Abstract
The scalable production of two-dimensional (2D) materials is needed to accelerate their adoption to industry. In this work, we present a low-cost in-line and enclosed process of exfoliation based on high-shear mixing to create aqueous dispersions of few-layer graphene, on a large scale with a Yw ~ 100% yield by weight and throughput of ϕ ~ 8.3 g h−1. The in-line process minimises basal plane defects compared to traditional beaker-based shear mixing which we attribute to a reduced Reynolds number, Re ~ 105. We demonstrate highly conductive graphene material with conductivities as high as σ ∼ 1.5 × 104 S m−1 leading to sheet-resistances as low as Rs ∼ 2.6 Ω □−1 (t ∼ 25 μm). The process is ideal for formulating non-toxic, biocompatible and highly concentrated (c ∼ 100 mg ml−1) inks. We utilise the graphene inks for inkjet printable conductive interconnects and lithium-ion battery anode composites that demonstrate a low-rate lithium storage capability of 370 mAh g−1, close to the theoretical capacity of graphite. Finally, we demonstrate the biocompatibility of the graphene inks with human colon cells and human umbilical vein endothelial cells at high c ∼ 1 mg ml−1 facilitating a route for the use of the graphene inks in applications that require biocompatibility at high c such as electronic textiles.
Similar content being viewed by others
Introduction
Graphene and other 2D materials are expected to find major commercial applications in the coming years1. By utilising the unique electrical, optical, mechanical, chemical and thermal properties of 2D materials, additional functionality or improved performance can be added to many applications. Graphene could be useful in over 40 major application areas such as composites, energy storage, thermal management, sensors and coatings2. For example, graphene can be used as a barrier material for anti-corrosion3, an additive for mechanical reinforcement in polymers4, or as a conductive material in sensors5. Many of these applications will require few-layer graphene flakes (<10 layers) in large multi-tonne quantities if successfully commercialised2. For example, if graphene flakes are used at low loading (~1%) in the 300 million tonnes per year global plastic industry6, or used to replace graphite (~106 tonnes per year)7 in Li-ion batteries for the electric vehicle market it would create a demand of >1 kilotonne, far exceeding the global graphene supply8. Few-layer graphene flakes could also be utilised as a cost-reduction replacement material for metal components in applications such as interconnects or electrodes, particularly where form factor is important such as electronic textiles9.
Currently, printable metal inks (e.g. silver and gold) are commonly used as interconnect or electrode materials and are made of precious metals costing ~£1000 per litre on average and can have oxidation issues10, toxicity11 or nanoparticle migration12, degrading device performance. Graphene inks could be potentially produced for as little as ~£20 per litre once scaled due to carbon’s elemental abundance while retaining several functional advantages when applied, such as flexibility, biocompatibility, environmental stability or weight reduction. The Hummers method emerged as one of the first methods to produce graphene in the form of graphene oxide (GO) flakes13. However, the GO flakes differ from pristine graphene, containing a population of functional groups that disrupt the sp2 structure of graphite14. Liquid phase exfoliation (LPE) is established as the primary method to produce dispersions of pristine graphene flakes at scale and low cost15. The most common techniques to undertake exfoliation in liquid are ultrasonication16, ball milling17, shear-mixing18, electrochemical exfoliation19, wet-jet milling20 and microfluidization21. To evaluate each technique’s effectiveness, figures-of-merit (FOM) are frequently used to characterise the resulting dispersions. The yield by weight (Yw) is the ratio between the weight of the final graphene material and the starting graphite flakes22, while the throughput (ϕ) is defined as the mass of graphene obtained per hour. In the literature, Yw and ϕ are commonly used as FOM to assess the production process. Electrochemical exfoliation involves intercalating a 2D material such as graphite with an ionic species that expands and exfoliates the bulk material into flakes19,23,24. The process has high Yw ~ 10–75% with ϕ ~ 0.3 g h−1 when post-processing (e.g. material washing) is considered19,23,24. Ultrasonication is the most widely studied technique and typically involves using a bath sonicator16, which has been used to exfoliate graphene in toxic solvents (e.g. n-methyl-2-pyrrolidone, NMP)16 and non-toxic (e.g. water) solvents25. However, ink concentrations, c up to ~ 1 mg ml−1 in <24 h22 equating to ϕ ~ 1 g h−1 and Yw ~ 3–5% has limited ultrasonication to lab-scale studies. Ball milling involves mixing graphite powder, solvent and zirconia/metal balls together in a rotatory mill. The friction and shear forces with the balls enable the exfoliation of graphite17. The ϕ of the process is ~ 0.2 g h−1 with a Yw ~ 12%17. Microchannel-based techniques such as microfluidization and wet-jet milling have also been used in cyclic processes to exfoliate graphene nanoplatelets and 2D materials with Yw ~ 100%20,21. Microfluidization involves using high pressure (~250 MPa) to push a liquid through an interaction chamber with several micron-sized (~87 μm) channels generating high shear (>106 s−1)21. The liquid is cycled back through the interaction chamber over several hours to reach ϕ ~ 9.3 g h−1 21. Wet-jet milling is a similar technique which uses a hydraulic mechanism and piston at high pressure (~250 MPa) to push liquid through perforated (~100 μm) discs20. The process has reached ϕ ~ 23 g h−1 20. However, in practice, the interaction chamber in these microchannel-based techniques can frequently become blocked by graphite. Unless addressed, this would decrease ϕ in a commercial environment. Moreover, microfluidization systems are currently expensive (>£40,000) and can overheat easily (even with active cooling) risking system damage. Processes such as ultrasonication, high-shear mixing and electrochemical exfoliation provide cheaper alternatives for 2D material production however their low ϕ ~ 0.2–5.3 g h−1 has made high-volume production of 2D materials difficult. Furthermore, ultrasonication and high-shear mixing have had challenges to scale as the hydrodynamic processes can damage the graphene basal plane after 2 h of processing26. Therefore, there is a need for a graphene production process which is reliable, produces pristine flakes free of basal plane defects and at a high Yw and ϕ. High-shear mixing could provide a route forward as it currently has minimal maintenance requirements and high ϕ. Shear-mixing involves using a rotor (rotating blades) and stator (stationary screen) to generate shear forces to exfoliate material18. The shear-mixing process is typically undertaken in an open beaker (<1 L) or industrial-sized (>300 L) container18, and has demonstrated ϕ ~ 5.3 g h−1 but with a low Yw ~ 0.001%. Therefore, the graphene production process is still in need of significant improvement to fulfil the potential industrial scale demand. Furthermore, the low electrical conductivities of the resultant films (σ ~ 400 S m−1) need to be improved for practical applications18. The engineering of conductive ink with a c > 1 mg ml−1, σ > 103 S m−1, ϕ > 5 g h−1, defect-free and in a non-toxic solvent is highly desirable. In this work, we adapt the shear mixing process to recycle unexfoliated material to maximise ϕ, c and YW. While minimising Re < 106 in our system reduces basal plane defects and maximises σ which enables the creation of applications such as inkjet printed interconnects and Li-ion batteries.
Results
In-line shear-mixing process
We used an in-line shear mixing system (Silverson) to produce graphene flake dispersions (Fig. 1). In-line shear mixing is an enclosed, cyclic process where the rotor head’s high-speed generates suction and drives the dispersion upwards into the rotor head. Centrifugal force pushes the graphite towards the rotor head’s periphery and between the rotor (i.e. rotating blades) and the stator (i.e. a metal screen). The graphite experiences hydrodynamic stress which exfoliates the layered material and pushes it out and away from the rotor and stator. The system is self-pumping, and fresh material continually is drawn into the rotor head, which results in a cyclic flow of material through the system. Using this method, unexfoliated graphite is recycled by undergoing repeated hydraulic shearing. An ice bath or chiller can be used on the rubber tubing to maintain the system temperature. Furthermore, unlike standard shear mixing, the liquid is entirely enclosed.
We use graphite flakes as a starting material for the inks. We mix the flakes (100 mg ml−1) with sodium deoxycholate (SDC, 5 mg ml−1) (Sigma-Aldrich) in deionised water (~1 L) before adding to the in-line system. The temperature of the system increases over time once material passes through the rotor head. Therefore, an ice bath is used to keep the temperature at ∼30 °C. Without cooling, the deionised water will evaporate, increase the liquid viscosity, decrease the liquid flow rate, and potentially damage the motor of the in-line shear-mixer. We define one cycle as the complete passthrough of the starting liquid volume (~1000 ml) through the rotor head. Each cycle takes approximately 10.8 s to complete at 8000 rpm (i.e. 1000 ml passes through the system in 10.8 s, see Methods). We process the graphite and SDC dispersion for multiple cycle sets (2000, 4000 and 6000 cycles). The process yields litre-scale dispersions of graphene ink with a high c of 100 mg ml−1 (measured on a microbalance, see Methods), Yw ~ 100% and ϕ ~ 8.3 g h−1.
Investigating flake lateral size, thickness and quality
After depositing the graphene ink on a silicon/silicon oxide (Si/SiO2) substrate, we use scanning electron microscopy (SEM) in Fig. 2 to determine the lateral size <L> distribution of the graphite starting material (Fig. 2a) and its change in <L> as a function of the number of processing cycles: 2000 (Fig. 2b), 4000 (Fig. 2c) and 6000 (Fig. 2d). We define the lateral size as <L> = (xy)−1/2, where x and y are the length and width of the flake27. For each processing cycle, we measure 50 flakes. The average lateral size is 4.8 ± 1.72 μm, 2.69 ± 1.89 μm, 1.47 ± 1.14 μm and 0.65 ± 0.48 μm for the dispersions of graphite, 2000, 4000 and 6000 cycles respectively, indicating a decreasing <L > with processing (Fig. 2e). The size distribution characterised by standard deviation (σsd) changes with processing cycles. The graphite’s σsd before processing is 1.72 μm which decreases to 0.48 μm after 6000 cycles. It might be possible to narrow the lateral size distribution further by increasing the processing cycles. However, it might not be desirable as further processing will likely damage the basal plane of the graphene (see Raman spectroscopy section). We use atomic force microscopy (AFM) to further characterise the flakes and determine their apparent thickness and confirm <L>. In Fig. 2f, AFM micrograph of a typical flake reveals thickness of 8 nm and <L> ~ 270 nm. We undertake AFM statistics in Fig. 2g and find the <L> of 30 flakes after 6000 cycles. The flakes have a log-normal distribution28 which peaks at 386 nm and has an average <L> ~ 492 nm, consistent with the SEM measurements. The flakes’ apparent thickness is measured in Fig. 2h and shows a peak flake thickness of 6.7 nm, indicating that we have made few-layer graphene.
a SEM images of graphite. b 2000 cycles. c 4000 cycles. d 6000 cycles. e The lateral size distribution is plotted as a function of processing cycles, error is calculated as the standard deviation (n = 50). f An AFM micrograph displaying a typical profile of a 6000 cycles graphene flake with a thickness of 8 nm and lateral size of 270 nm. The scale bar is 230 nm. g Lateral size and h thickness of the 6000 cycles graphene ink obtained from AFM analyses.
We use Raman spectroscopy to identify the flakes’ quality as a function of the number of processing cycles. Figure 3a shows the spectra of the graphene with 2000 cycles (red curve), 4000 cycles (blue curve), 6000 cycles (green curve) and graphite (black curve). In the graphene spectra, the G peak located at ~1581 cm−1, corresponds to the E2g phonon29. The D peak at ~1350 cm−1 is due to the breathing modes of six-atom rings and requires a defect for its activation30. Typically in few-layer graphene dispersions, the D peak corresponds to flake edges rather than defects within the flakes’ basal plane31,32. The 2D peak located at ~2700 cm−1 is the second-order resonance of the D peak33. In the graphite sample (black curve), the 2D peak split into several components. As the number of layers decreases, the 2D peak narrows to a single Lorentzian for single-layer graphene. For 2000 cycles, we observe a shoulder on the 2D peak (~ 2690 cm−1) indicating the presence of multilayer graphene. However, after 4000 and 6000 cycles, the 2D peak is a single Lorentzian fit, indicating that even if the flakes are multilayers, they are electronically decoupled and behave as a collection of single layers33. In disordered carbon, the position of the G peak, Pos (G) increases when the excitation wavelength, λL decreases from the IR to UV30,34. Additionally, the dispersion of the G peak, Disp(G) = ΔPos(G)/ΔλL and the FWHM of the G peak, FWHM(G), increase with disorder35,36. Combining the intensity ratio of the D and G peaks, I(D)/I(G), with FWHM(G) and Disp(G) can discriminate between disorder localised at the edges and disorder in the basal plane of the flakes. If the disorder is in the graphene basal plane, I(D)/I(G) will increase with FWHM(G) and Disp(G). In the former case, I(D)/I(G) will be uncorrelated with FWHM(G) and Disp(G). In Fig. 3b we find that I(D)/I(G) is uncorrelated with Disp(G). Therefore, the D peak originates from the flake edges rather than the basal plane. Furthermore, the average Disp(G) ~ 0.019 cm−1nm−1, which is significantly lower than the Disp(G) for disordered carbons (>0.1 cm−1nm−1)34,37. Therefore, the in-line shear mixing method produces pristine basal plane graphene flakes. In Fig. 3c, I(D)/I(G) is plotted as a function of FWHM(G). We find the FWHM is 20 ± 1 cm−1 for each sample, indicating that the sp2 grain size (La) ~ 40 nm remains constant before and after exfoliation and that the cyclic process does not significantly damage the graphene35,37. Since the D peak originates from the edge of the flakes and FWHM(G) remains constant in our samples, the I(D)/I(G) ratio can be used to determine <L>, where I(D)/I(G) increases as a function of inverse <L>18,38. In Fig. 3c, the I(D)/I(G) ratio increases with processing time from I(D)/I(G) ~ 0.1 for bulk graphite (black), I(D)/I(G) ~ 0.25 after 2000 cycles (red) and up to I(D)/I(G) ~ 0.5 after 4000 (blue) and 6000 (green) processing cycles. We attribute the increase in I(D)/I(G) with processing time to a decrease in flake lateral size. We can estimate <L> using the formula I(D)/I(G) ≈ (I(D)/I(G))graphite + k/<L>, where k is the slope of I(D)/I(G) as a function of <L>−1 measured by transmission electron microscopy18 Using I(D)/I(G) ~ 0.5 for 4000 and 6000 cycles, average I(D)/I(G) ~ 0.11 for our graphite and a slope of k = 0.17 we can estimate that <L> ~ 0.43 μm which is consistent with the SEM and AFM data18. In contrast, a correlation between I(D)/I(G) and FWHM (G) is observed with a graphene ink made by conventional shear mixing in a beaker under similar exfoliation parameters (Supplementary Note 1) indicating that in-plane defects are present where La ~ 20 nm, which will decrease graphene conductivity. We attribute the introduction of in-plane defects to an increased Reynolds number (Rebeaker = ρνL η−1 ~ 1.3 × 106) compared to our in-line shear mixing process (Reinline ~ 6 × 105), where ρ is the liquid density, ν is the liquid velocity, L is the characteristic length and η is the liquid viscosity. The reduced Reinline is attributed to the decreased L of the in-line shear mixing system Linline ≃ 0.46Lbeaker (Supplementary Note 2) and suggests that reducing the turbulence of the system reduces the defects present in graphene flakes produced by shear mixing. Therefore, in-line shear mixing could offer a route to manufacture higher quality graphene material than is traditionally produced by shear mixing.
Investigating flake chemical, electrical and thermal properties
We use X-ray photoelectron spectroscopy (XPS) analysis to investigate the oxygen and sodium content in the 6000 cycles graphene ink. The XPS survey spectra (Supplementary Fig. 3a) shows very high carbon content (92.5 ± 0.1 at.%) and low oxygen content (5.7 ± 0.1 at.%), attributed primarily to graphene flakes and sodium content (~1.8 at.%), which we attribute to residual surfactant. The relatively low oxygen content and the position of the main peak in O 1 s spectra at ~533 eV (Supplementary Fig. 3b) suggest C-O component dominance over C=O. Careful investigation of the high-resolution XPS spectra (Fig. 4a) for carbon 1s reveals a peak at ~285 eV attributed to the sp2 carbon component and indictive of the hexagonal lattice structure, also the satellite peak (π–π*) contribution, corresponding to the broad peak at ~290 eV, is a characteristic feature of sp2 hybridisation of carbon39. The π–π* transition occurs due to the delocalisation of the π-electrons in the carbon aromatic ring40. We observe that the π–π* peak is more pronounced in the 6000 cycles graphene ink than conventional shear mixing in a beaker (Supplementary Fig. 3), suggesting that 6000 cycles graphene ink should have reduced basal plane defects. In general, the more pronounced π–π* satellite peak, the greater the degree of sp2 bonding41.
a XPS C 1s spectra of the 6000 cycles graphene ink. b A plot of Rs as a function of the graphene and graphite film thickness for the 6000 cycles graphene ink with different post-processing conditions such as centrifugation and annealing. Error is calculated by standard deviation of mean (SDOM, n = 3). c A graph of the σ as a function of thickness for the 6000 cycles graphene ink with post-processing. d Thermogravimetric analysis is used to find thermograms of the graphene powder and SDC surfactant. The weight change is plotted as a function of temperature.
To examine the graphene inks’ electrical properties, we create films of the 6000 cycles graphene ink and graphite flakes by drop-casting onto glass slides with a spacer to control the wet film thickness. The films are dried at ∼100 °C on a hotplate to remove excess water. The dry film thickness (t) depends on the c of the ink and wet film thickness. Therefore, we vary t by increasing the number of droplets cast (e.g. 5 to 50) from a syringe. We find the sheet resistance (Rs) of each sample using a four-point probe (Jandel Probe) in three different locations to obtain an average. We use a profilometer to determine t for each sample and measure three areas to obtain an average. In Fig. 4b, we find a Rs of ∼ 324 Ω □−1 for the graphite film at t ∼ 27 μm. Consequently, the conductivity (σ) can be estimated using σ−1 = Rs t, equating to σ ∼ 100 S m−1. Without any post-processing or centrifugation, we find that the 6000 cycles graphene ink has an Rs of ∼ 37 Ω □−1 at t ∼ 24 μm, corresponding to σ ∼ 1.1 × 103 S m−1, one order of magnitude higher than σ of graphite, indicating graphite’s exfoliation to few-layer graphene. Recently, Fernandes et. al has determined through semi-automated AFM measurements that uncentrifuged graphene LPE dispersions could have a small number (∼5%) of graphite flakes, and the majority mass (∼90%) would be attributed to the thicker (>40 nm) material42. Therefore, we centrifuge the material at low speed (1k rpm, g ~ 125) to remove the small number of thicker flakes (>40 nm), and the film σ increases to ∼ 5 × 103 S m−1. We show the σ as a function of t in Fig. 4c. At t < 4 μm, the σ is thickness dependant, and at t ∼ 4 μm, the flakes form a percolative network, and the bulk σ is reached. Therefore, for electronic devices requiring high conductivity >103 S m−1 a t of at least 4 μm should be used. The residual surfactant can degrade the electrical properties of a deposited network of flakes43. Therefore, we then anneal the samples at 400 °C for 30 min to combust the SDC used in the graphene ink. We find σ increases even further to ∼ 1.5 × 104 S m−1 and demonstrates Rs ∼ 2.6 Ω □−1 (t ∼ 25 μm). The σ of the film is comparable to state-of-the-art printable graphene inks20,44,45. To investigate the removal of residual SDC surfactant after annealing, we investigate the SDC decomposition temperature with thermogravimetric analysis (TGA) in Fig. 4d. The 6000 cycles graphene ink is subjected to a freeze-drying process to obtain a graphene powder (see Methods). The SDC and graphene powder are heated from ~ 25 °C up to 1000 °C ramped at a rate of ~ 10 °C min−1 in an air atmosphere. We plot the weight change as a function of temperature, which shows that SDC significantly decomposes at temperatures >380 °C. The graphene powder has a carbon combustion temperature between 570 and 670 °C (maximum mass decomposition rate ≈ 627 °C), which is associated with the carbon decomposition temperature of few-layer graphene46.
Inkjet printable interconnects
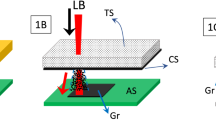
To make an inkjet printable ink requires the optimisation of c, surface tension, γ and η to ensure satellite droplet free ejection, and morphologically uniform (i.e. roughness minimisation) printed films43,47. We engineer the η of the graphene ink to ensure it is within an optimal 1–10 mPa s range required for inkjet printing43. We measure η with a parallel plate rotational rheometer (see Methods). A known shear rate is applied, and the resultant torque or shear stress is measured. The shear stress divided by the shear rate calculates the liquid viscosity shown in Fig. 5a43. We find pseudoplastic behaviour with an infinite-rate η of ~ 1 mPa s within the optimal η range (~ 1–10 mPa)43. The decrease in viscosity is attributed to the reduction in the Brownian motion of the graphene flakes48. In Fig. 5b, we determine the ink γ using the pendant drop method. The pendant drop method involves using a camera to visualise the shadow image of a droplet dispensed from a needle. The droplet’s radius of curvature is found from the shadow images that can be used with the Young–Laplace equation to determine the liquid surface tension49,50. We find a graphene ink γ of ~ 63 mN m−1, lower than the γ of water ~ 72 mN m−1, which we attribute to the presence of SDC surfactant. High c is needed to minimise the number of printing layers required to reach a thick network of flakes, thus increasing the ϕ of a printing process to create devices. In contrast, high σ is desirable to improve the performances of devices that require high σ, such as electrodes for micro-supercapacitors51, antennas52 or transistor electrodes53. Pristine inkjet printable graphene inks have commonly had low c (< 3 mg ml−1), to help minimise nozzle clogging. Identification of stabilising solvents by solubility parameter-matching16 has proven to be a useful starting point for ink formulation. However, the resultant c can be low, < 0.2 mg ml−1 44; therefore, many (>50) printing passes are required to build a percolating film of material. Other strategies have been developed to improve ink’s c. For example, vacuum filtration can be utilised with flocculation agents51 or a solvent exchange53 to redisperse graphene at high c (> 10 mg ml−1). However, the additional vacuum filtration step reduces ϕ < 1 g h−1 53 Alternately solvent interface trapping has been used engineer high c (> 100 mg ml−1) ink by trapping graphene between a polar and non-polar solvents54. GO powders also been used to directly disperse material at a high (> 1 mg ml−1) c but with low σ (< 1000 S m−1) due to the defective nature of the flakes55,56. To increase the σ, printed films are typically annealed at high temperatures >300 °C45,57. However, this process is incompatible with many textile (e.g. cotton) or polymer (e.g. polyethylene terephthalate, PET) substrates which degrade at temperatures >100 °C43. Therefore inkjet printable graphene inks with high σ (> 103 S m−1) and high c (> 10 mg ml−1), which do not clog the inkjet nozzle have been desirable but difficult to achieve. The 6000 cycles graphene ink achieves these requirements (σ > 103 S m−1, c ∼ 100 mg ml−1), however it is not compatible with most inkjet nozzles due to the flake <L > > 1 μm. Typically the flakes should be 1/50th of the nozzle diameter (a = 21 μm) to minimise nozzle clogging (∼ 420 nm)44. Therefore we centrifuge the 6000 cycles graphene ink at low speed (1k rpm, g ~ 125) to create a graphene ink Grinkjet with c ~ 5.9 mg ml−1, (Yw ∼ 6%) which minimises the probability of nozzle clogging and increases σ ∼ 5 × 103 S m−1 without annealing. We compare the σ and c of Grinkjet to others (Fig. 5c) and find that both c and σ are higher than most inkjet printable inks in the literature, where an optimal ink would be in the top right corner of the graph, assuming that the ink would not cause nozzle clogging issues or satellite droplets due to its concentration44,45,51,53,55,56,57,58,59. We use a drop-on-demand inkjet printer to print the ink. We image the jetting of the ink with a stroboscopic camera (Fig. 5d), the black dot at 0 μm represents the inkjet nozzle. The ink is observed to eject from the nozzle without satellite droplets, which is important to achieve well resolved printed films with resolution <100 μm. In Fig. 5e, we deposit the ink onto a PET substrate coated with aluminium oxide nanoparticles (Novele, Novacentrix) which have a low roughness (~18 nm). We print a 2 cm2 layout of interconnect array. Through optical inspection, we observe morphological uniformity of the print and evenly distributed flakes in the form of a film. We also inkjet print transistor contacts Fig. 5e demonstrating a ~ 50 μm gap between two graphene electrodes. The resolution of ~ 50 μm is as expected and comparable to previous works on inkjet printing47,53. These results indicate that the high concentration (5.9 mg ml−1) printable graphene inks can be produced using in-line shear mixing and can be inkjet printed at high resolution (~ 50 μm) in desirable patterns for flexible electronics.
a Ink viscosity as a function of applied shear rate, demonstrating non-Newtonian shear thinning behaviour. b Pendant drop of the ink used to calculate the surface tension. c Literature review of other inkjet printable conductive inks comparing their σ with the ink c. d Jetting of the ink observed with a stroboscopic camera. e Optical image of the inkjet-printed transistor contacts (left, dark field) and interconnect (right, bright field). The scale bar is 100 μm (left) and 1 cm (right).
Li-ion battery anode
Graphene is an attractive material to replace graphite in lithium-ion battery anodes since Li-ion battery anodes need high in-plane conductivity (>103 S m−1) to improve rate performance, and thus, charging speed60,61. Here, we demonstrate the potential of the graphene flakes produced using in-line shear mixing method as Li-ion anodes. Rather than using a polymeric binder, we add a small amount (15%) of single-walled carbon nanotubes (SWCNT) to the electrode. Using SWCNT is known to simultaneously maximise the electrode’s mechanical and electrical properties and tends to yield electrodes that perform near their theoretical capability62. The resultant graphene/SWCNT anodes have an active mass loading of ~1 mg cm−2 combined with a SWCNT loading of ~0.18 mg cm−2 and thickness of ~20 μm (see Methods).
To evaluate the electrochemical Li storage mechanism of graphene produced using in-line method, cyclic voltammetry (CV) data is measured for a graphene/SWCNT anode at a scanning rate of 0.1 mV s−1, shown in Fig. 6a. During the 1st cycle, an irreversible cathodic peak at ~1 V is observed, which can be attributed to the formation of a solid electrolyte interface (SEI) and the reaction of lithium ions with residual oxygen-containing functional groups on the graphene flakes63,64,65,66. In the range of 0.01–0.25 V, we observe Li+ insertion into graphene flakes67. In the second cycle, the cathodic peak at 1 V is not observed, indicating the oxygen-containing functional groups on the graphene flakes are completely reduced. After five cycles, the redox peaks between 0.01 and 0.25 V display no apparent changes, illustrating reversible Li+ intercalation/deintercalation in the graphene layer. Thus, the CV data confirms that the 6000 cycles graphene flakes store lithium effectively68.
a Cyclic voltammograms at a scan rate of 0.1 mV s−1 for various cycles. b Cycling capacity versus cycle number for composite anodes based on graphene flakes cycled initially at 30 mA g−1 and then at 100 mA g−1. Coulombic efficiency (CE) is shown in the inset. c Galvanostatic charge-discharge curves measured at different charging currents. d Specific capacity as a function of charging current. In all cases, CSp is normalised to the graphene mass.
For cyclability measurement, the galvanostatic charge and discharge are performed initially with a 43-cycle of activation at 30 mA g−1 followed by 375 cycles at 100 mA g−1, as shown in Fig. 6b. All data is normalised to the active mass of graphene (see Methods). The first activation cycle (30 mA g−1) specific capacity (Csp) was ~646 mAh g−1 for discharge and ~363 mAh g−1 for charge, with an initial coulomb efficiency (CE) of ~56%. After the activation cycles, the cells are tested for 375 cycles at 100 mA g−1. The graphene/SWCNT anodes show a lithium storage capability of ~228 mAh g−1 and ~243 mAh g−1 for discharge and charge, with a CE of ~100% for the first cycle at ~100 mA g−1 (Fig. 6b, inset). The charging and discharging capacity changed in the first 4 cycles before stabilising at a capacity of ~233 mAh g−1 for both discharge and charge with a CE of ~100% over 365 cycles.
The rate performance of our anodes is tested with voltage profiles shown in Fig. 6c and rate dependent cycling data in Fig. 6d. The anodes exhibited an initial performance of 641 mAh g−1 for discharge and 376 mAh g−1 for first cycle charge at 10 mA g−1 with an initial CE of ~58.6% that approaches ~99% for subsequent cycles. The specific charge capacity decreased with increasing specific currents whereby at 180 mA g−1, a specific charge capacity of ~107 mAh g−1 was achieved. In contrast, the specific discharge capacities fell off to 374, 287, 197 and 116 mAh g−1 for each rate’s first cycle, respectively. The low-rate capacity (20 mAh g−1) was ~370 mAh g−1, very close to graphite’s theoretical capacity (372 mAh g−1)69. The fact that we can reach close to the theoretical value attests to the high quality of graphene flakes produced using in-line method while facilitated by the presence of SWCNT60,62.
Biocompatibility with human cells
Understanding the biocompatibility of the graphene flakes with human cells is essential to utilise the material for applications that come into contact with humans, such as electronic textiles, bioscaffolds or drug delivery9,70,71. To test if there is any acute toxicity, we performed viability assays using increasing graphene flake concentrations (6000 cycles graphene ink) in Human umbilical vein endothelial cell (HUVECs) and standard human cancer cell line SW948. In Fig. 7a, the upper left quadrant represents acute toxicity by induction of cell necrosis stained by 7-AAD. In contrast, the lower right quadrant shows cells undergoing programmed cell death (Annexin V staining), i.e. apoptosis. Cells stained by both dyes are in a transitional phase between apoptosis and necrosis and fall in the right upper quadrant. Repeated measurements show no acute toxicity found when using the highest concentration of graphene (1 mg ml−1) in 48 h cell culture treatments (N = 2 for SW948, N = 3 for HUVECs). This was comparable to a control, where no graphene was added.
a Muse ™ Apoptotic profile using Annexin V for apoptosis staining and 7-Aminoactinomycin D (7-AAD) fluorescence intensity for necrosis is shown in a flow scatter using same cut offs for all measurements (N = 3 for HUVECs and N = 2 for SW948). b CCK8 viability normalised to 0 μg ml−1 control over increasing concentrations of graphene (0–1000 μg ml−1) (N = 9 from three independent experiments for HUVECs in blue and N = 6, from two independent experiments for SW948 in red). c Laser scanning confocal microscopy with the addition of propidium iodide (PI), used here to image whole cells, and image overlay with Hoechst cell nuclear staining with bright field transmittance of graphene pre-treated fixed cells at ×63 magnification, scale bar 50 μm for HUVECs and 25 μm for SW948 cells.
To confirm our cellular apoptotic measurements, we used a cell counting kit-8 (CCK8) assay based on a tetrazolium salt reduced by viable cells to a soluble formazan dye. The resulting dye absorbs light at 450 nm, which correlates with cellular viability (Fig. 7b). After 48 h of graphene treatment, the cell cultures did not show any dose-dependent toxicity up to the highest concentration tested at 1 mg ml−1 for SW948 and no significant toxicity up to 500 μg ml−1 for HUVECs. We compared our measurements against a control of deionised water and SDC, which did not show any dose-dependent toxicity up to 38.46 μg ml−1 of SDC (relative to the amounts contained in 1 mg ml−1 graphene suspension, Supplementary Fig. 4). To the best of our knowledge, this is one of the highest c that has demonstrated LPE graphene’s biocompatibility with human cells to date, which is essential for applications in printed electronics that use c of at least 1 mg ml−1 43 We attribute the biocompatibility on the ink to the lack of oxygen functional groups (~5.7%, determined by XPS) as graphene oxide dispersions have previously demonstrated toxicity to human cells as low as 1–10 µg ml−1 72,73 attributed to its high oxygen content (~47%) that induces the formation of reactive oxygen species that can be toxic to cells74. To detect any cell deformities due to graphene treatment, we imaged the cells using laser scanning confocal microscopy with propidium iodide to stain whole fixed cells with the nuclear Hoechst dye (Fig. 7c). Representative images show cells with similar morphology to control at high graphene treatments (Fig. 7c).
Discussion
We demonstrated an enclosed, cyclic exfoliation of graphene with in-line shear-mixing, which has a higher ϕ ∼ 8.3 g h−1 than previous graphene production techniques such as ultrasonication and ball milling. The Yw ∼ 100% is several orders of magnitude increase compared to previous work on shear mixing18, and can be used to create dispersions with extremely high concentration c ∼ 100 mg ml−1, achieving σ ∼ 103 S m−1 without post-processing and σ ∼ 1.5 × 104 S m−1 after centrifugation and annealing, which is state of the art for graphene inks. We achieve high σ by reducing Re < 106 which minimises basal plane topological defects. Therefore, in-line shear mixing could offer a route to manufacture higher quality graphene or potentially other 2D materials, than is traditionally produced by shear mixing. We demonstrate the graphene inks’ versatility by making anode electrodes with SWCNTs for Li-ion energy storage, achieving a low-rate capacity of ~370 mAh g−1. We show that highly concentrated (5.9 mg ml−1) and inkjet printable inks can be manufactured for use in inkjet-printed conductive interconnects with a resolution ∼50 μm. As a final demonstration, we examine the toxicity of the graphene flakes with human colon cells and HUVEC cells. We observe no dose-dependent toxicity up to 48 h indicating the graphene flakes’ biocompatibility at high concentrations ∼1 mg ml−1, which is essential to utilise graphene in textile electronics, composites and printed interconnects that could involve human contact with high concentrations of graphene.
Methods
Inkjet printing
We used a Fujifilm Dimatix DMP-2800 inkjet printer with a 21 µm diameter nozzle (Fujifilm DMC-11610) for the printing of interconnects. The inkjet printing platen temperature was kept at ambient conditions (20 °C). An inter-drop spacing of 25 μm was used for the interconnects and an inter-drop spacing of 40 μm for the transistor electrodes. We print 100 layers of ink to construct the interconnect pattern and 10 layers of ink for the transistor electrodes. We used a maximum jetting frequency of 2 kHz.
X-ray diffraction
XRD patterns were recorded with a D2 Phaser (Bruker) powder diffractometer equipped with LynxEye detector using Cu Kα radiation (λ = 1.54 Å) at a 2θ scan step of 0.03° and 1 s dwell time. XRD samples were prepared by drying the graphene dispersions under room condition and transferring the powder obtained to the Bruker sample holder.
X-ray photoelectron spectroscopy
Samples were prepared by depositing graphene dispersions on Si/SiO2 substrates mounted on aluminium stubs using conductive silver paint. XPS measurements were conducted using a K-Alpha™ X-ray Photoelectron Spectrometer (Thermo Scientific). High-resolution C1s, O 1s, and Na 1s spectra were collected at 40 eV pass energy with a 0.05 eV step size. Spectra were acquired using a monochromatic Al Kα X-ray (hν = 1486.6 eV) with an X-ray beam spot size of 400 μm, average of 3 points per sample were taken. Any energy shifts were calibrated using gold foil attached to the films and measuring the Au 4f binding energy at 84 eV as a standard. The XPS measurement data were analysed using the CasaXPS software (Casa Software Ltd).
Profilometry
We used a stylus profilometer (Bruker DektakXT) to measure the thickness of the deposited films on a quartz substrate. A stylus force of 3 mg was used with a tip of radius 12.5 μm over the sample.
Thermogravimetric analysis
To prepare the graphene sample, we take 30 ml of the 6000 cycles graphene ink and froze it at −18 °C. The frozen sample was then placed inside a freeze dryer (Telstar LyoQuest) to remove ice crystal by sublimation under vacuum overnight to make a graphene powder. We then undertake thermal analysis of the graphene powder and SDC surfactant using a thermogravimetric analyser (TA Instruments Q50) in air with a ramping temperature of 10 °C min−1 from ~25 °C up to 1000 °C.
Raman spectroscopy
We acquired the Raman spectra at 514.5 nm with a Reinshaw InVia equipped with a 50× objective. We kept the power on the samples below ~1 mW to minimise thermal damage from the laser. About 20 spectra are taken for each map. All measurements are acquired after drop-casting sample solution on Si/SiO2 wafer. The resolution of our spectrometer is ~1 cm−1 and our spot size was ~2 μm. For measurement of the Disp(G) we acquired additional Raman spectra at 457 nm and 633 nm, respectively.
Shear-mixing and ink preparation
We used a Silverson Model L5M, 250 W single phase motor high-shear laboratory mixer. An interchangeable in-line mixing assembly was used for in-line processing. It was equipped with a 4-blade rotor placed inside a stator with a rotor-stator gap (ΔR) of 300 μm and a rotor diameter (D) of 31.1 mm. We use graphite flakes (Imerys Graphite) as a starting material for the inks. We mix the flakes (100 mg ml−1) with sodium deoxycholate (SDC, 5 mg ml−1) (Sigma-Aldrich) in deionised water at 8000 rpm. The throughput was calculated as 1000 ml of 100 mg ml−1 ink (i.e. 100 g) processed for 4000 cycles (i.e. 12 h). The volume of the ink reservoir can be increased (>1 L) if further scale is required. The shear rate γ̇ ≈ πND ΔR−1 ≈ 4 × 104 s−1, where N is the revolutions per second of the shear mixer (8000 rpm = 133 s−1)18. Optical inspection of the ink reveals a homogeneous stable dispersion of graphene flakes.
Scanning electron microscopy
The ink was diluted 1:999 and drop-cast on 1 cm2 Si/SiO2 wafer. We acquired scanning electron microscopy images with a Magellan 400 L SEM. We used an accelerating voltage of 5 kV and gun current of 25pA during operation and obtained the images in secondary electron detection mode using an immersion lens and through-lens-detector. We measured <L> in ImageJ.
Atomic force microscopy
The ink was diluted 1:999 and drop-cast onto clean 1 cm2 Si/SiO2 wafers. We used a Bruker Dimension Icon in peakforce mode to scan a 20 μm × 20 μm area of the wafer. We then find the <L> and thickness of 30 flakes by manual counting in NanoScope Analysis.
Surface tension
We used a contact angle goniometer to measure the graphene ink’s surface tension through the pendant drop method. A suspended droplet from a needle forms a pendant’s shape resulting from the downward force due to gravity and the upward force due to surface tension. We used drop-shape analysis to calculate the surface tension from a shadow image of the droplet.
Rheometry
We used a parallel-plate rotational rheometer (DHR Rheometer, TA instruments) to find the viscosity as a function of the shear rate. We use a plate-to-plate distance of 500 μm and we loaded the ink between the plates by capillary action at a gap of 550 μm. We find the infinite-rate viscosity for the ink.
Microbalance
To find the c of the ink we use a microbalance (Sartorius ME5), we placed 1 ml of ink in a metal container and weighed the graphene flakes after evaporation of the solvent.
Electrochemical characterization
The 6000 cycles graphene ink was mixed with a SWCNT aqueous dispersion (0.4 wt% SWCNT in water, ~0.6 wt% carboxymethyl cellulose (CMC) as a surfactant stabiliser, Tuball, OCSiAl), which increases conductivity and functions as a binder. A mortar and pestle ground the mixed solution to obtain a uniform slurry, and then the slurry was cast onto copper foil using a doctor blade. The slurry cast anodes were slowly dried at 40 °C overnight in an oven to remove residual water. The films produced were cut to the desired dimensions (area = 1.131 cm2) for electrochemical testing. The anodes were annealed at 700 °C for 2 h in a vacuum tube furnace to remove CMC. The mass loading of graphene was ~1 mg cm−2, with 15 wt.% SWCNT. The anode thickness was ~20 μm.
Half-cell coin cells were assembled with Li-metal discs (diameter: 14 mm, MTI Corp.) as counter/reference electrodes. The electrolyte used was 1.2 M LiPF6 in a mixture of ethylene carbonate/ethyl methyl carbonate (EC/EMC, 1:1 in v/v, BASF) with 10 wt% fluoroethylene carbonate (FEC). A Celgard 2320 (thickness 20 μm) was used as the separator. The cells were assembled in a glovebox filled with highly pure argon gas (UNIlab Pro, Mbraun, with O2 and H2O levels <0.1 ppm).
Cyclic voltammetry
Cyclic voltammetry of the cells was carried out using a galvanostat–potentiostat between 0.01 and 1.2 V vs. Li+/Li at a scan rate of 0.1 mV s−1 for 10 cycles. For cycling capability tests, the cells were performed at 100 mA g−1 for 375 cycles after 3 cycles activation at 30 mA g−1. For rate capability measurement, cells were running at different current rates of 10, 20, 45, 90, 180 mA g−1 and then went back to 10 mA g−1. There are only 4 cycles at 10 mA g−1, then there are 5 cycles for the next steps at different current rates.
Cell viability study
SW948 cells were seeded in 96 well plates at 15k cells per well in Dulbecco’s Modified Eagle Medium (DMEM) media (5 mM glucose), with added 10% Foetal Bovine Serum (FBS), 5mM L-Glutamine (L-Glu), 5 mM penicillin (PC) and 5 mM streptomycin (SP), and left overnight in a humidified CO2 infused incubator at 37 °C. Graphene ink was added to the wells to reach c ranging between 7.8 μg ml−1 to 1000 μg ml−1. After 48 h, the cells were gently washed three times with PBS, where cells in one of the plates were resuspended in cell media (i.e. DMEM/glucose/FBS/L-Glu/PC/SP), containing 10% of the viability reagent cell counting kit 8 (CCK-8, Dojindo Molecular Technologies, Inc.). According to the assay protocol, one plate was incubated at 37 °C for 2 h before measuring absorbance at 450 nm using Spectramax Paradigm plate reader (Molecular Devices, Sunnyvale, CA 95134). After measuring absorbance, these wells were rewashed with phosphate-buffered saline (PS) at 0.01 M in deionised water, before another absorbance measurement was conducted at 450 nm to determine any background absorbance resulting from cellular internalised graphene, which was subsequently subtracted. An identical 96 well cell plate was fixed with 4% w/v paraformaldehyde (PFA) solution containing 15 μg ml−1 Hoechst for 30 min, washed once with PBS, before Hoechst fluorescence was measured at 360nmExcitation/500nmEmission on a Spectramax Paradigm plate reader (Molecular Devices, Sunnyvale, CA 95134).
Cell microscopy
Cells were imaged using Leica SP8 confocal microscope in epifluorescence mode for full well overviews and laser scanning confocal mode for high-resolution imaging.
Muse™ flow cytometry viability and apoptosis assays
SW948 cells were seeded in sterile 24 well plates at 45k cells per well in cell culture media (complete DMEM), and left to attach in a humidified CO2 infused incubator at 37 °C overnight. The next day, graphene ink was added to the cell growth media in each well to make a final c of 125, 250, 500, 1000 µg ml−1 before being left for 48 h in the incubator as described above. Following 48-h treatment with graphene inks, cells were washed with pre-heated phosphate-buffered saline (PBS), and detached from culture wells using disodium ethylenediaminetetraacetic acid (EDTA) and 0.25% Trypsin (w/v%) (Thermo Fisher 25200056). 20 µL of cell suspension was added to 380 µL of count and viability Muse reagent and left for 5 min before measuring 1000 cellular event using Muse™ cell analyser. For apoptosis 100 µL of cell suspension was added to 100 µL of Muse® Annexin V & Dead Cell Reagent and left for 20 mins at room temperature before measuring 5000 cellular event using Muse™ cell analyser.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. Data are also available from the corresponding author upon reasonable request.
References
Kong, W. et al. Path towards graphene commercialization from lab to market. Nat. Nanotechnol. 14, 927–938 (2019).
Barkan, T. Graphene: the hype versus commercial reality. Nat. Nanotechnol. 14, 904–906 (2019).
Bohm, S. Graphene against corrosion. Nat. Nanotechnol. 9, 741–742 (2014).
Papageorgiou, D. G., Kinloch, I. A. & Young, R. J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 90, 75–127 (2017).
Carey, T., Jones, C., Le Moal, F., Deganello, D. & Torrisi, F. Spray-coating thin films on three-dimensional surfaces for a semitransparent capacitive-touch device. ACS Appl. Mater. Interfaces 10, 19948–19956 (2018).
Posen, I. D., Jaramillo, P., Landis, A. E. & Griffin, W. M. Greenhouse gas mitigation for U.S. plastics production: energy first, feedstocks later. Environ. Res. Lett. 12, 034024 (2017).
Moradi, B. & Botte, G. G. Recycling of graphite anodes for the next generation of lithium ion batteries. J. Appl. Electrochem. 46, 123–148 (2016).
Spasenovic, M. in Graphene Technology (eds Nazarpour, S. & Waite, S. R.) 177–187 (Wiley‐VCH, 2016).
Qiang, S. et al. Wearable solid-state capacitors based on two-dimensional material all-textile heterostructures. Nanoscale 11, 9912–9919 (2019).
Magdassi, S., Grouchko, M. & Kamyshny, A. Copper nanoparticles for printed electronics: routes towards achieving oxidation stability. Materials (Basel) 3, 4626–4638 (2010).
Karlsson, H. L., Cronholm, P., Gustafsson, J. & Möller, L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 21, 1726–1732 (2008).
Domanski, K. et al. Not All that glitters is gold: metal-migration-induced degradation in perovskite solar cells. ACS Nano 10, 6306–6314 (2016).
Hummers, W. S. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339–1339 (1958).
Mattevi, C. et al. Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 19, 2577–2583 (2009).
Ferrari, A. C. et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 7, 4598–4810 (2015).
Hernandez, Y. et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 3, 563–568 (2008).
Yao, Y. et al. Large-scale production of two-dimensional nanosheets. J. Mater. Chem. 22, 13494–13499 (2012).
Paton, K. R. et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 13, 624–630 (2014).
Parvez, K. et al. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 136, 6083–6091 (2014).
Del Rio Castillo, A. E. et al. High-yield production of 2D crystals by wet-jet milling. Mater. Horiz. 5, 890–904 (2018).
Karagiannidis, P. G. et al. Microfluidization of graphite and formulation of graphene-based conductive inks. ACS Nano 11, 2742–2755 (2017).
Bonaccorso, F. et al. Production and processing of graphene and 2d crystals. Mater. Today 15, 564–589 (2012).
Dalal, M. H., Lee, C.-Y. & Wallace, G. G. Simultaneous anodic and cathodic exfoliation of graphite electrodes in an aqueous solution of inorganic salt. ChemElectroChem 8, 3168–3173 (2021).
Munuera, J. M. et al. Electrolytic exfoliation of graphite in water with multifunctional electrolytes: en route towards high quality, oxide-free graphene flakes. Nanoscale 8, 2982–2998 (2016).
Lotya, M. et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131, 3611–3620 (2009).
Bracamonte, M. V., Lacconi, G. I., Urreta, S. E. & Foa Torres, L. E. F. On the nature of defects in liquid-phase exfoliated graphene. J. Phys. Chem. C 118, 15455–15459 (2014).
Backes, C. et al. Equipartition of energy defines the size–thickness relationship in liquid-exfoliated nanosheets. ACS Nano 13, 7050–7061 (2019).
Kouroupis-Agalou, K. et al. Fragmentation and exfoliation of 2-dimensional materials: a statistical approach. Nanoscale 6, 5926–5933 (2014).
Tuinstra, F. & Koenig, L. Raman spectrum of graphite. J. Chem. Phys. 53, 1126–1130 (1970).
Ferrari, A. C. & Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 61, 14095–14107 (2000).
Casiraghi, C. et al. Raman spectroscopy of graphene edges. Nano Lett. 9, 1433–1441 (2009).
Bianchi, V. et al. Terahertz saturable absorbers from liquid phase exfoliation of graphite. Nat. Commun. 8, 15763–15763 (2017).
Ferrari, A. C. et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97, 187401–187401 (2006).
Ferrari, A. C. & Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 64, 075414–075414 (2001).
Ferrari, A. C., Rodil, S. E. & Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B 67, 155306 (2003).
Cançado, L. G. et al. Quantifying defects in graphene via raman spectroscopy at different excitation energies. Nano Lett. 11, 3190–3196 (2011).
Ferrari, A. C. Diamond-like carbon for magnetic storage disks. Surf. Coat. Technol. 180-181, 190–206 (2004).
Khan, U., O’Neill, A., Lotya, M., De, S. & Coleman, J. N. High-concentration solvent exfoliation of graphene. Small 6, 864–871 (2010).
Al-Gaashani, R., Najjar, A., Zakaria, Y., Mansour, S. & Atieh, M. A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 45, 14439–14448 (2019).
Hayes, W. I., Joseph, P., Mughal, M. Z. & Papakonstantinou, P. Production of reduced graphene oxide via hydrothermal reduction in an aqueous sulphuric acid suspension and its electrochemical behaviour. J. Solid State Electrochem 19, 361–380 (2015).
Kelemen, S. R., Rose, K. D. & Kwiatek, P. J. Carbon aromaticity based on XPS II to II∗ signal intensity. Appl. Surf. Sci. 64, 167–174 (1993).
Fernandes, T. F. D. et al. A semi-automated general statistical treatment of graphene systems. 2D Mater. 7, 025045 (2020).
Torrisi, F. & Carey, T. Graphene, related two-dimensional crystals and hybrid systems for printed and wearable electronics. Nano Today 23, 73–96 (2018).
Torrisi, F. et al. Inkjet-printed graphene electronics. ACS Nano 6, 2992–3006 (2012).
McManus, D. et al. Water-based and biocompatible 2D crystal inks for all-inkjet-printed heterostructures. Nat. Nanotechnol. 12, 343–350 (2017).
Farivar, F. et al. Unlocking thermogravimetric analysis (TGA) in the fight against “Fake graphene” materials. Carbon 179, 505–513 (2021).
Carey, T. et al. Inkjet printed circuits with 2D semiconductor inks for high-performance electronics. Adv. Electron. Mater. 7, 2100112 (2021).
Bossis, G. & Brady, J. F. The rheology of Brownian suspensions. J. Chem. Phys. 91, 1866–1874 (1989).
Yuan, Y. & Lee, T. R. Contact Angle and Wetting Properties (Springer, 2013).
Liu, H. & Cao, G. Effectiveness of the Young-Laplace equation at nanoscale. Sci. Rep. 6, 23936 (2016).
Hyun, W. J. et al. Scalable, self-aligned printing of flexible graphene micro-supercapacitors. Adv. Energy Mater. 7, 1700285 (2017).
Kim, S. et al. Inkjet-printed antennas, sensors and circuits on paper substrate. IET Microw. Antennas Propag. 7, 858–868 (2013).
Carey, T. et al. Fully inkjet-printed two-dimensional material field-effect heterojunctions for wearable and textile electronics. Nat. Commun. 8, 1202 (2017).
Woltornist, S. J. et al. Preparation of conductive graphene/graphite infused fabrics using an interface trapping method. Carbon 81, 38–42 (2015).
Huang, L., Huang, Y., Liang, J., Wan, X. & Chen, Y. Graphene-based conducting inks for direct inkjet printing of flexible conductive patterns and their applications in electric circuits and chemical sensors. Nano Res. 4, 675–684 (2011).
Kong, D., Le, L. T., Li, Y., Zunino, J. L. & Lee, W. Temperature-dependent electrical properties of graphene inkjet-printed on flexible materials. Langmuir 28, 13467–13472 (2012).
Gao, Y., Shi, W., Wang, W., Leng, Y. & Zhao, Y. Inkjet printing patterns of highly conductive pristine graphene on flexible substrates. Ind. Eng. Chem. Res 53, 16777–16784 (2014).
Finn, D. J. et al. Inkjet deposition of liquid-exfoliated graphene and MoS2 nanosheets for printed device applications. J. Mater. Chem. C 2, 925–932 (2014).
Seekaew, Y. et al. Low-cost and flexible printed graphene-PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 15, 2971–2981 (2014).
Park, S.-H. et al. High areal capacity battery electrodes enabled by segregated nanotube networks. Nat. Energy 4, 560–567 (2019).
Tian, R. et al. Quantifying the effect of electronic conductivity on the rate performance of nanocomposite battery electrodes. ACS Appl. Energy Mater. 3, 2966–2974 (2020).
Tian, R. et al. Liquid exfoliated SnP3 nanosheets for very high areal capacity lithium-ion batteries. Adv. Energy Mater. 11, 2002364 (2021).
Zhou, H., Zhu, S., Hibino, M., Honma, I. & Ichihara, M. Lithium storage in ordered mesoporous carbon (CMK-3) with high reversible specific energy capacity and good cycling performance. Adv 15, 2107–2111 (2003).
Lian, P. et al. Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim. Acta 55, 3909–3914 (2010).
Aurbach, D. Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 89, 206–218 (2000).
Xing, W. & Dahn, J. R. Study of irreversible capacities for Li insertion in hard and graphitic carbons. J. Electrochem. Soc. 144, 1195–1201 (1997).
Levi, M. D. & Aurbach, D. The mechanism of lithium intercalation in graphite film electrodes in aprotic media. Part 1. High resolution slow scan rate cyclic voltammetric studies and modeling. J. Electroanal. Chem. 421, 79–88 (1997).
Zhao, X. et al. Electrochemical exfoliation of graphene as an anode material for ultra-long cycle lithium ion batteries. J. Phys. Chem. Solids 139, 109301 (2020).
Guo, Y.-G., Hu, J.-S. & Wan, L.-J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv 20, 2878–2887 (2008).
Taale, M. et al. Biomimetic carbon fiber systems engineering: a modular design strategy to generate biofunctional composites from graphene and carbon nanofibers. ACS Appl. Mater. Interfaces 11, 5325–5335 (2019).
Liu, J., Cui, L. & Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 9, 9243–9257 (2013).
Vranic, S. et al. Live imaging of label-free graphene oxide reveals critical factors causing oxidative-stress-mediated cellular responses. ACS Nano 12, 1373–1389 (2018).
Hoyle, C. et al. Small, thin graphene oxide is anti-inflammatory activating nuclear factor erythroid 2-related factor 2 via metabolic reprogramming. ACS Nano 12, 11949–11962 (2018).
Das, S. et al. Oxygenated functional group density on graphene oxide: its effect on cell toxicity. Part. Part. Syst. Charact. 30, 148–157 (2013).
Acknowledgements
XPS data were taken at NEXUS, Newcastle University, facility that was funded by UK Engineering and Physical Sciences Research Council (EPSRC), grant number NS/A000015/1. We have also received support from the Science Foundation Ireland (SFI) funded centre AMBER (SFI/12/RC/2278_P2) and availed of the facilities of the SFI-funded AML and ARM labs. J.M.K acknowledges funding from Smart Quantum Dot Lighting (EPSRC, EP/P027628/1). T.C. acknowledges funding by a Marie Skłodowska-Curie Action “MOVE” (Grant Number 101030735).
Author information
Authors and Affiliations
Contributions
T.C. conceived the experiments. T.C. and J.C. directed the research and designed experiments. T.C. manufactured the inks and undertook Raman Spectroscopy with assistance from AD.A. and J.M.K. T.C. undertook Inkjet printing, Rheology, Surface tension and electrical measurements. H.K. and T.C. undertook AFM measurements. T.C. and J.M. undertook TGA measurements. A.A. and H.R.H. did all biological testing. T.C., A.K., D.H. and E.C. undertook SEM. R.T. and D.H. undertook the Li-ion battery testing. S.S. and L.S. undertook XRD and XPS measurements. The manuscript was written by T.C. in close consultation with other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carey, T., Alhourani, A., Tian, R. et al. Cyclic production of biocompatible few-layer graphene ink with in-line shear-mixing for inkjet-printed electrodes and Li-ion energy storage. npj 2D Mater Appl 6, 3 (2022). https://doi.org/10.1038/s41699-021-00279-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41699-021-00279-0
This article is cited by
-
Implementation of an alternative graphene-based electrode
Journal of Materials Science: Materials in Electronics (2023)