pediatric practice, and it is, therefore, crucial that all stakeholders are provided with relevant information on the purpose and nature of proposed AI/ML studies, and given examples of how their data may be utilized in the future. It is crucial that both chronological and developmental ages are factored into communication methods, given their relevance in several pediatric diseases.
When educating both parents and minor subjects, investigators should incorporate educational best practices. Where developmental delay is present in the subject or guardian, communication methods must be tailored appropriately. At the level of consultation with the child and family, investing in evidence-based decision aids has proven beneficial in enhancing decision-making capabilities23.
At the level of the community, efforts should be taken to improve digital literacy for young persons and parents or guardians inclusive of those from racial and ethnic minorities, rural and remote regions, and underrepresented disease groups. Collaborations with formal educational bodies to facilitate this through education on broad concepts of AI/ML health research to CYP may improve familiarity, promote transparency, clarity of research intentions, and enable exchange of ideas. Once an algorithm has been developed, further engagement in focus groups, where relevant permissions are in place, may help the iteration of working models. ACCEPT-AI emphasizes the importance of communication to improve digital literacy and engagement through the AI life cycle, at individual, parental, and community levels (Fig. 4).
Levels of communication to improve digital literacy with key stakeholders as proposed by the ACCEPT-AI framework, adapted from McLeroy et al.27.
Case 3: Data protection and identification
Researchers review a large public skin image database for the training of an ML algorithm that aims to diagnose skin lesions. They noticed unlabeled pediatric data mixed into the dataset.
Pediatric data must only be utilized when the data and technology addresses a clear need for the pediatric population. Researchers must be transparent about needs and potential benefits for data use in their protocols, and should clearly describe measures taken to minimize risk to pediatric subjects. Adverse events should be clearly documented, with plans in the protocols for clinical evaluation using validated pediatric tools, where possible. Currently, data protection laws involving de-identifiable data In the United States do not separate adult and pediatric data. Differentiating de-identifiable and identifiable data is a key consideration for safe data regulation, as legislation surrounding consent and data protection differ for the respective categories. In the United States, HIPAA supports applying the “Safe Harbor Rule” to remove key identifiers from clinical patient data for secondary research use, or alternatively, suggests expert consensus to determine adequate de-identification for study inclusion24. In Europe, the General Data Protection Regulation (GDPR) stipulates the need for explicit consent, a prerequisite to data usage, and permits by the patient25. While the development of specific laws that are tailored to pediatric data usage may be beneficial, existing legal processes must be optimized for transparency with both pediatric subjects, their parents or legal guardians. Further, researchers must make clear in their protocols the measures taken to protect data security and be familiar with local laws for adolescent consent given their geographical variance26. New pediatric data collection for AI/ML should meet the highest standards for data security without compromising patient privacy, as proposed by key recommendations in ACCEPT-AI.
Case 4: Key technological considerations for age-related algorithmic bias
Researchers train a predictive diagnostic algorithm using chest X-rays available on a public dataset. Images contain no age labels. Both adult and pediatric X-rays are used to train the ML model. The algorithm is then applied to an adult-only population.
Combining data across adults and children introduces age-related algorithmic bias, and risks compromising the applicability, generalizability, and effectiveness of a study, with potential impact on both populations. Clear documentation of the objective for which pediatric data will be collected and used in line with the ACCEPT-AI recommendations will help ensure key safety measures have been taken to avoid mixing of data unless there are clear indications to do so. Reporting the AI/ML technique applied in each study or approved device is important so that pediatric data use maps to the needs of the research question. Further, researchers should provide details on whether an algorithm has been trained to work with adult data, pediatric data, or both. While necessary at every stage of evaluation, ACCEPT-AI recommends three crucial checkpoints during an algorithmic cycle, that can be used to proactively assess for age-related bias; in dataset curation, training, and testing (including deployment and post-deployment phases) (Fig. 5).
Conclusions
Pediatric populations face many challenges in healthcare and research settings. As the research community develops consensus guidelines for AI/ML algorithms and refines the ethical and principled use of AI, specific protections for pediatric populations are essential. Legal protections and federal mandates (e.g., enforced by the FDA) regarding the development and deployment of AI/ML algorithms for pediatric populations have also yet to be established. Here, we propose the ACCEPT-AI framework, which is a set of principles and key recommendations that can be used independently or flexibly embedded in existing, emerging, and future consensus guidelines9,10,11,12,13. The examples of ethical challenges in pediatric data utilization highlighted demonstrate the pressing need for a greater understanding of age-related bias, data source composition (e.g., combining pediatric and adult data without labeling), their analysis, and the implications for autonomy, beneficence, non-maleficence, transparency, explainability, generalizability, and fairness across pediatric and adult populations.
References
Center for Devices and Radiological Health. Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (U.S. Food and Drug Administration, 2023).
Sounderajah, V. et al. Ethics methods are required as part of reporting guidelines for artificial intelligence in healthcare. Nat. Mach. Intell. 4, 316–317 (2022).
Srikumar, M. et al. Advancing ethics review practices in AI research. Nat. Mach. Intell. 4, 1061–1064 (2022).
Paulus, J. K. & Kent, D. M. Predictably unequal: understanding and addressing concerns that algorithmic clinical prediction may increase health disparities. NPJ Digit. Med. 3, 99 (2020).
Speer, E. M. et al. The state and future of pediatric research—an introductory overview. Pediatr Res. https://doi.org/10.1038/s41390-022-02439-4 (2023).
Piccini, P., Montagnani, C. & de Martino, M. Gender disparity in pediatrics: a review of the current literature. Ital. J. Pediatr. 44, 1 (2018).
Slopen, N. & Heard-Garris, N. Structural racism and pediatric health—a call for research to confront the origins of racial disparities in health. JAMA Pediatr. 176, 13–15 (2022).
Lockhart, J. W., King, M. M. & Munsch, C. Name-based demographic inference and the unequal distribution of misrecognition. Nat. Hum. Behav. https://doi.org/10.1038/s41562-023-01587-9 (2023).
Nature Publishing Group. Why Nature is updating its advice to authors on reporting race or ethnicity. https://www.nature.com/articles/d41586-023-00973-7 (2023).
Liu, X. et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat. Med. 26, 1364–1374 (2020).
Cruz Rivera, S. et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Nat. Med. 26, 1351–1363 (2020).
Collins, G. S. et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open. 11, e048008 (2021).
Sounderajah, V. et al. Developing a reporting guideline for artificial intelligence-centred diagnostic test accuracy studies: the STARD-AI protocol. BMJ Open. 11, e047709 (2021).
Sounderajah, V. et al. A quality assessment tool for artificial intelligence-centered diagnostic test accuracy studies: QUADAS-AI. Nat. Med. 27, 1663–1665 (2021).
CDC. How Children are Different. https://www.cdc.gov/childrenindisasters/differences.html (2019).
Chen, I. Y. et al. Ethical machine learning in healthcare. Annu. Rev. Biomed. Data Sci. 4, 123–144 (2021).
Vulnerable Populations. HHS.gov. (n.d.) [online]. https://www.hhs.gov/ohrp/regulations-and-policy/guidance/vulnerable-populations/index.html.
Martinez-Castaldi, C., Silverstein, M. & Bauchner, H. Child versus adult research: the gap in high-quality stussdy design. Pediatrics 122, 52–57 (2008).
Hoodbhoy, Z. et al. Machine learning for child and adolescent health: a systematic review. Pediatrics 147, e2020011833 (2021).
Reddy, C. D. et al. Video-based deep learning for automated assessment of left ventricular ejection fraction in pediatric patients. J. Am. Soc. Echocardiogr. 7, S0894–S7317 (2023).
Andreotta, A. J., Kirkham, N. & Rizzi, M. AI, big data, and the future of consent. AI Soc. 37, 1715–1728 (2022).
Visram, S. et al. Engaging children and young people on the potential role of artificial intelligence in medicine. Pediatr. Res. 93, 440–444 (2023).
Shirley, E. et al. Helping families make difficult choices: creation and implementation of a decision aid for neuromuscular scoliosis surgery. J. Pediatr. Orthop. 35, 831–837 (2015).
HHS. Guidance Regarding Methods for De-identification of Protected Health Information in Accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html (2023).
Art. 17 GDPR. Right to Erasure (‘right to be forgotten’). https://gdpr.eu/article-17-right-to-be-forgotten/.
Sharko, M. et al. State-by-state variability in adolescent privacy laws. Pediatrics 149, e2021053458 (2022).
McLeroy, K. R. An ecological perspective on health promotion programs. Health Educ. Q. 15, 351–377 (1988).
Acknowledgements
S.R. was supported by a National Institute of Health grant DP1-LM014278. The authors thank Dr. Justin Ko MD, MBA, Dr. Albert Chiou MD, MBA, Dr. Susan Swetter MD, and Dr. Vasiliki Ramidezah Ph.D. for support of their digital health and equity research at Stanford University.
Author information
Authors and Affiliations
Contributions
Conceptualization: V.M. (lead), A.B., R.D., and S.R. (equal contribution). Investigation: V.M. (lead), A.B., R.D., and S.R. (equal contribution). Project administration: V.M. (lead), A.B., R.D., and S.R. (supporting, equal contribution). Resources and software: V.M. (lead), A.B., R.D., and S.R. (equal contribution). Verification: S.R., R.D. (co-lead), V.M., and A.B. (supporting, equal contribution). Writing—original draft preparation: V.M. (lead), A.B., R.D., and S.R. (supporting). Writing—review and editing: R.D. (lead, equal contribution), S.R. (lead, equal contribution), and A.B. (supporting, equal contribution).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muralidharan, V., Burgart, A., Daneshjou, R. et al. Recommendations for the use of pediatric data in artificial intelligence and machine learning ACCEPT-AI. npj Digit. Med. 6, 166 (2023). https://doi.org/10.1038/s41746-023-00898-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-023-00898-5