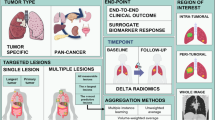
Abstract
Personalized medicine has revolutionized approaches to treatment in the field of lung cancer by enabling therapies to be specific to each patient. However, physicians encounter an immense number of challenges in providing the optimal treatment regimen for the individual given the sheer complexity of clinical aspects such as tumor molecular profile, tumor microenvironment, expected adverse events, acquired or inherent resistance mechanisms, the development of brain metastases, the limited availability of biomarkers and the choice of combination therapy. The integration of innovative next-generation technologies such as deep learning—a subset of machine learning—and radiomics has the potential to transform the field by supporting clinical decision making in cancer treatment and the delivery of precision therapies while integrating numerous clinical considerations. In this review, we present a brief explanation of the available technologies, the benefits of using these technologies in predicting immunotherapy response in lung cancer, and the expected future challenges in the context of precision medicine.
Similar content being viewed by others
The challenge of personalizing treatment
In oncological practice, personalized medicine—which has traditionally relied on the molecular characterization of tumors using genomic, and proteomic techniques, aims to customize treatment and ultimately guide clinical decisions and therapeutic interventions1. However, this approach has presented substantial difficulties for clinical oncologists, who are expected to develop a precise treatment plan for each patient based on these complex clinical data.
Personalized medicine in oncology traditionally involves invasive procedures to gain access to cancerous tissues, which only provides insight into small parts of the tumor and occasionally may be complicated by tumor placement and the potential risks of surgical biopsies2,3,4. While ‘liquid’ biopsies are a safer alternative to procuring solid tumor tissue and have shown promise in personalized medicine owing to the presence of circulating tumor DNA/RNA5,6,7,8, both liquid and tissue biopsies capture only a small spatiotemporal snapshot of notably heterogeneous and constantly evolving solid tumors9. Thus, tumor heterogeneity presents a barrier to personalized treatment10
AI-based analyses of radiological features from standard-of-care (SOC) images can serve as biomarkers and could play a considerable role in overcoming the challenges of personalized medicine. Although there are currently limitations to the technology, particularly with smaller tumors, the next-generation imaging analysis could ultimately be used to predict responses to therapies and develop more precise treatment plans for individual cancer patients in the era of personalized medicine. More recently, efforts to standardize radiomics via the integration of AI algorithms have steered the field of radiomics away from handcrafted features, thereby reducing bias and increasing accuracy and generalizability11,12,13,14,15,16. This will only serve to increase the diagnostic and prognostic accuracy of radiomics and strengthen associations between extracted data and biological and clinical endpoints to expedite and further optimize personalized treatment.
Why next-generation medical imaging analysis?
Diagnostic imaging—a qualitative and non-invasive method for assessing internal structures—has historically been the cornerstone of analytical and diagnostic workflows in oncology and helps to inform the course of treatment in clinical oncology practice10,17. While routine medical imaging (i.e., ultrasound [US], computed tomography [CT], magnetic resonance imaging [MRI], and positron emission tomography [PET]) can capture the overall spatial and temporal heterogeneity of solid tumors, qualitative visual assessments lack the granularity and objectivity to assess inter- and intratumour heterogeneity within the complex tumor microenvironment (TME)9.
Solid tumors not only vary in size and shape over time but are also known to be both phenotypically and genotypically heterogeneous, where tumor cells can vary in cell type, genomic sequence, gene expression, vascularization, oxygenation, metastatic potential, and response to treatment within the TME18,19,20,21,22,23,24. Furthermore, it has been established that heterogeneous tumor features are valuable prognostic indicators of variability in tumorigenesis, treatment efficacy, metastatic potential, and patient outcomes9,10,25,26,27,28.
Radiomics is an evolving field of study in which large numbers of quantitative features are extracted from standard-of-care (SOC) radiographic images and linked to clinical outcomes using either handcrafted or machine learning methodologies29. First proposed in 2012, radiomics leverages the heterogeneous nature of solid tumors by converting medical images into “mineable data” via the high-throughput extraction of high-dimensional quantitative features30,31,32. Burgeoned by increasing computational capabilities, radiomics has been used for nearly a decade to link quantitative features of tumors (i.e., intensity, texture, shape, volume, and wavelets) to clinical endpoints, such as response to therapy, metastases, and survival in a variety of cancers using multiple imaging modalities3,12,16,33,34,35. Despite the utility of this approach3,36, issues such as inconsistencies in image acquisition; motion artifacts; variability in segmentation and image processing; the limited reproducibility of features across centers, studies, and software tools; and analytical variability have been barriers to the adoption of radiomics into clinical workflows (please refer to the Supplemental Material for more technical details)9,14,37,38.
Deep learning—a subset of machine learning that uses multilayered artificial neural networks to transform input data (e.g., images) to output data (i.e., diagnostic parameters) while learning increasingly higher-level features in each layer—has been used to automate and optimize the extraction of features from medical images for nearly a decade39,40. The integration of DL into radiomics workflows (i.e., deep radiomics) bridges a gap in the field and mitigates many of the prevailing inconsistencies with “handcrafted” radiomics, in which human variability can introduce error at any point in the radiomics pipeline12,14,38. Notably, although radiomics and deep radiomics have been well investigated in a variety of cancers and have been used successfully to assess treatment response and predict survival in patients enrolled in clinical trials41, to the best of our knowledge, radiomics and deep radiomics have not yet been utilized in real-world clinical workflows.
Thus, while the integration of radiomics and AI offers oncologists a unique opportunity to predict personalized responses to immunotherapy in lung cancer prior to treatment, there are some barriers to incorporating these techniques into real-world clinical scenarios. The present paper aims to briefly review the current application of these technologies in lung cancer, as well as current obstacles to integrating radiomics techniques into clinical lung cancer workflows (please refer to the Supplemental Material for more technical details).
Clinical applications
There is an indisputable link between the radiomic features of tumor heterogeneity—both handcrafted and AI-enabled—and biological and clinical end points. In lung cancer, radiomic biomarkers have been shown to predict distant metastases42,43,44, malignancy in pulmonary nodules45,46, primary tumor stage10, histology10,47, pathological response after chemoradiation48, disease recurrence49, somatic mutations50,51,52,53,54, gene expression profiles and molecular pathways10,55, adverse events56, and survival54,57,58,59,60. Likewise, radiomic signatures can also be used to optimize treatment plans in NSCLC patients, such as chemoradiotherapy61, immunotherapy34,54,62, and tyrosine kinase and immune checkpoint inhibitors54,63. In the following sections, we will summarize the potential roles of handcrafted and deep radiomics in predicting personalized responses to immunotherapy in lung cancer (Fig. 1).
Currently, medical images play a predominant role in evaluating treatment responses. However, the introduction of innovative strategies such as radiomics and artificial intelligence provides a domain of predictive capabilities, augmenting treatment decision-making and complementing traditional tissue and liquid biopsy approaches.
Biomarkers for response to immunotherapy: beyond PD-L1
Immune checkpoint inhibitors that target biological biomarkers such as programmed death ligand 1 (PD-L1) have revolutionized lung cancer treatment as an alternative to cytotoxic chemotherapies64,65. However, despite the success of immunotherapy for some patients, a substantial number of patients do not experience clinical benefit, even in highly selected cohorts65,66. In this section, we will explore how the integration of radiomic biomarkers with SOC biomarkers could substantially impact patient care by helping to predict the response to immunotherapy.
Delta radiomic (DelRadX) features—changes in radiomic features over time—have been used to predict clinical outcomes in a variety of cancers67. A DelRadX signature based on CT images at baseline and at the end of the second treatment cycle performed satisfactorily in distinguishing between responders and nonresponders when used in combination with the clinical factor of distant metastasis (area under the curve [AUC] of 0.83 vs 0.81, respectively)34. Furthermore, this DelRadX signature was significantly more predictive of the response to immunotherapy than PD-L1 expression alone (p < 0.001). Khorrami et al.68 found that DelRadX using intranodular and perinodular texture features of malignant NSCLC nodules from CT images predicted the response to immunotherapy and overall survival (OS). Sun et al.69 determined the radiomic signature of CD8 cells using RNA-seq data combined with a radiomic analysis of solid tumors in a variety of cancers (including lung cancer) and validated it using two independent cohorts of patients. The radiomic signature of CD8 cells was predictive of the immune phenotype (dense vs low CD8 cell infiltration). In this study, a higher radiomics score—derived from five radiomic features extracted from each lesion, two discrete labels related to lesion location, and one imaging acquisition-related variable—was significantly associated with the response to anti-PD-1 or anti-PD-L1 monotherapy at both 3 and 6 months after the start of treatment, as well as OS.
Additionally, Trebeschi et al.70 developed a radiomics biomarker from CT imaging by training a model on all lesions (i.e., progressive, stable, and responding) to discern progressive disease. This biomarker was significantly associated with response vs nonresponse to immunotherapy in NSCLC patients. More specifically, tumors with increased morphological heterogeneity, nonuniform density patterns, and compact borders were more likely to respond to immunotherapy, while more compact and spherical profiles were associated with better response in nonresponding tumors. A gene enrichment analysis was performed to define the biological basis of the radiomic biomarker and found significant associations with pathways involved in mitosis, indicating a relationship between increased cell division and response to immunotherapy. Several other studies have used radiomic features to stratify NSCLC patients and to significantly predict survival outcomes in patients treated with immunotherapy71,72. Furthermore, other studies demonstrate that prognostic biomarkers perform best when combining radiomic, genetic, and clinical data, highlighting the complementary nature of these analyses55.
Tumor mutational burden (TMB) has also been shown to be a significant predictor of immunotherapy efficacy73. The deep learning model 3D-DenseNet was used to estimate the target tumor area in CT images from 327 NSCLC patients with TMB data and identified 1020 deep features to distinguish between patients with high and low TMBs. The TMB radiomic biomarker was a significant predictor of immunotherapy efficiency and distinguished between high-TMB and low-TMB patients in the training and test cohorts better than histological subtype. Moreover, the TMB radiomic marker was more robust than both the radiomic and clinical models.
Last, as somatic mutations, such as EGFR, are known to be associated with low response rates to anti-PD-1/PD-L1 immunotherapy74, DL models that predict mutation status from imaging could also predict response to immunotherapy. A study that used a PET/CT-based DL model with high accuracy in predicting EGFR mutation status across patient cohorts demonstrated a significant association between high EGFR mutation status and low durable clinical benefit, low PD-L1, high hyperprogression, and lower progression-free survival (PFS) in immunotherapy patients, indicating that EGFR mutation status assessed using SOC imaging could indeed serve as a biomarker to predict response to immunotherapy54.
Predicting the best combination therapy
Owing to the heterogeneous nature of lung cancer and the substantial number of patients who do not experience clinical benefit from immunotherapy alone, combining therapies can be an effective way to improve outcomes75,76. In this subsection, we will discuss the use of radiomics and DL technologies in supporting treatment decision making and predicting the best combination therapy to suit each patient.
A study by Sun et al.77 used a CD8 T-cell-associated radiomics signature to predict lesion response in irradiated and abscopal lesions using clinical data from patients with advanced solid tumors (including lung) from six independent clinical studies of combined radiotherapy and immunotherapy. The authors found that CD8 radiomics scores exhibited significantly higher tumor responses (i.e., decrease in lesion size) and that more heterogeneous CD8 radiomics scores across lesions were associated with mixed response or uniform progression, poor PFS, and OS. Additionally, heterogeneous CD8 radiomics scores based on the entropy of the distribution were significantly associated with the response evaluation criteria in solid tumors (RECIST)-based response in abscopal tumors. Not only can this study help to inform prognostic features for combined therapy and inform the choice of target lesion, but this study demonstrates that a radiomics score previously validated in a cohort treated with immunotherapy alone could be predictive in the context of combined therapies69.
Another study that used radiomics to study the response to combined therapies showed that a radiomic risk score (RRS), calculated using radiomic textural patterns within and around NSCLC nodules from pretreatment CT images, was found to be significantly associated with PFS and OS (p < 0.05) in patients treated with both chemoradiation and chemoradiation + immunotherapy78. The RRS also effectively stratified between low and high risk and was significantly associated with OS in the low PD-L1 group.
Predicting resistance to therapy
Acquired and inherent resistance mechanisms continue to be a considerable factor in poor lung cancer prognosis79. Most patients with NSCLC develop primary resistance during PD-1/PD-L1 monotherapy, of which only 15–20% exhibit a partial or complete response80. Acquired resistance can also occur despite initial clinical benefits. Notably, there are numerous mechanisms of resistance to immunotherapy in NSCLC beyond PD-L1 expression that could serve as predictive radiomic biomarkers in precision therapy, such as high microsatellite instability/defective DNA mismatch repair; tumor mutational burden; DNA polymerase (POLE) mutations; cytokine expression (e.g., interferon-gamma [IFN-γ], tumor necrosis factor-alpha [TNF-α], and interleukins); and point mutations, deletions, or homozygous or heterozygous loss of beta-2-microglobulin (B2M)81,82,83,84. Similarly, molecular mechanisms and tumor characteristics such as the overexpression of oncogenes (e.g., MDM285), EGFR mutations and associated changes in the TME86, or tumor hypoxia87 can also be predictive of the response to immunotherapy.
While there is a growing body of research that links radiomic features of tumors and the TME to tumor genotype, histology, immune state and clinical end points, studies that investigate radiomic biomarkers of resistance — both handcrafted and AI-enabled — are sorely lacking. The use of DelRadX to elucidate genotypic and phenotypic changes in response to treatment over time could expose underlying mechanisms of resistance. Public datasets for gene expression88 in NSCLC could be leveraged to identify prognostic biomarkers or to assess the multivariate performance of radiomic signatures10. Furthermore, existing knowledge of somatic mutations involved in resistance to targeted therapies74 or the role of immune cells (i.e., CD8 T cells) in tumor growth, metastasis, and resistance to immunotherapy89 could be leveraged to develop radiomic signatures that predict resistance to therapy.
Predicting side effects
The early detection of treatment-related adverse events is crucial for improving patient outcomes. Radiomics can also be applied to predictions of life-threatening adverse events such as cardio-toxicity, pneumonitis, and hyperprogression, as well as the misinterpretation of pseudo progression, to optimize patient care.
Several studies suggest that PET and CT-based radiomic features in NSCLC patients undergoing immunotherapy could be used to predict inflammatory conditions such as immunotherapy-induced pneumonitis90,91 or risk for developing severe immune-related adverse events (irSAEs)92. By capturing features at baseline that are predictive of potential irSAEs, treatment plans could be optimized early to minimize risk. For example, Mu et al.92 found that creating a radiomics nomogram that included a radiomics score based on features extracted from baseline (i.e., pretreatment), the type of immunotherapy, and dosing schedule effectively predicted patients with and without irSAEs with AUCs of 0.92, and 0.88 in the testing, and validation sets, respectively. However, the sample size of this study was relatively small for positive cases. As rule-of-thumb guides for binary classification studies suggest that sample size should be 10–15 times that of the number of features used, studies with smaller samples sizes should be interpreted with caution93.
Biomarkers that differentiate between pseudoprogression, progression, and hyperprogression during immunotherapy are lacking. Hyperprogression is an adverse reaction to anti-PD-1/PD-L1 immunotherapy in some NSCLC patients and is associated with significantly shorter survival. Vaidya et al.94 extracted 198 intratumoural and peritumoural radiomic textural patterns and tortuosity features of the nodule-associated vasculature from pretreatment CT scans. They found that the top features associated with hyperprogression were able to distinguish between hyperprogression and other patterns with AUCs of 0.85 in the training set and 0.96 in the validation set. A study by Tunali et al.95 created rapid disease progression phenotypes composed of time to progression (TTP)/tumor growth rates and hyperprogression in NSCLC patients being treated with immunotherapy using the following baseline predictors: patient demographics, clinical data, driver mutations, hematology data, and radiomic features from CT scans. As a result, the authors identified several effective clinical-radiomic models that predicted rapid disease phenotypes with AUC values of 0.80–0.86 and classified patients with TTP of <2 months and hyperprogression with 73 and 82% accuracy, respectively. Finally, DL biomarkers for somatic mutations such as EGFR have also been shown to be associated with high hyperprogression in patients undergoing immunotherapy54 and thus show promise as noninvasive biomarkers for predicting adverse events.
Tumor microenvironment
The tumor microenvironment (TME) is known to promote the growth and metastasis of lung tumors and has recently gained recognition as an important factor in understanding tumor behavior and response to immunotherapy96. Here, we will discuss the role of radiomics and DL in elucidating TME characteristics related to treatment outcomes.
Peritumoral radiomics—the use of radiomic techniques to assess heterogeneity of the peritumoral environment—has been investigated in a wide variety of cancers, in which the inclusion of the peritumoral region increases the predictive power of radiomic signatures compared to intratumoral signatures alone in a variety of cancers (see ref. 16 for review). Features of the TME have been specifically shown to have significant predictive and prognostic value in lung cancer68,94. For example, radiomic features of the tumor rim (i.e., 3 mm outside the tumor border) have been shown to be predictive of distant metastases in NSCLC, where the combination of clinical data and rim signatures was the most effective for stratifying patients97. Likewise, Hosny et al.60 used DL to create prognostic signatures of quantitative imaging features and found that the tumor–stroma interfaces exhibited the largest contributions to the prognostic signature, highlighting the importance of the TME in patient stratification. Furthermore, the authors created activation heatmaps overlaid on CT images to visualize the “importance” of each node or voxel relative to the final prediction, both within and beyond the tumor. A subsequent analysis that disregarded the data beyond the tumor resulted in a substantial drop in prognostic power, thereby confirming the importance of textural features in the tumor-surrounding region. A radiomics model developed by Tang et al.98 characterized the immune state of the TME in NSCLC patients using baseline CT images, percent tumor PD-L1 expression, and the density of tumor-infiltrating lymphocytes (CD3) to stratify patients into four clusters that were significantly correlated with overall survival (OS)82. The most favorable outcome group was characterized by low CT intensity and high heterogeneity, low PDL1, and high CD3 infiltration, suggesting a high immune-activated state. Finally, the infiltration of CD4 and CD8 T cells is also known to be an important mediator of responses to immune checkpoint inhibition96 and could make effective targets as biomarkers.
Radiogenomics
Radiogenomics is the study of the connections between SOC radiographic images and tumor genomics. While tumor molecular profiling is becoming SOC for NSCLC and provides a vast amount of information for personalized treatment, traditional molecular biomarker analyses often fail to capture the full picture of spatial and temporal intratumoural heterogeneity. Handcrafted and deep radiomics can bridge the gap between imaging phenotypes and tumor genomics to identify noninvasive, image-based genetic biomarkers to elucidate the underlying mechanisms of resistance and response to therapy and ultimately improve patient care.
Tumor genotype plays an important role in personalized treatment for lung cancer patients, where mutations in common proto-oncogenes and oncogenes such as EGFR, ALK, ROS1, and RET have been associated with radiomic signatures see 11 for review. In particular, EGFR has been widely investigated as a noninvasive biomarker using both handcrafted50,53 and deep radiomic methods52,54,59. Aerts et al.10 found that radiomic data were strongly prognostic and were associated with underlying patterns of gene expression in a lung cancer dataset using a gene-set enrichment analysis (GSEA) of 21,766 genes. Notably, features III and IV of the four-feature radiomic signature were strongly correlated with cell cycling pathways. Grossman et al.55 linked numerous imaging features based on intratumoural heterogeneity to RNA polymerase expression, the autodegration pathway E3 ubiquitin ligase COP1, p53, cell cycle regulation checkpoints, TGF-β signaling, mitochondrial pathways, lipoprotein metabolism, TRAF6-mediated NFkB activation, and axon guidance. Furthermore, several imaging features were linked to EGFR, KRAS, and TP53 mutants, as confirmed by Sanger sequencing. Another study used a 3D-CNN and transfer learning approach to identify both prognostic signatures using NSCLC CT images and correlations between the radiographic phenotypes quantified by CNN and global gene expression patterns using a pre-ranked GSEA60. Similar to other radiogenomic analyses in NSCLC, the authors found that the most significantly enriched pathways were linked to the cell cycle and transcriptional processes.
Predicting brain metastases
Lung cancer is one of the most common primary tumors leading to brain metastases (BrMs), accounting for more than 50% of all brain tumors99. The presence of BrMs plays a major role in guiding treatment, as brain-penetrating therapies are generally necessary in combination with immunotherapy to improve sensitivity to treatment. Radiomics has been used extensively to predict the local response for BrMs after stereotactic radiosurgery100, differentiate between BrMs and glioblastoma101, predict the primary tumor of origin102, assess the diagnostic ability of BrMs to predict EGFR mutation status in primary lung cancer BrMs103, and predict survival104. While the risk of BrMs in NSCLC patients has been predicted using a nonradiomic nomogram105 and based on total lesion glycolysis and metabolic tumor volume106, very few studies have used radiomics or DL to predict the development of brain metastases. To the best of our knowledge, no studies have leveraged DL to this end.
One study found that a radiomics score—based on seven potential predictors of BrMs in curatively resected locally advanced NSCLC patients—was significantly associated with BrMs in both the training and validation cohorts, in which combining clinical risk factors and radiomics data improved performance107. Additionally, patient smoking status and histology were both independent predictors of BrMs. In contrast, another study found that CT-based radiomics features of primary NSCLC did not improve a model based on clinical data108. Nonradiomic prognostic biomarkers of BrMs have been developed by characterizing the functional gene expression signatures of lung tumor tissue, BrMs, and their respective TMEs, indicating that the immune and fibrosis status of BrMs should guide therapeutic strategies109. The TMEs of both lung tumors and BrMs were enriched for genes associated with cancer-associated fibroblasts and the extracellular matrix and endothelium, suggesting that fibrosis and angiogenesis play a key role in tumor progression and metastasis. The expression of tumor proliferation genes was higher in the primary tumor. The authors also noted significantly higher expression of epithelial to mesenchymal transition (EMT) genes (i.e., laminins, integrins, and inflammatory and neutrophil-acting chemokines) in both tumors than in the TMEs, as EMT is thought to be the most common mechanism for metastasis110. Assessing differentially expressed genes from lung tumor cores between slow and fast metastatic cohorts revealed a metastatic signature gene set that also individually predicted survival in both the study cohort and a lung adenocarcinoma cohort (n = 501). These molecular biomarkers could potentially be incorporated into radiomics and deep learning models to predict BrMs in lung cancer patients.
Future challenges and implementation
While new technologies such as radiomics and DL have substantially advanced the field of personalized immunotherapy treatment in lung cancer, integrating these technologies into clinical workflows will presumably be an uphill climb. Several challenges will need to be addressed before we can create a roadmap to clinical application.
While numerous studies have improved the quality and interpretation of radiomics studies3, have worked to standardize radiomic features37, and have devised methods of moderating ‘center effect’ to reduce multi-center variability111,112, there are still multiple points in the handcrafted radiomics pipeline where errors and variability can be introduced37. While DL methods do mitigate issues with handcrafted radiomics—such as time-consuming manual feature selection and inter-observer and intra-observer variability—by minimizing operator input31,113, working with DL algorithms is a specialized skill and may not be readily available to radiologists and clinicians. To this end, public dataset repositories such as RIDER10,60 and The Cancer Imaging Archive114 can be leveraged to validate the performance of radiomic signatures, while open-source toolkits—such as PyRadiomics115, RaCaT116, and ImaGene117—allow researchers to use radiomics without having to develop feature pipelines from scratch16,118. Additionally, “how to” guides for implementing radiomics in medical imaging118,119 that include tools for both handcrafted and deep radiomics provide a valuable roadmap for those starting out. The use of AI-based tools such as I3Lung—which uses biological, molecular, radiological, and clinical data from more than 2000 NSCLC patients to predict individual responses to immunotherapy—could be a straightforward and cost-effective alternative to developing de novo DL models120. Lastly, using a transfer learning paradigm on pretrained DL models trained on large datasets makes DL more accessible while mitigating the need for large datasets in clinical workflows (see Supplemental Material for more detail).
Koçak et al.118 outlined three-tiered suggestions for radiologists interested in using radiomics: (i) paid software programs, (ii) free programs [see paper for suggestions] that allow radiomic feature extraction using a graphical user interface, or (iii) the development of coding skills necessary to use MATLAB or Python platforms. To become involved in AI, the authors offer a similar three-tiered approach: (i) become part of a data science collaboration, (ii) acquire statistical skills necessary to perform AI tasks without code, and (iii) learn coding language such as Python. Bera et al.16 Note that the pathway to regulatory approval is the main roadblock to adopting AI-based predictive and prognostic tools into clinical workflows. Furthermore, the authors state that while billing and reimbursement present yet another challenge, pursuing a regulatory pathway for lab-based diagnostic tests could be a viable option. It is also possible that efforts to unpack the “black box” of AI will increase transparency and explainability on the road to approval (see Supplemental Material for more detail).
No two cancers are alike. Deep radiomics holds the promise of predicting personalized responses to immunotherapy in lung cancer patients using SOC images, thereby expediting treatment and tailoring treatment to individuals. Although the road to adoption could be long, precision oncology will undoubtedly benefit from more objective and accurate characterizations and predictions of disease, ultimately serving to improve patient outcomes.
Data availability
All data is available by contacting the corresponding author LCR.
References
Ettinger, D. S. et al. NCCN guidelines® insights: non–small cell lung cancer, version 2.2023: featured updates to the NCCN guidelines. J. Natl Compr. Cancer Netw. 21, 340–350 (2023).
Overman, M. J. et al. Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J. Clin. Oncol. 31, 17 (2013).
Lambin, P. et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762 (2017).
Bechara, R. et al. Practice and complications of flexible bronchoscopy with biopsy procedures. J. Bronchol. 12, 139–142 (2005).
Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017).
Laufer-Geva, S. et al. The clinical impact of comprehensive genomic testing of circulating cell-free DNA in advanced lung cancer. J. Thorac. Oncol. 13, 1705–1716 (2018).
Rolfo, C. et al. Liquid biopsy for advanced NSCLC: a consensus statement from the international association for the study of lung cancer. J. Thorac. Oncol. 16, 1647–1662 (2021).
Aggarwal, C. et al. Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non–small cell lung cancer. JAMA Oncol. 5, 173–180 (2019).
Yip, S. S. & Aerts, H. J. Applications and limitations of radiomics. Phys. Med. Biol. 61, R150 (2016).
Aerts, H. J. W. L. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 5, 4006 (2014).
Avanzo, M., Stancanello, J., Pirrone, G. & Sartor, G. Radiomics and deep learning in lung cancer. Strahlenther. Onkol. 196, 879–887 (2020).
Hosny, A., Aerts, H. J. & Mak, R. H. Handcrafted versus deep learning radiomics for prediction of cancer therapy response. Lancet Digit Health 1, e106–e107 (2019).
Hosny, A., Parmar, C., Quackenbush, J., Schwartz, L. H. & Aerts, H. J. W. L. Artificial intelligence in radiology. Nat. Rev. Cancer 18, 500–510 (2018).
Bettinelli, A. et al. A novel benchmarking approach to assess the agreement among radiomic tools. Radiology 303, 533–541 (2022).
Primakov, S. P. et al. Automated detection and segmentation of non-small cell lung cancer computed tomography images. Nat. Commun. 13, 1–12 (2022).
Bera, K., Braman, N., Gupta, A., Velcheti, V. & Madabhushi, A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 19, 132–146 (2022).
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: images are more than pictures, they are data. Radiology 278, 563 (2016).
Prager, B. C., Xie, Q., Bao, S. & Rich, J. N. Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell 24, 41–53 (2019).
Janiszewska, M. The microcosmos of intratumor heterogeneity: the space-time of cancer evolution. Oncogene 39, 2031–2039 (2019).
Goveia, J. et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell 37, 21–36.e13 (2020).
Lugano, R., Ramachandran, M. & Dimberg, A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 77, 1745–1770 (2020).
Marusyk, A., Janiszewska, M. & Polyak, K. Intratumor heterogeneity: the rosetta stone of therapy resistance. Cancer Cell 37, 471–484 (2020).
Junttila, M. R. & De Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Kleppe, M. & Levine, R. L. Tumor heterogeneity confounds and illuminates: assessing the implications. Nat. Med. 20, 342–344 (2014).
Marusyk, A. & Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta (BBA) - Rev. Cancer 1805, 105–117 (2010).
Wu, D. et al. Roles of tumor heterogeneity in the development of drug resistance: a call for precision therapy. Semin. Cancer Biol. 42, 13–19 (2017).
Davnall, F. et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3, 573–589 (2012).
Rogers, W. et al. Radiomics: from qualitative to quantitative imaging. Br. J. Radiol. 93, 20190948 (2020).
Liu, Z. et al. The applications of radiomics in precision diagnosis and treatment of oncology: opportunities and challenges. Theranostics 9, 1303–1322 (2019).
Kumar, V. et al. Radiomics: the process and the challenges. Magn. Reson Imaging 30, 1234–1248 (2012).
Lambin, P. et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 48, 441–446 (2012).
Pavic, M. et al. FDG PET versus CT radiomics to predict outcome in malignant pleural mesothelioma patients. EJNMMI Res. 10, 1–8 (2020).
Liu, Y. et al. Imaging biomarkers to predict and evaluate the effectiveness of immunotherapy in advanced non-small-cell lung cancer. Front. Oncol. 11, 773 (2021).
Conti, A., Duggento, A., Indovina, I., Guerrisi, M. & Toschi, N. Radiomics in breast cancer classification and prediction. Semin. Cancer Biol. 72, 238–250 (2021).
Park, J. E. et al. Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur. Radiol. 30, 523–536 (2020).
Zwanenburg, A. et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295, 328–338 (2020).
Ibrahim, A. et al. Radiomics for precision medicine: current challenges, future prospects, and the proposal of a new framework. Methods 188, 20–29 (2021).
Litjens, G. et al. A survey on deep learning in medical image analysis. Med Image Anal. 42, 60–88 (2017).
Shen, D., Wu, G. & Suk, H. I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 19, 221 (2017).
Dercle, L. et al. Early readout on overall survival of patients with melanoma treated with immunotherapy using a novel imaging analysis. JAMA Oncol. 8, 385–392 (2022).
Coroller, T. P. et al. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother. Oncol. 114, 345–350 (2015).
Zhou, H. et al. Diagnosis of distant metastasis of lung cancer: based on clinical and radiomic features. Transl. Oncol. 11, 31–36 (2018).
Wu, J. et al. Early-stage non–small cell lung cancer: quantitative imaging characteristics of 18F fluorodeoxyglucose PET/CT allow prediction of distant metastasis. Radiology 281, 270 (2016).
Liu, Y. et al. Radiological image traits predictive of cancer status in pulmonary nodules. Clin. Cancer Res. 23, 1442–1449 (2017).
Hawkins, S. et al. Predicting malignant nodules from screening CT scans. J. Thorac. Oncol. 11, 2120–2128 (2016).
Wu, W. et al. Exploratory study to identify radiomics classifiers for lung cancer histology. Front. Oncol. 6, 71 (2016).
Coroller, T. P. et al. Radiomic-based pathological response prediction from primary tumors and lymph nodes in NSCLC. J. Thorac. Oncol. 12, 467–476 (2017).
Huynh, E. et al. Associations of radiomic data extracted from static and respiratory-gated CT scans with disease recurrence in lung cancer patients treated with SBRT. PLoS ONE 12, e0169172 (2017).
Liu, Y. et al. Radiomic features are associated with EGFR mutation status in lung adenocarcinomas. Clin. Lung Cancer 17, 441–448.e6 (2016).
Wang, S. et al. Predicting EGFR mutation status in lung adenocarcinoma on computed tomography image using deep learning. Eur. Respir. J. 53, 1800986 (2019).
Yu, D. et al. Convolutional neural networks for predicting molecular profiles of non-small cell lung cancer. Proceedings - International Symposium on Biomedical Imaging 569–572 https://doi.org/10.1109/ISBI.2017.7950585 (2017).
Rios Velazquez, E. et al. Somatic mutations drive distinct imaging phenotypes in lung cancer. Cancer Res. 77, 3922–3930 (2017).
Mu, W. et al. Non-invasive decision support for NSCLC treatment using PET/CT radiomics. Nat. Commun. 11, 5228 (2020).
Grossmann, P. et al. Defining the biological basis of radiomic phenotypes in lung cancer. Elife 6, e23421 (2017).
Mu, W. et al. Radiomics of 18F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur. J. Nucl. Med Mol. Imaging 47, 1168–1182 (2020).
Hawkins, S. H. et al. Predicting outcomes of nonsmall cell lung cancer using CT image features. IEEE Access 2, 1418–1426 (2014).
Parmar, C., Grossmann, P., Bussink, J., Lambin, P. & Aerts, H. J. W. L. Machine learning methods for quantitative radiomic biomarkers. Sci. Rep. 5, 1–11 (2015).
Wang, S. et al. Unsupervised Deep Learning Features for Lung Cancer Overall Survival Analysis. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2018-July, 2583–2586 (2018).
Hosny, A. et al. Deep learning for lung cancer prognostication: a retrospective multi-cohort radiomics study. PLoS Med. 15, e1002711 (2018).
Cook, G. J. R. et al. Are pretreatment 18F-FDG PET tumor textural features in non–small cell lung cancer associated with response and survival after chemoradiotherapy? J. Nucl. Med. 54, 19–26 (2013).
Wu, M. et al. A combined-radiomics approach of CT images to predict response to anti-PD-1 immunotherapy in NSCLC: a retrospective multicenter study. Front. Oncol. 11, 688679 (2022).
Song, J. et al. A new approach to predict progression-free survival in stage IV EGFR-mutant NSCLC patients with EGFR-TKI therapy. Clin. Cancer Res. 24, 3583–3592 (2018).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Grigg, C. & Rizvi, N. A. PD-L1 biomarker testing for non-small cell lung cancer: Truth or fiction? J. Immunother. Cancer 4, 1–10 (2016).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 541, 321–330 (2017).
Fave, X. et al. Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancer. Sci. Rep. 7, 1–11 (2017).
Khorrami, M. et al. Changes in CT radiomic features associated with lymphocyte distribution predict overall survival and response to immunotherapy in non–small cell lung cancer. Cancer Immunol. Res. 8, 108–119 (2020).
Sun, R. et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 19, 1180–1191 (2018).
Trebeschi, S. et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann. Oncol. 30, 998–1004 (2019).
Tunali, I. et al. Hypoxia-related radiomics and immunotherapy response: a multicohort study of non-small cell lung cancer. JNCI Cancer Spectr. 5, pkab048 (2021).
Nardone, V. et al. Radiomics predicts survival of patients with advanced non-small cell lung cancer undergoing PD-1 blockade using Nivolumab. Oncol. Lett. 19, 1559–1566 (2020).
He, B. et al. Predicting response to immunotherapy in advanced non-small-cell lung cancer using tumor mutational burden radiomic biomarker. J. Immunother. Cancer 8, 550 (2020).
Gainor, J. F. et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin. Cancer Res. 22, 4585–4593 (2016).
Rosell, R. & Karachaliou, N. Optimizing lung cancer treatment approaches. Nat. Rev. Clin. Oncol. 12, 75–76 (2014).
Huang, M. Y., Jiang, X. M., Wang, B. L., Sun, Y. & Lu, J. J. Combination therapy with PD-1/PD-L1 blockade in non-small cell lung cancer: strategies and mechanisms. Pharmacol. Ther. 219, 107694 (2021).
Sun, R. et al. Radiomics to predict outcomes and abscopal response of patients with cancer treated with immunotherapy combined with radiotherapy using a validated signature of CD8 cells. J. Immunother. Cancer 8, e001429 (2020).
Jazieh, K. et al. Original research: Novel imaging biomarkers predict outcomes in stage III unresectable non-small cell lung cancer treated with chemoradiation and durvalumab. J. Immunother. Cancer 10, 3778 (2022).
Boyero, L. et al. Primary and acquired resistance to immunotherapy in lung cancer: unveiling the mechanisms underlying of immune checkpoint blockade therapy. Cancers (Basel) 12, 1–36 (2020).
Topalian, S. L. et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer treated with nivolumab. JAMA Oncol. 5, 1411–1420 (2019).
Boutsikou, E. et al. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther. Adv. Med Oncol. 10, 1758835918768238 (2018).
Ren, D. et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol. Cancer 19, 1–19 (2020).
Gettinger, S. et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 7, 1420–1435 (2017).
Sade-Feldman, M. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1–11 (2017).
Hou, H., Sun, D. & Zhang, X. The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int. 19, 1–8 (2019).
Madeddu, C. et al. EGFR-mutated non-small cell lung cancer and resistance to immunotherapy: role of the tumor microenvironment. Int. J. Mol. Sci. 23, 6489 (2022).
Kopecka, J. et al. Hypoxia as a driver of resistance to immunotherapy. Drug Resist. Updat. 59, 100787 (2021).
Gevaert, O. et al. Non-small cell lung cancer: identifying prognostic imaging biomarkers by leveraging public gene expression microarray data - Methods and preliminary results. Radiology 264, 387–396 (2012).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568 (2014).
Colen, R. R. et al. Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Investig. N. Drugs 36, 601–607 (2018).
Tohidinezhad, F. et al. Computed tomography-based radiomics for the differential diagnosis of pneumonitis in stage IV non-small cell lung cancer patients treated with immune checkpoint inhibitors. Eur. J. Cancer 183, 142–151 (2023).
Mu, W., Tunali, I., Qi, J., Schabath, M. B. & Gillies, R. J. Radiomics of 18F fluorodeoxyglucose PET/CT images predicts severe immune-related adverse events in patients with NSCLC. Radio. Artif. Intell. 2, e190063 (2020).
Shur, J. D. et al. Radiomics in oncology: a practical guide. Radiographics 41, 1717–1732 (2021).
Vaidya, P. et al. Novel, non-invasive imaging approach to identify patients with advanced non-small cell lung cancer at risk of hyperprogressive disease with immune checkpoint blockade. J. Immunother. Cancer 8, 1343 (2020).
Tunali, I. et al. Novel clinical and radiomic predictors of rapid disease progression phenotypes among lung cancer patients treated with immunotherapy: an early report. Lung Cancer 129, 75–79 (2019).
Altorki, N. K. et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer 19, 9–31 (2018).
Dou, T. H., Coroller, T. P., van Griethuysen, J. J. M., Mak, R. H. & Aerts, H. J. W. L. Peritumoral radiomics features predict distant metastasis in locally advanced NSCLC. PLoS ONE 13, e0206108 (2018).
Tang, C. et al. Development of an immune-pathology informed radiomics model for non-small cell lung cancer. Sci. Rep. 8, 1–9 (2018).
Nayak, L., Lee, E. Q. & Wen, P. Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 14, 48–54 (2012).
Mouraviev, A. et al. Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro Oncol. 22, 797–805 (2020).
Bijari, S., Jahanbakhshi, A., Hajishafiezahramini, P. & Abdolmaleki, P. Differentiating glioblastoma multiforme from brain metastases using multidimensional radiomics features derived from MRI and multiple machine learning models. Biomed. Res. Int. 2022, 1–10 (2022).
Lohmann, P. et al. PET/MRI radiomics in patients with brain metastases. Front. Neurol. 11, 1 (2020).
Ahn, S. J. et al. Contrast-enhanced T1-weighted image radiomics of brain metastases may predict EGFR mutation status in primary lung cancer. Sci. Rep. 10, 1–9 (2020).
Chen, B. T. et al. Predicting survival duration with MRI radiomics of brain metastases from non-small cell lung cancer. Front Oncol. 11, 520 (2021).
Won, Y. W. et al. A nomogram to predict brain metastasis as the first relapse in curatively resected non-small cell lung cancer patients. Lung Cancer 88, 201–207 (2015).
Shang, J. et al. Predictive value of baseline metabolic tumor burden on 18F-FDG PET/CT for brain metastases in patients with locally advanced non-small-cell lung cancer. Front Oncol. 12, 1029684 (2022).
Sun, F., Chen, Y., Chen, X., Sun, X. & Xing, L. CT-based radiomics for predicting brain metastases as the first failure in patients with curatively resected locally advanced non-small cell lung cancer. Eur. J. Radio. 134, 109411 (2021).
Keek, S. A. et al. Investigation of the added value of CT-based radiomics in predicting the development of brain metastases in patients with radically treated stage III NSCLC. Ther. Adv. Med Oncol. 14, 1–18 (2022).
Zhang, Q. et al. The spatial transcriptomic landscape of non-small cell lung cancer brain metastasis. Nat. Commun. 13, 1–19 (2022).
Tyler, M. & Tirosh, I. Decoupling epithelial-mesenchymal transitions from stromal profiles by integrative expression analysis. Nat. Commun. 12, 1–13 (2021).
Da-ano, R. et al. Performance comparison of modified ComBat for harmonization of radiomic features for multicenter studies. Sci. Rep. 10, 1–12 (2020).
Farwell, M. D. & Mankoff, D. A. Analysis of routine computed tomographic scans with radiomics and machine learning: one step closer to clinical practice. JAMA Oncol. 8, 393–394 (2022).
Kumar, D. et al. Discovery radiomics for pathologically-proven computed tomography lung cancer prediction. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 10317 LNCS, 54–62 (2017).
Vaidya, P. et al. CT derived radiomic score for predicting the added benefit of adjuvant chemotherapy following surgery in stage I, II resectable non-small cell lung cancer: a retrospective multicohort study for outcome prediction. Lancet Digit. Health 2, e116–e128 (2020).
Van Griethuysen, J. J. M. et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 77, e104–e107 (2017).
Pfaehler, E., Zwanenburg, A., de Jong, J. R. & Boellaard, R. RaCaT: an open source and easy to use radiomics calculator tool. PLoS ONE 14, e0212223 (2019).
Sukhadia, S. S. et al. ImaGene: a web-based software platform for tumor radiogenomic evaluation and reporting. Bioinform. Adv. 2, vbac079 (2022).
Koçak, B., Durmaz, E. Ş., Ateş, E. & Kılıçkesmez, Ö. Radiomics with artificial intelligence: a practical guide for beginners. Diagn. Interv. Radiol. 25, 485 (2019).
van Timmeren, J. E., Cester, D., Tanadini-Lang, S., Alkadhi, H. & Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 11, 1–16 (2020).
Prelaj, A. et al. The EU-funded I3LUNG Project: integrative science, intelligent data platform for individualized LUNG cancer care with immunotherapy. Clin. Lung Cancer 24, 381–387 (2023).
Acknowledgements
This study was supported by the award of the European Commission | Horizon 2020 Framework Program (EU Framework Program for Research and Innovation H2020), 101057695. The authors would like to thank Dr. Jennifer Head for her contribution to the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. Conception and design of the study by L.C.R., co-first authors L.C.R. and W.K., all authors read and approved the final manuscript: L.C.R., W.K., A.A., V.F., M.S., R.S., L.K., G.R., I.F., N.P. and N.B.
Corresponding authors
Ethics declarations
Competing interests
N.P. has received honoraria and worked as advisor and researcher with AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Foundation Medicine, Guardant360, Imagene, Merck, MSD, Novartis, Novocure, Pfizer, Roche, Rhenium and Takeda. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roisman, L.C., Kian, W., Anoze, A. et al. Radiological artificial intelligence - predicting personalized immunotherapy outcomes in lung cancer. npj Precis. Onc. 7, 125 (2023). https://doi.org/10.1038/s41698-023-00473-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-023-00473-x