Abstract
Current prognostic tools cannot clearly distinguish indolent and aggressive prostate cancer (PC). We hypothesized that analyzing individual contributions of epithelial and stromal components in localized PC (LPC) could improve risk stratification, as stromal subtypes may have been overlooked due to the emphasis on malignant epithelial cells. Hence, we derived molecular subtypes of PC using gene expression analysis of LPC samples from prostatectomy patients (cohort 1, n = 127) and validated these subtypes in two independent prostatectomy cohorts (cohort 2, n = 406, cohort 3, n = 126). Stroma and epithelium-specific signatures were established from laser-capture microdissection data and non-negative matrix factorization was used to identify subtypes based on these signatures. Subtypes were functionally characterized by gene set and cell type enrichment analyses, and survival analysis was conducted. Three epithelial (E1-E3) and three stromal (S1-S3) PC subtypes were identified. While subtyping based on epithelial signatures showed inconsistent associations to biochemical recurrence (BCR), subtyping by stromal signatures was significantly associated with BCR in all three cohorts, with subtype S3 indicating high BCR risk. Subtype S3 exhibited distinct features, including significantly decreased cell-polarity and myogenesis, significantly increased infiltration of M2-polarized macrophages and CD8 + T-cells compared to subtype S1. For patients clinically classified as CAPRA-S intermediate risk, S3 improved prediction of BCR. This study demonstrates the potential of stromal signatures in identification of clinically relevant PC subtypes, and further indicated that stromal characterization may enhance risk stratification in LPC and may be particularly promising in cases with high prognostic ambiguity based on clinical parameters.
Similar content being viewed by others
Introduction
Prostate cancer (PC) is the third leading cause of cancer-associated mortality and the most commonly diagnosed non-skin cancer among men in the west1. While indolent PC can often be managed by active surveillance, early stage aggressive PC requires active treatment by radical prostatectomy (RP) or radiation therapy to avoid metastatic spread2. Several prognostic nomograms based on clinical variables, e.g., Gleason Grade, tumor stage and serum prostate specific antigen (PSA), have been developed in an attempt to stratify localized PC (LPC) patients into low, intermediate or high-risk groups2,3. However, models based exclusively on clinical parameters offer limited accuracy and cannot readily distinguish between aggressive and indolent PC at the early disease stage. This is evident by the overtreatment of indolent PC that can lead to unnecessary side effects4, and undertreatment or delayed treatment of potentially aggressive PC resulting in ~30% of patients treated by RP experiencing biochemical recurrence (BCR) within a 10-year period5,6. Thus, there is a need for a better risk stratification tool to improve decision-making in LPC.
While PC originates in the prostate epithelium, the stromal composition has become increasingly recognized as an important contributor to disease initiation, progression, and response to treatment7. The environment of cells around the PC cells, known as the tumor microenvironment (TME), can act reciprocally with the cancer cells to influence aggressiveness8. Various characteristics of the TME, such as infiltration of specific immune cell types and transformation of resident stromal cells, have been associated with recurrence and metastatic spread across cancer types, including PC9,10,11. Previous studies have reported high abundance of cancer-associated fibroblasts, changes to the extracellular matrix, and increased vessel formation as indications of aggressive PC12,13. Increases in specialized macrophages (M2-polarized macrophages) and regulatory T-cells have also been associated to invasion and higher risk of metastatic spread in PC14,15, while other subsets of T-cells (e.g., CD8 + T-cells) may affect PC progression as suggested by their association to poor metastasis-free survival16. Hence, the specific cell type composition of the TME as well as the interactions between different cell types may reflect PC aggressiveness, and further investigations are required to fully understand this.
For some cancers, e.g., pancreatic cancer and colorectal cancer, it has been suggested that patients can be grouped into several subtypes with distinct prognosis based on their TME composition, including differences in the presence of certain immune and stromal cell types (e.g., fibroblasts, neutrophils and CD8 + T-cells) and in certain cancer cell signaling pathways (e.g., NOTCH1 signaling)17,18. However, limited attention has been given to the importance of TME subtypes in PC biology and progression19. Thus, we aimed to investigate if separate analysis of epithelium- and stroma-specific gene expression patterns could identify novel and clinically-relevant subtypes of PC.
We used three large RP cohorts to identify and validate prognostic subtypes of LPC. Based on unsupervised clustering using epithelium or stroma-specific gene expression signatures from prostate samples, we identified three epithelial and three stromal subtypes of early-stage, clinically-localized PC. Both epithelial and stromal subtypes were characterized using clinicopathological characteristics (e.g., Gleason Grade, T-stage), gene set enrichment analysis, and stromal and immune cell infiltration patterns. Lastly, we used epithelial and stromal subtypes to identify high-risk subsets of PC patients and to improve the accuracy of risk prediction for patients classified as intermediate risk based on clinical factors (CAPRA-S nomogram).
Results
Definition of epithelium- and stroma-specific gene expression signatures and derivation of epithelial and stromal subtypes
We hypothesized that clinically relevant subtypes of PC could be identified by separating the contribution of stroma (TME) from that of the epithelium (PC cells). To investigate this, we searched for genes that were specifically expressed in either prostate epithelium or prostate stroma using data from laser capture microdissected healthy prostate epithelium and matched healthy stroma samples from Tyekucheva et al.19. (Fig. 1a). Identification of genes with expression specific for epithelium or stroma, and involved in Gene Ontology pathways related to epithelium- or stroma-specific functions, resulted in a prostate epithelium-specific gene signature (n = 86 genes, Supplementary Table 1) and a prostate stroma-specific gene signature (n = 88 genes, Supplementary Table 1).
First, to test the significance of the epithelium- and stroma-specific gene signatures, we generated total RNA sequencing data from 127 LPC tumor samples. To ensure the cohort was representative for PC, we further included 31 adjacent normal samples (AN) (cohort 1, discovery), allowing us to identify well-known PC-associated gene differences between LPC and AN. An overview of the study design is given in Fig. 1a. Unsupervised clustering, using Euclidean distance and complete clustering-linkage based on the 500 most variably expressed genes, resulted in two distinct clusters, with all but one AN sample in one cluster and the vast majority of LPC samples in the other cluster (Supplementary Fig. 1a). Differential gene expression (DGE) analysis identified 4954 downregulated and 6246 upregulated genes in LPC compared to AN samples (BH-adj. p < 0.05). The most significantly upregulated genes in LPC samples were PC-associated genes (e.g., DLX1, HPN, and SIM220,21,22), and well known PC-associated oncogenes such as ERG, MYC, and KLK323 were similarly upregulated in LPC samples (Supplementary Fig. 1b), supporting the validity of our data and indicating that it is a representative RP patient cohort.
Next, we used the epithelium and stroma-specific gene signatures to sub-classify the LPC tumor samples from cohort 1 based solely on their expression of epithelium or stroma specific-genes (Fig. 1a). Using non-negative matrix factorization (NMF) and consensus clustering, we evaluated a range of potential subtype partitions (2–8 clusters) and based on cophenetic and silhouette scores identified the optimal number of partitions to use for the final analysis. Specifically, we classified samples into three PC subtypes based on the epithelial (E) gene expression signature (E1, E2 and E3; n = 61, 34, and 29 patients, respectively; Fig. 1b; Supplementary Fig. 2a) and three PC subtypes based on the stromal (S) gene expression signature (S1, S2 and S3; n = 44, 43, and 37 patients, respectively; Fig. 1c; Supplementary Fig. 2b). Subtypes were subsequently validated by NMF and consensus clustering also in the external, publicly available cohort 2 (TCGA) and cohort 3 (MSKCC) (Fig. 1a). The overlap of epithelial (E1–E3) and stromal (S1–S3) subtypes in cohorts 1–3 is given in Supplementary Fig. 3, and clinicopathological characteristics for cohorts 1–3 are given in Table 1.
Epithelial subtypes have growth and hormone regulation characteristics but heterogeneous prognostic potential across cohorts
We initially sought to characterize the three epithelial subtypes, E1, E2 and E3, identified above in cohort 1 (Fig. 1b) and validated in cohorts 2 and 3 (Supplementary Fig. 4a).
For each subtype, we analyzed the distribution of clinical variables known to be associated with adverse PC disease course. Subtype E3 showed an association to higher pT-stage and higher ISUP grade group, although this trend was not significant across all cohorts (Fig. 2a, b). Next, the subtype associated with the least (E1) and the most (E3) adverse clinicopathological characteristics, respectively, were further compared.
Stacked bar plot in each cohort showing percentage of patients in each subtype according to (a) pathological T-stage, (b) 2014 ISUP Gleason grade. Fisher´s Exact test is used to determine significant differences in clinical characteristics. c Venn diagram and heatmap showing overlap of significantly enriched Hallmarks for GSEA of DGE between E1 and E3.
Gene set enrichment analysis (GSEA) based on genes differentially expressed between subtype E1 and E3 identified several Hallmark pathways important to PC biology (n = 30, 13, 38 in cohorts 1, 2, 3, respectively). Of these, 10 pathways were seen to be differentially expressed between E1 and E3 consistently in all 3 cohorts (encompassing 22% of unique enriched pathways across cohorts, p < 0.05, Fisher’s Exact Test; Fig. 2c), with crucial functions in cell growth (E2F and MYC target pathways) and a reduced hormone response (androgen and estrogen response pathways), indicating aggressive cancer growth and reduced sensitivity to hormone signaling in the E3 subtype. Overlap in pathways across cohorts likely indicate that the underlying disease biology differs between subtype E1 and E3.
Furthermore, cell-type enrichment analyses showed higher enrichment of M2-polarized macrophages and CD8 + T-cells in subtype E3 compared to E1 in both cohort 1 and cohort 2 (Supplementary Fig. 5). We and others have previously reported that high levels of infiltrating M2-polarized macrophages and CD8 + T-cells is associated with more aggressive PC16,24,25. No other cell type investigated (fibroblasts, smooth muscle cells, adipocytes, B-cells, CD4 + T-cells, dendritic cells, endothelial cells, eosinophils, epithelial cells, M1-polarized macrophages, mast cells, NK cells, and regulatory T-cells) showed significant difference in abundance between subtypes E1 and E3 that were consistent across both cohorts analyzed (Supplementary Fig. 5).
Lastly, prognostic evaluation showed significant association of subtype E3 with increased risk of post-operative BCR compared to E1 in cohort 1 (log-rank test, p = 0.032, Fig. 4a) and cohort 2 (log-rank test, p = 0.019, Fig. 4b) but not in cohort 3 (log-rank test, p = 0.433 Fig. 4c). These results were corroborated by univariate Cox regression analysis of time to BCR (Table 2). Subtype E3 was not significantly associated with BCR in any of the three cohorts when adjusting for clinical variables (CAPRA-S nomogram) in multivariate analysis (Table 3).
Thus, our data indicate unique transcriptional characteristics inherent to the different epithelial subtypes, potentially underlying distinct PC biology. However, heterogeneity between cohorts in terms of prognostic potential indicates limited clinical utility for subtyping on the epithelial gene expression signature.
Stromal subtypes are characterized by stromal dysfunction and changes in immune cell infiltration and predict prostate cancer recurrence
We next sought to characterize and validate the three stromal subtypes, S1, S2 and S3, identified earlier (Fig. 1c). Stromal subtype S3 was associated with higher pT-stage and higher ISUP grade group in all three cohorts, although ISUP grade did not reach statistical significance in cohort 1 (Fig. 3a, b). The subtype with the least (S1) and the subtype with the most (S3) adverse clinicopathological characteristics, respectively, were further compared. Genes from the stroma-specific signature with importance for sample classification into subtypes S1 and S3 have been listed in Supplementary Table 2.
Stacked bar plot in each cohort showing the percentage of patients in each subtype according to (a) pathological T-stage, (b) 2014 ISUP Gleason grade. Fisher´s Exact test was used to determine significant differences in clinical characteristics. c Venn diagram and heatmap showing overlap of significantly enriched Hallmarks for GSEA of DGE between S1 and S3. Raincloud plots show differences in enrichment score between S1 and S3. In cohort 1 for Fibroblasts (d), Smooth muscle (e), Immune Score (h), CD4 + T-cells (i), M2-polarized macrophages (l), and CD8 + T-cells (m). In cohort 2 for Fibroblasts (f), Smooth muscle (g), Immune Score (j), CD4 + T-cells (k), M2-polarized macrophages (l), and CD8 + T-cells (m). Wilcoxon rank-sum test was used to determine significant differences between subtypes S1 and S3. FDR-corrected p values are reported. # = axis is square root scaled.
GSEA based on genes differentially expressed between subtype S1 and S3 identified several Hallmark pathways important to PC biology (n = 18, 18, 36 in cohorts 1, 2, and 3, respectively). Of these, 11 pathways were seen to be differentially expressed between S1 and S3 consistently in all 3 cohorts (encompassing 26% of unique enriched pathways across cohorts, p < 0.05, Fisher’s Exact Test; Fig. 3c, Supplementary Fig. 6), with alterations such as increases in pathways related to cell growth (E2F and MYC targets), reduced stromal environment functions (e.g., myogenesis, hypoxia, and adipogenesis) and reduced cell polarity (apical junction and apical surface) (Fig. 3c). Thus, indicating that dysregulated stroma is associated with loss of epithelial cell polarity and enhanced tumor growth.
To further investigate this stromal dysregulation, we characterized the cell type compositional differences between S3 and S1 in cohorts 1 and 2 by computational cell type enrichment analysis26 (Fig. 3d–o and Supplementary Fig. 7). We observed significantly reduced fibroblast enrichment score in S3 compared to S1 in both cohorts and significantly reduced smooth muscle cell enrichment score in cohort 2 (Fig. 3d–g), corroborating the GSEA findings and indicating that fibroblasts and smooth muscle cells play an important role in maintaining a functional stroma. We did not observe any consistent difference in overall immune cell infiltration between subtype S3 and S1 in cohorts 1 and 2, although, increased immune infiltration was observed in cohort 2 (Fig. 3h, j). In contrast, we observed a significant decrease in CD4 + T-cells in subtype S3 compared to subtype S1 in both cohorts (Fig. 3i, k). Furthermore, a significant increase in M2-polarized macrophages and CD8 + T-cells was observed in subtype S3 in both cohorts (Fig. 3l–o). B-cells, dendritic cells, endothelial cells, eosinophils, epithelial cells, M1 polarized macrophages, mast cells, and regulatory T-cells did not change consistently across the two cohorts (Supplementary Fig. 7). Adipocyte levels were significantly lower in subtype S3, although with many samples in cohort 2 absent for adipocytes (Supplementary Fig. 7).
Interestingly, subtype S3 was significantly associated with increased risk of BCR compared to S1 in cohort 1 (log-rank test, p = 0.0019, Fig. 4d), cohort 2 (log-rank test, p = 0.0098, Fig. 4e), and cohort 3 (log-rank test, p = 0.0057, Fig. 4f). These results were corroborated by univariate Cox regression analyses of BCR-free survival in subtype S3 compared to S1 (Hazard Ratio (HR) = 3.2, HR = 2.14, HR = 3.42 in cohort 1, 2, and 3, respectively, p < 0.05; Table 2). In multivariate analyses, subtype S3 was significantly associated with BCR independent of CAPRA-S risk group in cohort 1, but not in cohorts 2 and 3 (Table 3).
To validate that the observed differences in recurrence risk between stromal subtypes was associated to stroma-specific gene expression and not to unexplored background processes inherent to the datasets, we stratified patients on the 500 most variably expressed genes in cohort 1 (Supplementary Fig. 1). Association of BCR risk with patient subgroups stratified on variably expressed genes would indicate that unexplored biological processes in the tissue could be responsible for the trends in the dataset attributed to stromal characteristics. Stratification of patients on the 500 most variably expressed genes revealed stable clusters in all three cohorts, but were not significantly associated with prognostic outcome (BCR) (Supplementary Fig. 8). Together, this indicates that although some background processes may be present in the dataset due to the stable clustering, these could not replicate the prognostic potential observed from stromal subtypes. Hence, the subtypes based on stroma specific gene expression could not be attributed to underlying background gene expression.
Taken together, our results indicate that stromal transcriptional dysregulation and cell type changes inherent to the stromal subtypes underlie distinct PC biology and disease aggressiveness. This was corroborated by the consistent prognostic potential of these subtypes across three large independent RP cohorts from multiple countries.
Stromal subtypes improve risk stratification of prostate cancer patients with intermediate risk
To further improve on the prognostic effect of the subtypes, we investigated prognostic ability of the overlap between high-risk epithelial and stromal subtypes, as well as between low-risk epithelial and stromal subtypes (Supplementary Fig. 9a-c). However, stratification of patients into combined high-risk (S3 + E3, S3 + E2, S2 + E3) or combined low-risk (S1 + E1, S1 + E2, S2 + E1) did not robustly increase prognostic accuracy compared to S3 alone (Fig. 4d–f compared to Supplementary Fig. 9a-c).
Instead, since we identified stromal subtypes to harbor robust prognostic potential, we asked if this stroma-derived subtype information could be used to further improve upon the current prognostic risk evaluation tools. To test this, we selected patients with an ambiguous, intermediate risk of BCR according to CAPRA-S. Interestingly, CAPRA-S intermediate risk patients with the S3 subtype had an increased BCR risk compared to CAPRA-S intermediate-risk patients with S1/S2 subtypes in cohort 1 (log-rank test, Supplementary Fig. 10a, p = 0.032). This was validated in cohort 2 and cohort 3 (Supplementary Fig. 10b, c, Supplementary Table 3) and could not be recreated using the epithelial subtypes instead (CAPRA-S intermediate + E1/E2 vs. CAPRA-S intermediate + E3) (Supplementary Fig. 10d-f, Supplementary Table 3). Additionally, univariate Cox regression showed the conjugated CAPRA-S intermediate + S3 subgroup to have BCR risk comparable to that of the CAPRA-S high risk group (cohort 1, delta HR = 1.4, Fig. 5). This re-stratification of the BCR risk in the CAPRA-S intermediate + S3 subgroup was further validated in cohort 2 and cohort 3 (cohort 2, delta HR = 2.3; cohort 3, delta HR = 4.2; Fig. 5, Supplementary Table 3).
Thus, our results suggest a potential for further stratification of clinically defined (CAPRA-S) intermediate risk PC based on stroma-derived molecular risk classification. Taken together our results indicate that, beyond cancer cell (epithelial) characteristics, the surrounding stromal environment holds unique biological characteristics that can be exploited for prognostication in conjunction with clinical variables.
Discussion
There is mounting evidence that characterization of the TME, with a focus on both immune cell and stromal characteristics, can increase our understanding of PC pathobiology and be used to improve prognostic accuracy7,24. To this end, we clustered LPC patients into subtypes based specifically on epithelial or stromal gene expression. The emerging subtypes showed unique characteristics for both the stromal and epithelial subtypes, however only the stromal subtypes showed consistent prognostic potential across the training cohort and two external validation cohorts.
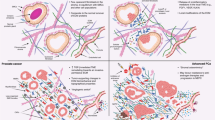
Characterization of the three stromal subtypes showed subtype S3 to be associated with adverse clinical changes and subtype S1 to be associated with more indolent characteristics (Fig. 3a, b), thus indicating that stroma-associated gene expression could be biologically relevant for determining PC aggressiveness. While previous studies have used gross stromal histology or the presence of individual cell types as markers for PC aggressiveness27,28, these have seen limited clinical relevance and consequently have not been translated into clinical practice. Interestingly, in our study, although subtype identification was without any information on malignancy, the stromal subtypes displayed clear and robust clinical and prognostic differences in multiple independent cohorts (Fig. 3a, b, Fig. 4d-f). To the best of our knowledge, the current study is the first to use unsupervised clustering to identify clinically relevant TME subtypes in PC and suggests that the stromal subtypes identified in this study could hold prognostic potential in clinically ambiguous PC, although evaluation of prognostic potential requires further studies.
Multiple transcriptional pathways and cell types differed between the stromal subtypes S1 and S3. Of these, the decreased fibroblast and smooth muscle cell enrichment in subtype S3 (Fig. 3d-g) likely reflects a larger change in the stromal composition, as also corroborated by the observed transcriptional changes in subtype S3 (e.g., myogenesis, adipogenesis, and hypoxia, Fig. 3c). Beyond the stromal changes, subtype S3 also had reduced infiltration of CD4 + T-cells, which have been associated to reduced risk of PC lymph node metastasis29. Furthermore, subtype S3 had increased infiltration of M2-polarized macrophages, which have previously been associated to metastatic potential and interactions with cancer associated fibroblasts in PC15,30, and increased CD8 + T-cells, where no consensus on influence on PC prognosis have been reached16,27,31,32. Thus, increased levels of M2 polarized macrophages likely reflects an immunosuppressive environment in subtype S333, while change in the specific T-cell subsets require additional investigation. Interestingly, the changes in stromal and immune cell composition and failure to maintain stromal functions were correlated to increased cell growth and a loss of epithelial cell polarity, processes that are invariably linked to cancer progression and metastatic spread34,35. This suggests a role of stromal and immune cells in the dysregulation to the stromal environment that may be necessary for the transformation of LPC into metastatic PC.
While the stromal subtypes predicted disease aggressiveness across multiple cohorts (Fig. 4d-f), these results could not be replicated using the epithelial subtypes (Supplementary Fig. 10d-f) nor substantially improved by addition of epithelial subtype information to stromal subtypes (Supplementary Fig. 9a-c, compared to Fig. 4d-f). Furthermore, by adding the stromal subtype information to the CAPRA-S risk groups we saw increased prognostic ability (Fig. 5), highlighting the possible utilization of integrating stromal information for patients where tumor-centric information (such as Gleason grading, T-stage, and serum PSA level) is inadequate to provide a clear outcome prediction.
Indeed, Gleason grade group and T-stage evaluation may already explain much of the malignant transformation in PC epithelial cells that genomic subtypes in LPC attempt to identify36,37. While the molecular heterogeneity of primary PC is well documented38, TME heterogeneity in PC is largely unexplored. Thus, the stromal changes we identify across cohorts may reflect more universal changes to the TME in aggressive cancer, beyond heterogeneous epithelial (malignant cell) characteristics. Our results support the hypothesis that although cancer cells have the capacity to disseminate and become metastatic, a feature that can somewhat be discerned from the histology (i.e., high Gleason Grade), an altered stromal environment is also required for further evolution of the cancer and metastatic spread19,39.
Pending further validation, the link between stromal composition and clinical outcome could potentially be utilized in a clinical setting in the future. Risk stratification and management of intermediate-risk PC with active surveillance is controversial and disputed as a viable treatment strategy40. Here, utilization of stromal subtyping may aid in improving risk stratification and tracking of metastatic potential. In line with this, offering active treatment to intermediate-risk patients with stromal high-risk disease may then improve the safety of referring the remaining intermediate-risk PC patients to AS. Furthermore, commercial gene expression tests available for PC treatment guidance have been investigated for use during active surveillance of intermediate-risk PC, but were not able to predict disease upstaging41. Inclusion of stromal subtype evaluation in the continuous surveillance of intermediate-risk PC patients may also have the potential to improve prediction of progression risk. However, clinical studies utilizing stromal subtyping for treatment selection or continuous evaluation of progression risk is needed to prove any such potential utility.
Although several commercial and non-commercial gene expression-based models are available for prostate cancer treatment guidance, only a few utilize stromal gene expression42,43,44. While most models rely on expression of cell-cycle or growth related genes, the Oncotype DX model includes multiple genes related to stromal composition and cellular organization45. Several of the Oncotype DX genes were present either in our stromal signature (COL1A1, FLNC, GSN and TPM2) or epithelial signature (KLK2 and AZGP1), with stromal signature genes being related to stromal response and cellular organization45. Likely, the stromal genes in Oncotype DX quantify some of the aspects captured by our stromal subtypes. Hence, other stromal subtype characteristics could further improve this model, and other prognostic models, especially for evaluation of intermediate risk PC. Future studies should investigate this further.
In addition to the gene-expression-based models, other studies have developed stroma-related PC signatures for prediction of high Gleason PC or metastatic potential following RP19,39. One study used LCM samples of epithelium and stroma from patients with low (3 + 3) or high (≥8) Gleason score PC to develop a 24-gene signature, which they named the Gleason stromal gene signature. Interestingly, where we observed limited prognostic utility between epithelial subtypes in the current study, this previous study identified only one epithelial gene with consistent expression change between low and high Gleason grade PC, corroborating the difficulty of identifying prognostic epithelial subtypes in PC. While the Gleason stromal gene signature was able to separate low and high Gleason grade PC, including in a recent validation study46, no further evaluation of its clinical utility beyond Gleason grade prediction has been performed.
Another study used a pre-clinical, patient-derived xenograft model of PC metastasis in mice to develop a 93-gene stroma-derived metastatic signature (SDMS)19. The SDMS stratified patients into high or low risk of metastasis following RP. Indeed, similar to how our S3 subtype improved risk stratification for patients with CAPRA-S intermediate risk PC (Fig. 5), the SDMS improved risk stratification for patients with Gleason score seven tumors. While the prognostic value of the SDMS was validated in multiple independent cohorts, the study used a murine model lacking several immune components for signature development potentially limiting insight into the stroma and immune cell interplay in PC progression. A total of 5 genes (AEBP1, C1S, COL1A1, LUM, PRELP) overlap between our stromal signature and the Gleason stromal gene signature by Tyekucheva et al 19., while seven genes (ACTG2, DES, MGP, SPARC, TPM1, CLU, COL14A1) overlap between our stromal signature and the SDMS signature reported by Mo et al. 39. The small overlap in genes between our stromal signature and both the previously reported Gleason stromal gene signature19 and SDMS39 highlight that both the methods used to identify the different stromal signatures, and the underlying biological differences identified, are different between the three studies. The previously identified signatures were based on specific clinical differences (i.e., low vs. high Gleason, metastatic vs. non-metastatic), while our stromal signature was based on benign prostate tissue, but turned out to be clinically relevant, likely due to identified differences in microenvironment composition and function. Nonetheless, the biological aspects of PC progression identified with the Gleason stromal gene signature, the SDMS, and our stromal subtypes all provide unique insights into a more holistic understanding of the PC TME and its role in malignancy.
Our study has certain limitations. First, the use of BCR as endpoint for prognostic evaluation instead of the more clinically appropriate metastatic spread or PC-specific death. However, as early-stage PC is characterized by a long time to progression47, it requires more than 10 years of follow-up to evaluate these outcomes. Secondly, no spatial or single cell data was available to perform more in-depth analysis of the subtypes. Instead, our study utilized several large, independent, and international cohorts of bulk tumor transcriptomic data to identify subtypes using unsupervised clustering, and to evaluate subtype aggressiveness using follow-up information. Single cell or high-resolution spatial transcriptomic dataset with fewer samples and short follow-up would have been inadequate for such analyses. Further work should investigate details of the now established stromal subtypes using single cell or high-resolution spatial transcriptomic analysis of PC tissue.
In conclusion, we have identified stromal subtypes in primary tumor samples of LPC patients and validated their characteristics in two independent cohorts. We have identified a stromal subtype (S3) that was consistently more aggressive in LPC, displayed specific stromal dysfunction at both the cellular and pathways level, and had increased M2-polarized macrophage and CD8 + T-cell infiltration. Furthermore, it improved prognostic stratification of patients with clinically intermediate risk PC, which suggest potential use in active surveillance management of intermediate risk disease. However, further studies validating the clinical utility of stromal subtypes in PC is required.
Methods
Patient cohorts
Cohort 1 included 127 fresh-frozen primary PC tissue specimens and 31 AN prostate specimens from 142 patients with LPC treated by RP (Table 1). Samples were collected at the Department of Urology, Aarhus University Hospital (2004–2017) or Regional Hospital West Jutland (2016–2019). RNA profiling was performed by total RNA-sequencing as previously described48,49.
All research for cohort 1 was carried out in accordance with the principles of the Helsinki Declaration and was approved by The Central Denmark Region Committees on Health Research Ethics [#2000/0299, #1–10–72–361–18, #1–10–72–367–13], The National Committee on Health Research Ethics [#1603543/66451] and notified to The Danish Data Protection Agency [#2013–4–2041, #1–16–02–330–13, #1–16–02–23–19, #1–16–02–248–14]. Written consent was obtained from all participants prior to their donation of tissue samples for a research biobank, while the requirement for patient consent to the specific analyses in this retrospective study was waived.
Cohort 2 included 406 tumor samples from patients with LPC treated by RP from The Cancer Genome Atlas Prostate Adenocarcinoma Dataset (TCGA-PRAD)36. RNA profiling of tumor samples was performed by poly(A) enriched RNA sequencing. Molecular and clinical data was publicly available and obtained from the TCGA data portal50 as described previously51.
Cohort 3 included 126 tumor samples from patients with LPC treated by RP from the Memorial Sloan-Kettering Cancer Center dataset by Taylor et al. (MSKCC)52. RNA profiling of tumor samples was conducted using an Affymetrix Human Exon 1.0 ST Array. Expression data was publicly available and obtained from the Gene Expression Omnibus (GEO) database (GSE21032).
The laser-capture microdissection dataset included 5 cystoprostatectomy samples from patients with bladder cancer from Tyekucheva et al. 19. Samples were reviewed for no incidental prostate cancer by a pathologist and laser capture microdissection was used to isolate areas of epithelium or stroma19. RNA profiling was performed using the Affymetrix Gene Array STA 1.0. Expression data was publicly available and obtained from the GEO database (GSE97284).
RNA extraction and sequencing
For cohort 1, total RNA extraction and library preparation was performed as previously described24,49. Briefly, immediately following RP, fresh prostate tissue biopsy samples were obtained and stored at −80 °C in TissueTek. Approximately 40 sections (20 µm thick) were cut from each sample. The first and last tissue sections were stained with Hematoxylin and Eosin (H&E) and evaluated by a pathologist for areas of malignant prostate tissue or benign prostate tissue absent of malignant cells. Total RNA extraction was performed on the remaining sections using the RNeasy Plus Mini Kit (QIAGEN, Cat#74036). RNA concentration was assessed on a NanoQuant Plate™ (TECAN). RNA quality was assessed using a 2100 Bioanalyzer (Agilent).
Sequencing libraries were generated using the ScriptSeq RNA-seq Library kit with RiboZero™ Magnetic Gold Kit (Illumina; AN = 11, LPC = 52) or the KAPA RNA HyperPrep Kit with KAPA RiboErase Kit (Roche; AN = 20, LPC = 75). Paired-end sequencing was performed using either Illumina HiSeq 2000, NextSeq 500, or NovaSeq 6000 ( ~ 25 million reads/sample; 2 × 75 bp, 2 × 75 bp, or 2 ×100 bp, respectively). All reads were QC checked and trimmed, and transcripts were quantified using Kallisto (version 0.46.2)53 with GrCh38.p13/hg38 as reference transcriptome. Transcripts were summarized to gene level counts using tximport54. Subsequently, normalization, filtering and log2 transformation was performed using edgeR55. Correction for batch effects was performed in the design formula for differential expression analyses or using the removeBatchEffect function from the R package Limma56.
Gene signature definition and subtype identification
Genes for the epithelium- and stroma-specific signatures were identified in an external laser-capture microdissection dataset (see above), based on two criteria: 1) differential gene expression analysis of epithelium vs. stroma using Limma56, where we selected genes significantly upregulated in either healthy prostate epithelium or healthy prostate stroma (false discovery rate (FDR) < 0.05, log2 fold-change (logFC) ≥ 2.0). And 2) Gene Ontology57,58 analysis for biological processes, where the final selection required significant enrichment of epithelium- or stroma-specific Gene Ontology terms (Fold enrichment ≥10, FDR < 0.05) when using epithelium or stroma genes, respectively, for the analysis. For the epithelium genes, one pathway was significantly enriched: Epithelial cell development. For the stromal genes, seven pathways were significantly enriched and all related to stromal functions: Regulation of complement activation, Regulation of transforming growth factor-beta secretion, Mesenchymal migration, Regulation of amyloid fibril formation, Regulation of smooth muscle cell migration, and Regulation of metallopeptidase activity. This resulted in 86 epithelium-associated genes for an epithelial signature and 88 stroma-associated genes for a stromal signature (Supplementary Table 1).
For the epithelial subtype and the stromal subtype discovery in cohort 1, patient clustering was performed on the epithelial signature (n = 86 genes) or the stromal signature (n = 88 genes), respectively, using NMF with consensus clustering via the R package NMF59. The optimal number of clusters/subtypes for each signature was evaluated based on the cophenetic and silhouette scores, indicative of clustering stability. Based on an initial evaluation of a range of clusters (2–8 clusters), having three clusters was deemed as being optimal both when clustering based on the epithelial and stromal signature and hence used for the final consensus clustering with 5000 clustering iterations. Genes important for sample placement into stromal subtype S1 or S3 were extracted using the feature selection method described in Carmona-Saez et al. 60. implemented in the NMF package in R (Supplementary Table 2).
Validation of epithelial and stromal subtypes
Subtype validation in cohort 2 and cohort 3 was performed on samples clustered on identical gene-expression signatures and clustering parameters to cohort 1 (n = 3 clusters, iterations = 5000). Cluster similarity across cohorts was validated using Spearman’s rank correlation. Spearman’s rank correlation analysis was used to evaluate the concordance of gene contribution to placement of samples into a specific subtype (gene weights) across cohorts. A positive correlation of gene weights confirmed that E1, E2 and E3 were defined based on the same epithelial genes across the three cohorts (Supplementary Fig. 4a). Similarly, correlation of gene weights validated that subtypes S1 and S3 were based on the same stromal genes across the three cohorts, while S2 could not be validated in all three cohorts (Supplementary Fig. 4b).
Differential gene expression analysis and gene set enrichment analysis
Transcriptomic differences between AN and LPC samples in cohort 1 were examined by DGE analysis using edgeR55. Transcriptomic differences between the high- and low-risk stromal subtypes (S3 and S1) and between the high- and low-risk epithelial subtypes (E3 and E1) were examined by DGE analysis. In cohorts 1 and 2 (RNA sequencing data) DGE was performed using edgeR, while in cohort 3 (microarray expression data) DGE was performed using Limma in R56. In all cases, adjustment for multiple testing was performed using the Benjamini-Hochberg (FDR) method61. Adjusted p values < 0.05 were considered significant.
Pre-ranked GSEA was subsequently performed on the output from either edgeR or Limma. GSEA was performed using the R package fgsea62 based on the cancer Hallmark Gene Set Collection from the Molecular Signature Database63. Gene sets with BH-adjusted p value < 0.05 were considered significantly enriched, while normalized enrichment scores were used to determine the level of enrichment.
Cell-type enrichment analysis
The cellular composition of the subtypes was estimated by cell type enrichment analysis using the xCell26 package for R. xCell uses bulk gene expression data to determine the enrichment of specific cell types in individual samples, based on cell type-specific expression signatures. Cell type enrichment analysis was evaluated in the cohorts with tumor RNA sequencing data (cohort 1 and cohort 2), as the microarray data format available from cohort 3 was not available to us in a format eligible for accurate xCell analysis64.
Statistical analysis
All statistical analyses were performed in R (v. 4.0.2) using R Studio (v. 1.1.463). Comparison of categorical variables (i.e., clinicopathological parameters) was conducted using Fisher’s exact Test, while comparison of continuous variables (e.g., cell type enrichment) was done using Wilcoxon rank-sum test with BH-corrected p values.
For comparison of stromal and epithelial subtypes to clinicopathologically defined risk groups, we used the established CAPRA-S nomogram3. CAPRA-S risk groups were defined based on scores [0–2] = low risk; [3–5] = intermediate risk; [6–12] = high risk3. Stromal subtypes, epithelial subtypes, and CAPRA-S risk groups were analyzed as categorical variables in survival analyses. The prognostic potential of the subtypes and CAPRA-S risk groups was evaluated by BCR-free survival analyses, using univariate and multivariate Cox regression, Kaplan-Meier and log-rank tests in the survival package in R65. BCR was defined as PSA ≥ 0.2 ng/mL. Patients without BCR were censored at their most recent PSA measurement. Patients with unknown BCR status (lost to follow-up) or BCR within 3 months of RP (likely to have residual tumor) were excluded from BCR-free survival analyses (cohort 1, n = 3; cohort 2, n = 9; cohort 3, n = 4). Prognostic accuracy was determined using Harrell’s C-index.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
As the requirement for patient consent was waived in the current study, we do not have permission to deposit the full raw and individual-level clinical data in a public repository. The raw data, individual-level clinical data, and processed gene expression files for the discovery cohort have instead been deposited in the controlled access repository the GenomeDK Data Library (ID GDK000002). All inquiries regarding data access should be made to the Data Access Committee as described on the project’s GenomeDK Data Library page (https://genome.au.dk/library/GDK000002/).
Code availability
The underlying code for this study is available from the corresponding author upon reasonable request.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 71, 209–249 (2021).
Parker, C. et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 31, 1119–1134 (2020).
Cooperberg, M. R., Hilton, J. F. & Carroll, P. R. The CAPRA-S score: a straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer 117, 5039–5046 (2011).
Albertsen, P. C. Treatment of localized prostate cancer: when is active surveillance appropriate? Nat. Rev. Clin. Oncol. 7, 394–400 (2010).
Roehl, K. A., Han, M., Ramos, C. G., Antenor, J. A. & Catalona, W. J. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3478 consecutive patients: long-term results. J Urol 172, 910–914 (2004).
Lin, X. et al. Assessment of biochemical recurrence of prostate cancer (review). Int. J. Oncol. 55, 1194–1212 (2019).
Pederzoli, F. et al. Stromal cells in prostate cancer pathobiology: friends or foes? Br. J. Cancer, https://doi.org/10.1038/s41416-022-02085-x (2022).
Turley, S. J., Cremasco, V. & Astarita, J. L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15, 669–682 (2015).
Väyrynen, S. A. et al. Composition, spatial characteristics, and prognostic significance of myeloid cell infiltration in pancreatic cancer. Clin. Cancer Res. 27, 1069–1081 (2021).
Soysal, S. D., Tzankov, A. & Muenst, S. E. Role of the tumor microenvironment in breast cancer. Pathobiology 82, 142–152 (2015).
Giannoni, E. et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 70, 6945–6956 (2010).
Erdogan, B. et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 216, 3799–3816 (2017).
Tuxhorn, J. A. et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 8, 2912–2923 (2002).
Lanciotti, M. et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. BioMed. Res. Int. 2014, 486798 (2014).
Comito, G. et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 33, 2423–2431 (2014).
Zhao, S. G. et al. The immune landscape of prostate cancer and nomination of PD-L2 as a potential therapeutic target. J. Natl Cancer Inst. 111, 301–310 (2019).
Ogawa, Y. et al. Three distinct stroma types in human pancreatic cancer identified by image analysis of fibroblast subpopulations and collagen. Clin. Cancer Res. 27, 107–119 (2021).
Jackstadt, R. et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell 36, 319–336.e317 (2019).
Tyekucheva, S. et al. Stromal and epithelial transcriptional map of initiation progression and metastatic potential of human prostate cancer. Nat. Commun. 8, 420 (2017).
Kelly, K. A. et al. Detection of early prostate cancer using a hepsin-targeted imaging agent. Cancer Res. 68, 2286–2291 (2008).
Lu, B., Asara, J. M., Sanda, M. G. & Arredouani, M. S. The role of the transcription factor SIM2 in prostate cancer. PLOS One 6, e28837 (2011).
Goel, S. et al. Transcriptional network involving ERG and AR orchestrates Distal-less homeobox-1 mediated prostate cancer progression. Nat. Commun. 12, 5325 (2021).
Rebello, R. J. et al. Prostate cancer. Nat. Rev. Disease Prim. 7, 9 (2021).
Andersen, L. B. et al. Immune cell analyses of the tumor microenvironment in prostate cancer highlight infiltrating regulatory T cells and macrophages as adverse prognostic factors. J. Pathol. 255, 155–165 (2021).
Glud, E. N. et al. Identification of a high-risk immunogenic prostate cancer patient subset as candidates for T-cell engager immunotherapy and the introduction of a novel albumin-fused anti-CD3 × anti-PSMA bispecific design. British Journal of Cancer 127, 2186–2197 (2022).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome biology 18, 220 (2017).
Ness, N. et al. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 74, 1452–1461 (2014).
Ruder, S. et al. Development and validation of a quantitative reactive stroma biomarker (qRS) for prostate cancer prognosis. Hum Pathol 122, 84–91 (2022).
Ntala, C. et al. Analysis of prostate cancer tumor microenvironment identifies reduced stromal CD4 Effector T-cell infiltration in tumors with pelvic nodal metastasis. European Urology Open Science 29, 19–29 (2021).
Salachan, P. V. et al. Spatial whole transcriptome profiling of primary tumor from patients with metastatic prostate cancer. Int J Cancer 153, 2055–2067 (2023).
McArdle, P. A. et al. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br. J. Cancer 91, 541–543 (2004).
Yang, Y. et al. High intratumoral CD8+ T-cell infiltration is associated with improved survival in prostate cancer patients undergoing radical prostatectomy. Prostate 81, 20–28 (2021).
Hao, N. B. et al. Macrophages in tumor microenvironments and the progression of tumors. Clin. Dev. Immunol. 2012, 948098 (2012).
Lee, M. & Vasioukhin, V. Cell polarity and cancer—cell and tissue polarity as a non-canonical tumor suppressor. J. Cell Sci. 121, 1141–1150 (2008).
Wang, X. et al. E-cadherin bridges cell polarity and spindle orientation to ensure prostate epithelial integrity and prevent carcinogenesis in vivo. PLOS Genet. 14, e1007609 (2018).
Abeshouse, A. et al. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Abida, W. et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision-making. JCO Precis. Oncol. 1–16, https://doi.org/10.1200/po.17.00029 (2017).
Løvf, M. et al. Multifocal primary prostate cancer exhibits high degree of genomic heterogeneity. Eur. Urol. 75, 498–505 (2019).
Mo, F. et al. Stromal gene expression is predictive for metastatic primary prostate cancer. Eur. Urol. 73, 524–532 (2018).
Walker, C. H., Marchetti, K. A., Singhal, U. & Morgan, T. M. Active surveillance for prostate cancer: selection criteria, guidelines, and outcomes. World J. Urol. 40, 35–42 (2022).
Lin, D. W. et al. 17-Gene genomic prostate score test results in the canary prostate active surveillance study (PASS) cohort. J. Clin. Oncol. 38, 1549–1557 (2020).
Cooperberg, M. R. et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J. Clin. Oncol. 31, 1428–1434 (2013).
Ross, A. E. et al. A genomic classifier predicting metastatic disease progression in men with biochemical recurrence after prostatectomy. Prostate Cancer Prostatic Dis. 17, 64–69 (2014).
Fredsøe, J. et al. Diagnostic and prognostic MicroRNA biomarkers for prostate cancer in cell-free urine. Eur. Urol. Focus 4, 825–833 (2018).
Klein, E. A. et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur. Urol. 66, 550–560 (2014).
Ma, C. et al. The prostate stromal transcriptome in aggressive and lethal prostate cancer. Mol. Cancer Res. 21, 253–260 (2023).
Albertsen, P. C., Hanley, J. A. & Fine, J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 293, 2095–2101 (2005).
Salachan, P. V. et al. Microbiota of the prostate tumor environment investigated by whole-transcriptome profiling. Genome Med. 14, 9 (2022).
Hansen, E. B. et al. The transcriptional landscape and biomarker potential of circular RNAs in prostate cancer. Genome Med. 14, 8 (2022).
Zhu, Y., Qiu, P. & Ji, Y. TCGA-Assembler: open-source software for retrieving and processing TCGA data. Nat. Methods 11, 599–600 (2014).
Schmidt, L. et al. Exploring the transcriptome of hormone-naive multifocal prostate cancer and matched lymph node metastases. Br. J. Cancer 119, 1527–1537 (2018).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Research 4, 1521 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Ritchie, M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
The Gene Ontology Consortium The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 49, D325–D334 (2020).
Gaujoux, R. & Seoighe, C. A flexible R package for nonnegative matrix factorization. BMC Bioinform. 11, 367 (2010).
Carmona-Saez, P., Pascual-Marqui, R. D., Tirado, F., Carazo, J. M. & Pascual-Montano, A. Biclustering of gene expression data by non-smooth non-negative matrix factorization. BMC Bioinform. 7, 78 (2006).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
Korotkevich, G. et al. Fast gene set enrichment analysis. bioRxiv, 060012, https://doi.org/10.1101/060012 (2021).
Liberzon, A. et al. The molecular signatures database hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Aran, D. Bioinformatics for Cancer Immunotherapy: Methods and Protocols (ed Sebastian Boegel) 263–276 (Springer US, 2020).
Therneau, T. M. A Package for Survival Analysis in R. https://CRAN.R-project.org/package=survival (2020).
Acknowledgements
We thank Pamela Celis and Jesper Boel Boulund Kristensen for excellent technical assistance in the laboratory. Christa Haldrup helped conceive the study. The Danish Cancer Biobank is acknowledged for biological material and for information regarding handling and storage. The GenomeDK cluster was utilized for some of the data analysis in this publication. We thank GenomeDK and Aarhus University for providing computational resources. Figure 1a was created with BioRender.com. This study was supported by grants from The Independent Research Fund Denmark, The Danish Cancer Society, Aarhus University, Graduate School at Health and Aarhus University, Department of Clinical Medicine. The funders played no role in the study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
M.R., J.F. and K.D.S. conceived and designed the study. M.R., S.H.L. and B.P.U. collected the data. P.V.S., M.P.L.B., S.H.L., B.P.U., J.B.J. and M.B. contributed data or analysis tools. M.R., J.F., P.V.S. and K.D.S. performed analysis and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rasmussen, M., Fredsøe, J., Salachan, P.V. et al. Stroma-specific gene expression signature identifies prostate cancer subtype with high recurrence risk. npj Precis. Onc. 8, 48 (2024). https://doi.org/10.1038/s41698-024-00540-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-024-00540-x