Abstract
Glioblastoma (GBM) remains a deadly tumor. Treatment with chemo-radiotherapy and corticosteroids is known to impair the functionality of lymphocytes, potentially compromising the development of autologous CAR T cell therapies. We here generated pre-clinical investigations of autologous anti-GD2 CAR T cells tested against 2D and 3D models of GBM primary cells. We detected a robust antitumor effect, highlighting the feasibility of developing an autologous anti-GD2 CAR T cell-based therapy for GBM patients.
Similar content being viewed by others
Glioblastoma multiforme (GBM) is the most frequent and deadly primary glial brain tumor in adults, characterized by a poor response to chemo- and radiotherapy1. Autologous chimeric antigen receptor (CAR) T cell therapy has been changing the therapeutic landscape of hematological malignancies2,3, and emerging as a promising treatment for solid tumors, including GBM4,5. Evidence of treatment feasibility and safety in humans has been shown in clinical studies involving PSMA-targeting CAR T cells against prostate cancer6, anti-claudin18.2 CAR T cells in gastrointestinal cancers7, and anti-GD2 CAR T cells for diffuse midline glioma (DMG), a peculiar brain tumor occurring in children and young adults8.
Regarding GBM, published CAR T cell-based clinical trials have been focused on cell surface targets such as epidermal growth factor receptor vIII (EGFRvIII), human epidermal growth factor receptor 2 (HER2), and IL-13 receptor α chain 2 (IL13Rα2)4,5. So far, clinical investigations have exploited the use of autologous CAR T cells based on pre-clinical data obtained from allogeneic settings5. The use of pre-clinical models based on allogeneic CAR T cells is often necessary due to lack of sufficient autologous material and this may be particularly true for GBM patients since current treatments often induce severe and long-lasting lymphocyte toxicity9,10. In addition, autologous pre-clinical models may be preferable during CAR T development, as the data acquired in these models offer a more robust basis for clinical translation that is currently linked with autologous CAR-T approved for clinical uses.
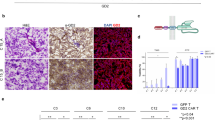
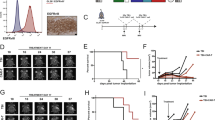
We and others have previously investigated the efficacy of allogeneic anti-GD2 CAR T cells in pre-clinical models of GBM highlighting a specific antitumor activity, although associated with significant allogeneic background activity11,12,13. In the current study, we tested the antitumor activity of autologous anti-GD2 CAR T cells against 2D and 3D cultures of matched GD2-positive primary tumor cell lines derived from four patients (identified as C6, C10, C12, and C15). Autologous T cells, isolated from cryopreserved PBMCs, were engineered with a vector expressing a second-generation anti-GD2 CAR11. The transduction efficiency of these autologous T cells (76.6 ± 14.2%) was comparable to our previous findings12 despite the PBMCs freezing/thawing processes, the long cryopreservation interval (up to 37 months) and the previous/current immunosuppressive treatments in three out of four patients included in the study (Table 1). Next to transduction efficiency, the immunophenotype of effector cells derived from two patients has been analyzed (C6 and C15) providing evidence of a mixed population of cytotoxic T lymphocytes (CTL, mostly NKT), CD8+ and CD4+ with variability among the two donors in terms of lymphocyte subsets (Supplementary Figs. 1 and 2). Despite the differences, autologous anti-GD2 CAR T effectors mediated a specific and robust killing in all conditions (Fig. 1a), except initially (24 h) against the GBM C6 cell line at the lowest E:T ratio (1:5) and at the earliest time point (24 h). The microscopic evaluations confirmed an anti-GD2 CAR mediated anti-GBM effect with the generation of activated lymphocyte clusters appearing overtime at all E:T ratios tested in C6, C10 and C15 patients (Fig. 1b).
a Patient-derived glioblastoma (GBM) cells (dsRED C6, dsRED C10) are co-cultured with autologous GFP T and anti-GD2 CAR T cells at three different E:T ratios. Tumor viability is evaluated by fluorescence viability assay at three different time points (24, 48, and 72 h). Data are shown as mean ± SD from six technical replicates; p values are calculated by unpaired two-tailed t-test. b Representative fluorescence micrographs of C6, C10, and C15 GBM cells (red) in co-culture with autologous anti-GD2 CAR T cell (green) and GFP T cells (green) at 2:1 and 5:1 E:T ratio at 24, 48, and 72 h showing a robust activation and cytotoxic activity of autologous anti-GD2 CAR T cells. GFP T cell population does not affect tumor growth. Scale bar: 1000 µm (C6 and C10); 400 µm (C15).
We then compared the anti-GBM activity of autologous and allogeneic cells with or without CAR transduction (Fig. 2a). Cells armored with the CAR construct generated a robust anti-GBM effect at 48 and 72 h, with the allogeneic cells performing better than the autologous for C6 and the autologous CAR T performing better than the allogeneic cells for C10. In all conditions, autologous GFP T cells did not impact tumor viability confirming that the CAR introduction drives a specific and robust anti-GBM effect. To better understand how previously frozen PBMCs perform after transduction and during anti-GBM activity, we evaluated cytokine release during cytotoxicity assay shown in Fig. 1b for C15. Thus, we compared autologous CAR T cells from C15 patient, generated after 20 months of storage, with freshly generated allogeneic CAR T cells derived from a healthy donor. We collected supernatants from co-cultures of both autologous and allogeneic effectors against GBM C15 target cells at three different time points (24, 48, and 72 h) and analyzed 7 different cytokines: IL-10, IL-2, Granzyme B, Granzyme A, IFNγ, TNFα, and perforin. The results show that autologous anti-GD2 CAR T cells have a comparable, if not even higher, cytokine release than allogeneic CAR T cells (Fig. 2b).
a Patient-derived dsRED-labeled glioblastoma (GBM) lines C6 and C10 are co-cultured with either autologous (AUTO) or allogeneic (ALLO) GFP T and anti-GD2 CAR T cells at 2:1 E:T ratio. Tumor viability is evaluated by fluorescence assay at 48 and 72 h. Data are shown as mean ± SD from three technical replicates; p values are calculated by unpaired two-tailed t-test. b Cytokine release profile comparing previously frozen autologous versus allogeneic effector cells in co-culture with GBM C15 cells. Supernatants of co-culture at 2:1 E:T ratio are assessed for cytokine release at 24, 48, and 72 h. Cytokine level is measured by Simple Plex ELLA (Bio-Techne), and the concentration is reported as pg/mL. §: out of range.
The antitumor effect of anti-GD2 CAR T cells against GBM cells of C6, C10, and C12 patients was then additionally evaluated in 3D spheroid models to further challenge the observed anti-GBM activity (Fig. 3). This approach was established accounting for (i) the more aggressive features of cells growing in 3D, (ii) the complexity of GBM growth, (iii) the heterogeneity of cell subsets in spheres with different levels of apoptosis resistance, (iv) the known immunosuppressive GBM microenvironment in 3D, and (v) the limits of CAR T penetration in solid tumors14,15,16. Confirming our previously reported data12, allogeneic anti-GD2 CAR T counteracted primary human GBM spheroids growth. In the autologous settings, GFP T lymphocytes surrounded the spheroids without penetrating the GBM spheres at all time points. On the contrary, the presence of the CAR allowed autologous T cells to enter and disrupt GBM spheroids starting from 24 h (Fig. 3).
Representative fluorescence microscopy images of 3D spheroid assays from C6, C10, and C12 patients. In the first column of each panel, GBM spheroids alone are showed; in the second and the third column the co-cultures at 2:1 E:T ratio with both (autologous or allogeneic) GFP T and anti-GD2 CAR T cells, respectively, are showed. Antitumor activity is monitored at 24, 48, and 72 h. Tumor cells in red, anti-GD2 CAR T cells and GFP T cells in green. Scale bar: 1000 µm.
Aiming at reinforcing our approach and getting closer to a more complex tumor microenvironment, we then generated a 3D spheroid co-culture of the C15 GBM line with either allogeneic or autologous CAR T cells with and without healthy microglial cells (HMC3 cell line with a GD2 expression of 10.4%, not shown). Microglia represent a relevant cell type of the brain playing a central role in the functioning of healthy brain tissue, being also associated with the establishment of an immunosuppressed niche in GBM17. Therefore, we considered microglia relevant for a CAR T cell-based strategy, not only for their possible inhibitory action but also for the potential risk of off-target effects due to the reported variable levels of GD2 in glial cells18. First, we verified whether C15 cells alone could generate GBM spheroids (Fig. 4a, upper-left pictures), then we successfully targeted them by either autologous or allogeneic CAR T lymphocytes (and controls; Fig. 4a, first and second row), confirming how GBM spheroids can be tackled by autologous CAR T cells. Afterward, we combined the C15 GBM cells (red) with HMC3 microglia cells (purple) with the evidence of a more compact structure (Fig. 4a, third and fourth row). These more complex spheroids were inefficiently penetrated by GFP T cells, despite their intra-GBM clusters. On the contrary, anti-GD2 CAR T cells were able to efficiently tackle GBM cells with their apparent complete eradication, suggesting that microglial cells do not abrogate the activity of both allogeneic and autologous anti-GD2 CAR T cells (Fig. 4a, third and fifth columns). To further confirm these findings, we approached the assay using Luciferase-labeled C12 cells combined with HMC3 microglia cells monitored by luminescence assay to better quantify the data in an additional GBM line (Supplementary Fig. 3). Even in this model and starting from 48 h of co-culture with autologous anti-GD2 CAR T, we were able to significantly reduce GBM viability even in the presence of glial cells (Supplementary Fig. 3). Moreover, taking advantage of our previous experience in testing cell-based approaches in 3D in vitro cultures19,20, we generated a GBM model where glial cells (purple) and two GBM primary cell lines (C6 and C12 in red) were respectively loaded into scaffold-based bioreactors (Fig. 4b, left and right panels). Once red and purple cells were engrafted into the 3D matrix (Fig. 4b, first and fourth columns), the systems were perfused by autologous anti-GD2 CAR T cells (and controls) generating a robust cytotoxic activity against GBM with the reduction of red signals even in the presence of microglia (Fig. 4b, third and sixth columns), once again confirming the therapeutic profile of our strategy against GBM.
a Representative fluorescence microscopy images of GBM C15 spheroids (red) with or without healthy microglia (HMC3 in purple), in co-culture with either autologous or allogeneic anti-GD2 CAR T or GFP T cells (green) at 2:1 E:T ratio. CAR T cells antitumor activity is monitored at 24 and 48 h. Scale bar: 257 μm. b Representative fluorescence microscopy images of dsRED C6 and C12 cells with HMC3 cells, forming a GBM-like structure in the VITVO® 3D bioreactor at 24, 48, and 72 h and then in co-culture with autologous anti-GD2 CAR T and GFP T cells at 3:1 E:T ratio. C6 and C12 cells in red, anti-GD2 CAR T cells and GFP control T cells in green, HMC3 in purple. Scale bar: 257 μm.
Reports on pre-clinical models utilizing matching autologous CAR T cells and primary tumor cell lines are uncommon21. This is mostly due to the difficulties in obtaining primary cancer cells and enough circulating lymphocytes to enable the generation of CAR T cells9,10, in particular from peripheral blood of chemotherapy/immunosuppressive therapy-treated GBM subjects. Our study represented a unique opportunity to evaluate the feasibility and potency of autologous CAR T for GBM, detecting antitumor activity in co-cultures by challenging 3D models, recently considered relevant for clinical translation by regulatory bodies22.
Despite the success, autologous CAR T cells have some drawbacks such as costs, production failure, and risk of treatment delay23. For these reasons, researchers have been starting to explore the possibility of manufacturing off-the-shelf allogeneic CAR T cells with several technological approaches. Although very promising, allogeneic CAR T cells may, in turn, be associated with major concerns: graft-versus-host disease (GVHD) and risk of limited antitumor activity because of rapid elimination of infused cells by the host immune system24. In addition, the use of allogeneic CAR T cells may not be ideal for brain cancer for the risk of allogeneic response causing CNS toxicity, a known side effect of CAR T therapies25. Thus, while research in this field is very active and off-the-shelf approaches might gain relevant space in the future, we presume autologous CAR T cells may still be very relevant in selected solid tumor types, such as GBM.
In this context, a sufficient number of T lymphocytes will be an essential requirement for successful CAR T manufacturing26 as well as the identification of factors that may affect characteristics of a leukapheresis product, as conceived for hematological diseases27. A basic requirement is the collection of a sufficient number of T lymphocytes, frequently impaired by previous/concurrent medications. In our study, we managed to produce active CAR T cells from PBMCs isolated (from 20 to 30 ml of peripheral blood) despite a relatively low number of lymphocytes at the time of blood collection. We believe that this is a relevant achievement in a pre-clinical setting. Certainly, when moving to the clinical scenario, it will be important to carefully consider the timing of PBMCs collection by leukapheresis to provide enough cells as a pre-requisite of a new therapeutic approach in GBM.
Indeed, standard treatments for both newly diagnosed and relapsed GBM patients struggle to provide a substantial survival benefit and new approaches are eagerly demanded. Our data, showing that autologous anti-GD2 CAR T cells mediated a powerful and specific antitumor response against matched human primary GBM cells, encourage the development of a CAR T-based therapeutic option for GBM indicating how this approach may be close to a possible clinical implementation.
Methods
Primary glioblastoma cell isolation, culture, and transduction
Primary GBM samples and peripheral blood were obtained from patients affected by high-grade glioma who underwent surgery at the Neurosurgery Unit (NOCSAE, Baggiovara, University Hospital of Modena). Samples were collected after signed informed consent from patients. The study was performed following the ethical standards as laid down in the Declaration of Helsinki and its later amendments. It was validated by the Ethical and Institutional Review Board at the University Hospital of Modena (prot. N.3600/C.E. September 2017). Tumor samples were dissociated and GBM cell lines were isolated as described12. Once cells started forming spheroids, the medium was replaced every 2 to 3 days, and the reseeding density was 20,000 cells/cm2. Primary tumor samples (ID: C6, C10, C12, and C15) were transduced by viral particles encoding for dsRED, as published12, or transiently marked with a fluorescent probe (for C15; CellTracker™ Orange CMRA; Thermo Fisher Scientific, Massachusetts, USA; #C34551). GBM primary cell lines were tested routinely for the absence of mycoplasma (MycoAlert Mycoplasma Detection Kit and MycoAlert Control Set, Lonza, Basel, Switzerland; #LOLT07318, #LOLT07518).
Healthy microglia culture
HMC3 cells (ATCC® #CRL-3304™) were cultured as recommended by ATCC. The base medium for this cell line is EMEM (ATCC® #30-2003™) supplemented with 10% heat-inactivated defined fetal bovine serum (FBS; 500 ml, Gibco, #10270-106, and 1% Penicillin/Streptomycin (Pen/Strep; 100 ml, Gibco, #15140-122). HMC3 cells were transiently marked with a fluorescent probe (CellTracker™ Deep Red; Thermo Fisher Scientific, Massachusetts, USA; #C34565).
CAR T cells manufacturing
CAR T cells were engineered starting from GBM patients’ PBMCs (as approved by the Institutional Review Board) which were separated by a density gradient (Lymphoprep, Alere Technologies AS, Oslo, Norway; #1114545) and then plated in RPMI 1640 (Gibco; #52400-017) with 1% FBS, 1% glutamine and 1% penicillin-streptomycin. Non-adherent cells were pre-stimulated for 48 h in 7 µg/mL Phytohemagglutinin (PHA-M, Sigma-Aldrich; #L8902) at a concentration of 1 × 106 cells/mL in a culture medium made by RPMI 1640 supplemented with 10% heat-inactivated defined FBS (HyClone Laboratories, Utah, USA; #SH30070.03), 500 UI/mL recombinant human Interleukin-2 (rhIL-2, Proleukin, Clinigen Healthcare Ltd, Staffordshire DE14 2WW, UK; #801313AY). Isolated T lymphocytes were transduced by a retroviral vector bearing second-generation anti-GD2 CAR and GFP control vector11,12.
Flow cytometry
The expression of GD2 antigen on isolated tumor cells was assessed by FACS analysis. Primary unconjugated mouse antihuman GD2 (BD, Franklin Lakes NJ, USA; #554272, 1:20) and a secondary APC-conjugated goat anti-mouse Ig (APC Goat Anti-Mouse Ig polyclonal multiple adsorptions; BD; #550826, 1:20) were used for staining. FACS analyses were also performed on anti-GD2 CAR T and GFP T cells manufactured from selected PBMCs of two GBM patients (C6 and C15). Three panels of antibodies were used to detect cell subsets, for cytotoxic T lymphocyte (CTL) labeling: iTCR/TCR γ/δ (APC, Biolegend, #331212), CD56 (PE, BD, #345812), CD3 (PerCP, BD, #345766), CD8 (PE-Cy7, Biolegend, #344750); for memory T lymphocyte labeling CD45RA (APC, Biolegend, #304112), CD3 (FITC, BD, #349201), CD4 (PE, Biolegend, #345769), CCR7 (PerCP, Biolegend, #353219), CD8 (PE-Cy7). Gating strategy is reported in Supplementary Fig. 2. Dilution of all the antibodies 1:20. The transduction efficiency of anti-GD2 CAR T and GFP T cells was determined by the percentage of GFP-positive cells. Data were collected by BD FACSAria III (BD) and analyzed using BD FACSDiva software (BD).
Cytotoxicity assay in 2D
Autologous anti-GD2 CAR T or GFP T cells were used as effectors (E) and human GD2-positive primary cell lines as targets (T). Different effector-to-target (E:T) ratios were used to assess the cell viability using 96-well black plates (Corning, Kennebuck, ME, USA; #3904) and GloMax Discover Multimode as microplate reader (Promega, Madison, WI, USA). Cultures containing the medium alone or 1% Triton X-100 were used as controls, representing 100% and 0% cell viability, respectively. Average viability was calculated as 100 × (experimental fluorescence − 0% viability fluorescence)/(100% viability fluorescence − 0% viability fluorescence). Co-cultures were also monitored by EVOS FL auto (Thermo Fisher Scientific) over 72 h.
ELISA
Cytokines in supernatants of cell co-cultures were assessed by automated immunoassay workflow. In detail, IL-2, IL-10, IFNγ, TNFα, Granzyme A, Granzyme B, and perforin were quantified by the automated ELISA platform “Simple Plex ELLA” (ProteinSimple, Bio-Techne, Minneapolis, MN, USA). Instrument calibration was performed using the in-cartridge factory standard curve, and supernatant samples were diluted 1:2 in Sample Diluent (ProteinSimple).
Cytotoxicity assays in 3D models
In vitro 3D cultures were performed through the formation of spheres. dsRED-positive GBM C6, C10, and C12 spheres were obtained by seeding 20,000 cells/well in a 96-well plate (Sphera Low-Attachment Surface, ThermoFisher; #174927). After 24 h, GBM spheres were co-cultured with transduced lymphocytes at an E:T ratio of 2:1 over 3 days. Co-cultures were also monitored by EVOS FL auto (Thermo Fisher Scientific) over 72 h. C15 cells transiently marked with a red fluorescent probe were seeded at 30,000 cells/well to form spheroids with or without microglia cells (HMC3) at 15,000 cells/well. HMC3 cells were transiently marked with a different fluorescent probe (CellTracker™ Deep Red). These spheroids were then co-cultured with transduced lymphocytes at an E:T ratio of 2:1 over 72 h. Representative images were collected by Leica SP8 confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany). In addition, a 3D bioreactor (VITVO®, EIR Biotherapies srl, Mirandola, Italy; #F000001) was used to evaluate the cytotoxic effect on target cells mediated by CAR T cells at an E:T ratio of 3:1 from 24 h to 72 h. The bioreactor was loaded with 5.0 × 105 dsRED C6 or C12 cells in combination with 2.5 × 105 Deep Red HMC3 cells in 1.4 mL of culture media by a 3 mL syringe (Becton Dickinson and Co, Franklin Lakes, NJ, USA). After 24 h, effector T cells were added. Representative fluorescence images of VITVO® were collected by Nikon A1R confocal microscope (Nikon Instruments Inc., Melville, NY, USA). Quantification of antitumor activity exerted by anti-GD2 CAR T cells and control GFP T cells was obtained seeding composite spheroids with C12Luc GBM cells and HMC3 cells, in co-culture with lymphocytes. We used GloMax Discover Multimode as microplate reader (Promega, Madison, WI, USA). Cultures containing the medium alone or 1% Triton X-100 were used as controls, representing 100% and 0% cell viability, respectively. Average viability was calculated as 100 × (experimental fluorescence − 0% viability fluorescence)/(100% viability fluorescence − 0% viability fluorescence).
Statistical analysis
GraphPad Prism version 6.0 (GraphPad Prism software, San Diego, CA, USA) was used to generate graphs and perform statistical analysis. In vitro data are expressed by means ± standard deviation (SD). To determine statistical significance, unpaired two-tailed Student’s t-test was used to determine statistical significance. A level of p value < 0.05 was used to designate significant differences.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Felthun, J. et al. How immunotherapies are targeting the glioblastoma immune environment. J. Clin. Neurosci. 47, 20–27 (2018).
Pan, K. et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 41, 119 (2022).
U.S. Food & Drug Administration. Carvykti. https://www.fda.gov/vaccines-blood-biologics/carvykti (2022).
Maggs, L. et al. CAR T cell-based immunotherapy for the treatment of glioblastoma. Front Neurosci. 15, 662064 (2021).
Elmadany, N. et al. Site-specific considerations on engineered T cells for malignant gliomas. Biomedicines 10, 1738 (2022).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28, 1189–1198 (2022).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Mendez, J. S. et al. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J. Neurooncol. 127, 329–335 (2016).
Grossman, S. A. et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 17, 5473–5480 (2011).
Prapa, M. et al. A novel anti-GD2/4-1BB chimeric antigen receptor triggers neuroblastoma cell killing. Oncotarget 6, 24884–24894 (2015).
Prapa, M. et al. GD2 CAR T cells against human glioblastoma. NPJ Precis Oncol. 5, 93 (2021).
Gargett, T. et al. GD2-targeting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma. J. Immunother. Cancer 10, e005187 (2022).
Wanigasekara, J. et al. Advances in 3D culture systems for therapeutic discovery and development in brain cancer. Drug Discov. Today 28, 103426 (2022).
Stanković, T. et al. In vitro biomimetic models for glioblastoma-a promising tool for drug response studies. Drug Resist. Updat. 55, 100753 (2021).
Choi, S. I. et al. Prospective approaches to enhancing CAR T cell therapy for glioblastoma. Front Immunol. 13, 1008751 (2022).
Ghoochani, A. et al. MIF-CD74 signaling impedes microglial M1 polarization and facilitates brain tumorigenesis. Oncogene 35, 6246–6261 (2016).
Marconi, S. et al. Expression of gangliosides on glial and neuronal cells in normal and pathological adult human brain. J. Neuroimmunol. 170, 115–121 (2005).
Candini, O. et al. A novel 3D in vitro platform for pre-clinical investigations in drug testing, gene therapy, and immuno-oncology. Sci. Rep. 9, 7154 (2019).
Golinelli, G. et al. A 3D platform to investigate dynamic cell-to-cell interactions between tumor cells and mesenchymal progenitors. Front Cell Dev. Biol. 9, 767253 (2022).
Owens, G. L. et al. Preclinical assessment of CAR T-cell therapy targeting the tumor antigen 5T4 in ovarian cancer. J. Immunother. 41, 130–140 (2018).
Wadman, M. FDA no longer needs to require animal tests before human drug trials. Science 379, 127–128 (2023).
Depil, S. et al. Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Dhakal, B. et al. Promise and pitfalls of allogeneic chimeric antigen receptor therapy in plasma cell and lymphoid malignancies. Br. J. Haematol. 197, 28–40 (2022).
Belin, C. et al. Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Sci. Rep. 10, 18997 (2020).
Qayed, M. et al. Leukapheresis guidance and best practices for optimal chimeric antigen receptor T-cell manufacturing. Cytotherapy 24, 869–878 (2022).
Tuazon, S. A. et al. Factors affecting lymphocyte collection efficiency for the manufacture of chimeric antigen receptor T cells in adults with B-cell malignancies. Transfusion 59, 1773–1780 (2019).
Acknowledgements
We are extremely grateful to Prof. Dario Campana (Department of Pediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore) for the collaboration in developing the CAR receptor. We thank CIGS (“Centro Interdipartimentale Grandi Strumenti” of the University of Modena and Reggio Emilia) for technical support when using Leica SP8 and Nikon A1R confocal microscopes. We are also grateful to Prof. Giuseppe Biagini who kindly provided us with the HMC3 cell line. This work was supported in parts by the following grants: “Dipartimenti Eccellenti MIUR 2022” (M. Dominici, G. Grisendi), Università di Modena e Reggio Emilia - Fondi Ateneo Ricerca (FAR) Fondazione di Modena (FoMo) 2021 (M. Dominici), Diana Laneri post-doctoral fellowship 2021 (C.C.), Mongiorgi-Leone Donation (C.C., M. Dominici), ASLEM 2021 (C.C., M. Dominici), Go4IT CRUI (G. Golinelli), the Associazione ASEOP (M.S.). The research leading to these results has received funding from the European Community - Next Generation EU DD3175/2021 and DD3138/2021 CN_3: National Center for Gene Therapy and Drugs based on RNA Technology, Project Code CN 00000041, to M. Dominici and G. Golinelli, CUP E93C22001080001. The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them. All authors express immense gratitude to the four GBM patients who generously donated cancer cells and peripheral blood lymphocytes. In lasting memory of Giulio.
Author information
Authors and Affiliations
Contributions
C.C. and M.P. (co-first authors): conception or design of the work, data collection, drafting the manuscript, data analysis, and interpretation; G.R. and M.S.: data collection, drafting the manuscript, data analysis, and interpretation; G.N., G. Pugliese, L.T., M. Dall’Ora, G. Golinelli, G. Grisendi, J.V., C.S., R.V.P., D.N.S., A.F., S.B., and C.I.: data collection, data analysis, and interpretation; M.B., R.D., K.D.E., and A.P.: data analysis and interpretation; G. Pavesi and M. Dominici (co-last authors): conception or design of the work, drafting the manuscript, data analysis, and interpretation. All authors: final approval of the completed version.
Corresponding author
Ethics declarations
Competing interests
G. Golinelli, G. Grisendi, C.S., and M. Dominici hold patents in the field of cancer and gene therapy. C.I. holds post-market surveillance counselling for Finceramica S.p.A. The other authors do not declare any competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiavelli, C., Prapa, M., Rovesti, G. et al. Autologous anti-GD2 CAR T cells efficiently target primary human glioblastoma. npj Precis. Onc. 8, 26 (2024). https://doi.org/10.1038/s41698-024-00506-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-024-00506-z