Abstract
Placental malaria vaccines (PMVs) are being developed to prevent severe sequelae of placental malaria (PM) in pregnant women and their offspring. The leading candidate vaccine antigen VAR2CSA mediates parasite binding to placental receptor chondroitin sulfate A (CSA). Despite promising results in small animal studies, recent human trials of the first two PMV candidates (PAMVAC and PRIMVAC) generated limited cross-reactivity and cross-inhibitory activity to heterologous parasites. Here we immunized Aotus nancymaae monkeys with three PMV candidates (PAMVAC, PRIMVAC and ID1-ID2a_M1010) adjuvanted with Alhydrogel, and exploited the model to investigate boosting of functional vaccine responses during PM episodes as well as with nanoparticle antigens. PMV candidates induced high levels of antigen-specific IgG with significant cross-reactivity across PMV antigens by enzyme-linked immunosorbent assay. Conversely, PMV antibodies recognized native VAR2CSA and blocked CSA adhesion of only homologous parasites and not of heterologous parasites. PM episodes did not significantly boost VAR2CSA antibody levels or serum functional activity; nanoparticle and monomer antigens alike boosted serum reactivity but not functional activities. Overall, PMV candidates induced functional antibodies with limited heterologous activity in Aotus monkeys, similar to responses reported in humans. The Aotus model appears suitable for preclinical downselection of PMV candidates and assessment of antibody boosting by PM episodes.
Similar content being viewed by others
Main
Pregnant women living in malaria-endemic areas become more susceptible to Plasmodium infection despite pre-existing immunity to malaria acquired during childhood1. Plasmodium falciparum malaria during pregnancy leads to placental malaria (PM), which is characterized by the sequestration of chondroitin sulfate A (CSA)-binding P. falciparum-infected erythrocytes (IE) in the placenta and is associated with adverse pregnancy outcomes2,3,4. Susceptibility to PM is more pronounced in first-time mothers and decreases over successive pregnancies, as women acquire functional anti-adhesion antibodies against CSA-binding IE5,6. Indeed, multigravid women who are less susceptible to PM acquire antibodies with broadly neutralizing activity against placenta-binding IE6,7,8. The anti-adhesion activity of antibodies in pregnant women has been associated with improved pregnancy outcomes9,10. These observations suggest that resistance to PM can be conferred to pregnant women through vaccination by inducing protective antibodies targeting the surface of placental IE6.
The placenta-sequestering P. falciparum-IE surface displays VAR2CSA, a member of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family and the main parasite ligand mediating IE adhesion to the key placental receptor CSA11,12,13,14. The large size of VAR2CSA stymies efforts to manufacture full-length recombinant protein, and studies of VAR2CSA domains or fragments that bind CSA have informed the design of constructs that induce anti-adhesion antibodies in small animals (mouse, rat and rabbit)15,16,17,18,19,20,21,22,23. Among these VAR2CSA constructs, PAMVAC and PRIMVAC, based on partially overlapping N-terminal fragments of VAR2CSA, have recently been tested in phase I clinical trials in Europe and Africa (ClinicalTrials.gov, NCT02647489 and NCT02658253). In rodents, PAMVAC and PRIMVAC induced cross-reactive binding-inhibitory antisera with activity similar to that detected in PM-resistant multigravid women16,21,24.
First-in-human reports from clinical trials demonstrated that both PAMVAC and PRIMVAC vaccines were safe, well tolerated and immunogenic and induced functional antibodies in malaria-naive and malaria-exposed women25,26. However, the level of anti-adhesion activity of antibodies induced in volunteers appeared lower compared to that found when PAMVAC and PRIMVAC were tested in rodents with the same adjuvants16,21,24,25,26, with limited cross-strain activity, suggesting that these VAR2CSA-based vaccines could be improved or combined to generate broader protection against PM. These observations also highlight the importance of relevant animal models that could better predict human immune responses to VAR2CSA antigens.
In PM-resistant multigravid women, other measures of antibody activity (including opsonizing and phagocytosis activity) have been linked to protection27,28. A recent study by Aitken et al. used a multivariate prediction model to identify antibody features associated with protection against PM in pregnant women29, highlighting IgG3 binding to IE that may inhibit placental sequestration and promote parasite clearance by antibody-dependent phagocytosis, while neutrophil and monocyte phagocytosis of IE were linked to parasite clearance. Antibody avidity to VAR2CSA has also been reported as another feature associated with reduced risk of placenta malaria infection30 and increased birthweight31. However, the antibody effector mechanism that primarily confers protection against PM remains unclear.
In this Article, we evaluate a nonhuman primate (NHP) model for assessing VAR2CSA-based placental malaria vaccines (PMVs). Many studies have indicated the Aotus nancymaae monkey model to be useful for supporting malaria vaccine development, since it is susceptible to P. falciparum infection, and shows antibody profiles similar to humans following a malaria infection32,33,34. Furthermore, we have recently established an Aotus model for PM that recapitulates key features of malaria infection and immunity in pregnant women, including placental sequestration, selective binding to CSA by placental parasites, and the acquisition of heterologous functional antibodies over successive pregnancies35. In the current work, we used the immunogenicity assays described in the human trial reports25,26 as a rationale for initial testing of PMV-induced antibodies in Aotus. Overall, this study provides evidence that PMV immunogenicity in the new Aotus model is similar to that of human responses.
Results
Local and systemic reactogenicity
Overall, vaccines were well tolerated by the Aotus monkeys. No death occurred during the vaccination period. Fifteen out of 40 monkeys showed some muscle firmness at the injection site after vaccination with no edema, redness or warmth. Hardened areas of the muscle were measured using a caliper to determine the time at which the reaction dissipated from the animals. This muscle induration was observed across all vaccination groups, including the Pfs25 control group, and resolved within 7 days post-vaccination. No sign of systemic reaction or change in behavior was observed. Detailed description of clinical observations post-immunization is presented as Supplementary Data File 1.
PMV-induced antibodies in Aotus exhibit strong homologous activity
Forty naive female Aotus monkeys received three immunizations of PAMVAC (n = 9), PRIMVAC (n = 9), ID1-ID2a_M1010 (n = 9) and Pfs25 (n = 13) at 4-week intervals (Fig. 1 and Supplementary Fig. 1). Sera were collected 2 weeks after the last vaccination (D70) from all immunized monkeys to study the immunogenicity of PMV candidates. The samples were tested by enzyme-linked immunosorbent assay (ELISA) for vaccine-specific antibody reactivity and all antisera showed IgG titers against their corresponding antigen (Fig. 2a). Antibodies from Pfs25-immunized monkeys showed no cross-reactivity to the VAR2CSA-based antigens. Similarly, none of the PMV-induced antibodies reacted to Pfs25 antigen (Fig. 2a). All PMV antisera showed significantly higher cross-reactivity to PMV antigens compared to Pfs25 antisera (Mann–Whitney test, P < 0.001 for all comparisons). Overall, PMV candidates induced a high level of vaccine-specific IgG to homologous antigen with some level of cross-reactivity to heterologous PMV antigens (Fig. 2a and Supplementary Fig. 2). PAMVAC-induced antibodies showed a similar level of heterologous reactivity to PRIMVAC and ID1-ID2a_M1010 antigens (Supplementary Fig. 2), whereas antibodies from PRIMVAC and ID1-ID2a_M1010 immunizations respectively had higher heterologous reactivity to ID1-ID2a_M1010 and PRIMVAC antigens than to PAMVAC (Wilcoxon matched-pairs signed-rank test, P = 0.02 for PRIMVAC-IgG and P = 0.004 for ID1-ID2a_M1010-IgG).
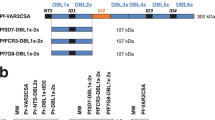
a, Illustration of the three PMV constructs of VAR2CSA used in this study. Boundaries of each construct are shown as amino acid residues from the corresponding VAR2CSA allele (FCR3 and 3D7) and M1010 composite sequence. b, Flow chart of the study, highlighting the different stages of the study and the number of animals involved in each stage. Timeline indicates three vaccinations (Vac1, Vac2 and Vac3) with PMV and Pfs25 antigens. ATS, acid terminal sequence; cVLP, capsid-based virus like particle; EPA, mutagenized Pseudomonas aeruginosa exoprotein A; TM, transmembrane.
a, The reactivity of vaccine-induced IgG 2 weeks after the last vaccination dose was assessed against PMV candidates PAMVAC (Pf-FCR3) (n = 9) and PRIMVAC (Pf-3D7) (n = 9) as well as recombinant antigens rID1-ID2a-M1010 (produced from the ID1-ID2a construct of VAR2CSAM1010 sequence) (n = 9) and rPfs25 (n = 13). b,c, Functional characterization of antibodies was evaluated for the ability of IgG to bind antigens expressed on the surface of the IEs by flow cytometry analysis (b), and for CSA-binding inhibition activity against different P. falciparum isolates (c). For each group of vaccinated monkeys, the geometric mean and 95% confidence interval of the antibody activity measured by ELISA and flow cytometry are shown (a and b). For BIA, the mean and s.e.m. are shown (c). Mann–Whitney test was used to compare activity of PMV-induced antibody to that of Pfs25 with P values indicated as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.005 and ****P ≤ 0.001.
CS2 (which carries the same VAR2CSA sequence as FCR3 variant used in PAMVAC) and NF54 (which displays the same VAR2CSA sequence as 3D7 variant used in PRIMVAC) isolates were used to assess the reactivity of these antibodies in plasma to native VAR2CSA expressed on the surface of the IE. For each test sample, the ratio median fluorescence intensity (rMFI) was obtained by normalizing the MFI value of the samples at D70 with the MFI of the pool of pre-bleed (D0) plasma samples. Functional characterization of PMV-induced IgG demonstrated strong ability to recognize the native VAR2CSA expressed by the homologous parasite strain (mean rMFI 2.4 for PAMVAC group against CS2 and mean rMFI 3.4 for PRIMVAC group against NF54) (Fig. 2b). Unlike the cross-reactivity of PMV-induced IgG to different VAR2CSA-based antigens measured by ELISA, no heterologous reactivity of antibodies binding to VAR2CSA-expressing CS2- and NF54-IE was observed in flow cytometry analysis. These findings revealed a weak or absent capacity of PMV-induced antibodies from Aotus to recognize epitopes shared by different variants of native VAR2CSA. No IE surface labeling of the child isolate (C216851) with binding capacity to CD36 (an endothelial receptor linked with cytoadherence of Pf-IE in non-pregnant individuals, reviewed in ref. 1) was observed in plasma from immunized monkeys, and IgG from Pfs25-immunized monkeys showed no reactivity to all three isolates.
This pattern of plasma reactivity to native surface antigen corresponded to no heterologous binding inhibition activity against CS2 and NF54 isolates (Fig. 2c). Plasma from PAMVAC-vaccinated monkeys at 1:5 dilution significantly inhibited CS2 binding to CSA (mean inhibition 21.9%) compared to Pfs25 (mean inhibition 1.2%; Mann–Whitney test, P = 0.0005), PRIMVAC (mean inhibition 1.6 %; Mann–Whitney test, P = 0.003) and ID1-ID2a_M1010 (mean inhibition 2.2%; Mann–Whitney test, P = 0.002) vaccine groups. Conversely, PRIMVAC-induced antibodies significantly blocked NF54 binding to CSA (mean inhibition 42.6%) compared to Pfs25 (mean inhibition 7.8%; Mann–Whitney test, P = 0.007)-, PAMVAC (mean inhibition 4.9%; Mann–Whitney test, P = 0.01)- and ID1-ID2a_M1010 (mean inhibition 10.4%; Mann–Whitney test, P = 0.02)-induced antibodies.
The relationship between PAMVAC- and PRIMVAC-induced antibody levels and functional activity against CS2 and NF54 isolates was analyzed. Overall, in the PAMVAC vaccine group, the level of antibodies binding to PAMVAC antigens measured by ELISA did not correlate with the level of IE-surface recognition and binding inhibition activity against the homologous parasite (CS2) (Fig. 3). In the PRIMVAC group, the level of antibody binding to PRIMVAC antigen strongly correlated with that of IE-surface recognition of NF54 (ρ = 0.92, P = 0.001), but not with the binding inhibition activity (Fig. 3). In addition, the levels of surface reactivity and binding inhibition activity to NF54 were significantly correlated for PRIMVAC-induced antibodies (ρ = 0.74, P = 0.03, Fig. 3). No significant correlation was found when heterologous IE-surface recognition and binding inhibition activities of the vaccine-induced antibodies against both isolates was analyzed (Supplementary Fig. 3).
a–c, Spearman (ρ) coefficients and P values are reported to describe the relationship between ELISA titers of PAMVAC antibodies in Aotus (n = 9) and surface reactivity (a) or binding inhibition activity (b) against CS2 parasite. The correlation between surface reactivity and BIA of PAMVAC antibodies is also shown (c). d–f, The same analyses were performed for PRIMVAC-induced antibodies in Aotus (n = 9). Data for animals immunized with PAMVAC (blue circle) and PRIMVAC (open red square) are shown.
A single acute malaria infection during pregnancy does not boost the activity of PMV-induced antibodies in Aotus
The Aotus PM model offers a unique opportunity to investigate the impact of P. falciparum infection during pregnancy on PMV-specific antibodies. Twenty-three immunized animals became pregnant and were infected with CS2 parasite. For this acute infection, a week-long infection was allowed before cesarean section (C-section) was performed (Fig. 1b).
Antibody reactivity and function were assessed before infection and 4 weeks post-delivery. Because pregnancy occurs sporadically in primates, the timing between the D70 post-vaccination serum sample and the day of CS2 inoculation during pregnancy varied by monkey and ranged from 6 to 584 days; however, there was no statistically significant difference between the groups in the median time to CS2 inoculation (Supplementary Fig. 4). The time interval between the day of CS2 inoculation and 4 weeks postpartum was 35 days for all animals. No response was observed in Pfs25-vaccinated animals (measured by ELISA on PMV antigens, flow cytometry and binding-inhibition assays (BIAs) on CS2 and NF54) when comparing D70 post-vaccination to 4 weeks postpartum measurements (Fig. 4), as might be expected because Pfs25 is not expressed by blood-stage parasites.
a–c, ELISA titers 2 weeks after the last dose in the primary vaccine series (D70) compared to those measured at the time of CS2 parasite inoculation and 4 weeks postpartum, for each of the vaccine groups (n = 6 for PAMVAC-immunized monkeys, n = 5 for PRIMVAC-immunized monkeys, n = 4 for ID1-ID2a_M1010-immunized monkeys and n = 7 for Pfs25-immunized monkeys). d,e, Flow cytometry for IE surface reactivity. f,g, BIA for parasite blocking activity. For each group of vaccinated monkeys, the geometric mean and 95% confidence interval of the antibody activity measured by ELISA and flow cytometry are shown (a–e). For BIA, the mean and s.e.m. are shown (f and g). Wilcoxon matched-pairs signed-rank test was used to evaluate antibody-boosting activity at 4 weeks postpartum following CS2 inoculation during pregnancy.
Overall, no significant decline was observed in the levels of vaccine-induced antibody for each PMV-immunized group between D70 post-vaccination to 4 weeks postpartum (Fig. 4 and Supplementary Figs. 5 and 6) However, a decrease in the levels of vaccine-induced antibody can be seen in most PAMVAC and ID1-ID2a-M1010 immunized monkeys between D70 post-vaccination and the time of CS2 inoculation, although not statistically significant (Supplementary Fig. 5). Following infection during pregnancy, no significant increase above the pre-infection level was observed by IgG ELISA for any PMV group to its corresponding PMV antigen (Fig. 4a–c); a slight nonsignificant increase in ELISA IgG reactivity of PAMVAC antisera to PAMVAC antigen was measured (Wilcoxon matched-pairs signed-rank test, P > 0.99). Similarly, the level of reactivity to NF54 and CS2 IE surface in sera from PRIMVAC- and PAMVAC-immunized monkeys, respectively, did not increase following CS2 infection during pregnancy (Wilcoxon matched-pairs signed-rank test, P = 0.06 for PRIMVAC group against NF54 and P = 0.3 for PAMVAC group against CS2) (Fig. 4d,e).
CSA-binding inhibition activity of antibodies from PMV-vaccinated groups against both homologous and heterologous parasites showed a different trend versus other assays: the level of inhibition from D70 post-vaccination to the day of CS2 inoculation increased (Wilcoxon matched-pairs signed-rank test, P = 0.03 and P = 0.12 for PAMVAC group against CS2 and NF54, respectively; P = 0.25 and P > 0.99 for ID1-ID2a_M1010 group against CS2 and NF54, respectively) or was unchanged (for PRIMVAC and Pfs25 groups) (Fig. 4f,g) Unexpectedly, a non-statistically significant decline of this activity in PAMVAC group against CS2 (Wilcoxon matched-pairs signed-rank test, P = 0.12) was observed at 4 weeks postpartum following CS2 inoculation during pregnancy. Overall, there was no significant change in the activity of PMV-induced antibodies between the D70 post-vaccination and 4 weeks postpartum, a time window ranging from 41 to 1,003 days. Remarkably, all homologous responses (measured by ELISA, flow cytometry and BIA) of PRIMVAC-induced antibodies against PRIMVAC antigen and NF54 parasite appeared stable over time from D70 post-vaccination through 4 weeks postpartum (Fig. 4).
The levels of PAMVAC- and PRIMVAC-induced antibodies binding to the homologous PMV antigen did not significantly correlate with the level of IE-surface recognition and binding inhibition activities against homologous parasites at the day of CS2 inoculation (Supplementary Fig. 7) and 4 weeks postpartum (Supplementary Fig. 8). Similarly, no significant relationship was observed between ELISA cross-reactivity and the heterologous functional activities induced by PAMVAC, PRIMVAC and ID1-ID2a_M1010 against CS2 and NF54 on the day of CS2 inoculation, nor with the background activity induced by Pfs25 control (Supplementary Fig. 9). At 4 weeks postpartum, the level of cross-reactive antibodies binding to PAMVAC and PRIMVAC antigens (generally, optical density (OD) <0.8) respectively correlated to IE-surface reactivity to CS2 (ρ = 0.59, P = 0.03) and NF54 (ρ = 0.64, P = 0.03) (Supplementary Fig. 10), probably because of pregnancy exposure to CS2 infection. However, no relationship was found between the binding inhibition activity and the ELISA cross-reactivity of the antibodies against heterologous antigens and parasites (Supplementary Fig. 10).
PMV can induce a delayed acquisition of cross-inhibitory activity against CS2 and NF54 parasites
To explore strain-transcending activity of PMV-induced antibodies in Aotus, we analyzed the cross-inhibitory and cross-recognition activities of the antibodies against both CS2 and NF54 parasites. These activities were assessed on samples collected at D70 post-vaccination, on the day of CS2 inoculation, and 4 weeks postpartum. When defining cross-inhibitory activity as >50% inhibition of both CS2 and NF54 binding to CSA, no serum samples showed such activity at D70 post-vaccination and 4 weeks postpartum (Fig. 5a,c). Three monkeys (Aotus AO12 and AO10 that received PAMVAC and AO13 that received ID1-ID2a_M1010 vaccine, and were respectively inoculated with CS2 8, 346 and 381 days after D70 post-vaccination samples were collected) had developed cross-inhibitory activity against CS2 and NF54 measured just before CS2 inoculation (Fig. 5b). Curiously, this cross-inhibitory activity waned by 4 weeks postpartum despite exposure to CS2 inoculation (Fig. 5c). Of note, some other monkeys maintained their homologous inhibitory activity from D70 post-vaccination through 4 weeks postpartum (Aotus AO1 from PAMVAC group; AO4 and AO5 from PRIMVAC group), while others showed newly acquired homologous activity at the time of CS2 inoculation that persisted at 4 weeks postpartum (Aotus AO11 and AO8 from PRIMVAC group).
a–c, The levels of binding inhibition activity of the PMV-induced antibodies against CS2 and NF54 parasites in samples collected at D70 post-vaccination (a), pre-CS2 inoculation (b) and 4 weeks postpartum (c) are shown. The dashed lines represent 50% inhibition, and the gray-colored area in each graph defines labeled samples with inhibitory activity. d–f, The recognition levels of surface-expressed VAR2CSA on CS2 and NF54 IE by the PMV-induced antibodies was also analyzed at D70 post-vaccination (d), pre-CS2 inoculation (e) and 4 weeks postpartum (f), with dashed lines representing rMFI 1.2 and the gray-colored area highlighting flow-positive samples. In a and d, n = 9 for PAMVAC-immunized monkeys, n = 9 for PRIMVAC-immunized monkeys, n = 9 for ID1-ID2a_M1010-immunized monkeys and n = 13 for Pfs25-immunized monkeys. In b, c, e and f, n = 6 for PAMVAC-immunized monkeys, n = 5 for PRIMVAC-immunized monkeys, n = 4 for ID1-ID2a_M1010-immunized monkeys and n = 7 for Pfs25-immunized monkeys. Data for animals immunized with PAMVAC (Pf-FCR3) (blue circle), PRIMVAC (Pf-3D7) (open red square), rID1-ID2a-M1010 (green triangle) and rPfs25 (black diamond) are shown and samples with cross-recognition activity are labeled. Spearman (ρ) coefficients and P values are reported for each graph.
Surface recognition of native VAR2CSA expressed on CS2 and NF54 IE followed a pattern different than that observed for the inhibitory activity profile. Limited cross-recognition activity of the PMV-induced antibodies in Aotus was detected at D70 post-vaccination, with only one monkey (AO14 from PAMVAC group) exhibiting such activity (Fig. 5d). By the time of CS2 inoculation, two additional monkeys (AO11 from PRIMVAC group and AO15 from ID1-ID2a_M1010 group) had acquired the cross-recognition activity (Fig. 5e). Following CS2 inoculation, four additional monkeys (AO9 and AO1 from PAMVAC group, AO11 and AO8 from PRIMVAC group, AO17 from Pfs25 group), developed IE surface cross-recognition activity (Fig. 5f). Cross-recognition activity detected on CS2 and NF54 parasites 5 weeks after exposure to CS2 was significantly correlated (ρ = 0.54, P = 0.014, Fig. 5f).
Nanoparticle or monomer PMV similarly boost ELISA reactivity but not function
On study day 999 (cohort 1) and day 853 (cohort 2) (943 and 797 days after completing the primary vaccination series for cohort 1 and 2, respectively), 18 monkeys were immunized with either nanoparticle (PAMVACcVLP or ID1-ID2a_M1010EPA) or monomer forms of PAMVAC or ID1-ID2a_M1010. Before re-immunization (pre-boost), PAMVAC monkeys displayed minimal or modest antibody responses to homologous antigen (Fig. 6a), while PRIMVAC monkeys maintained substantial levels of antibody to homologous antigen (Fig. 6b). Fourteen days after administration, serum IgG binding to PMV antigens by ELISA substantially increased in all groups, with highest reactivity to the antigen used for the primary vaccine series and high cross-reactivity to other antigens (Fig. 6a–c). PMV-induced antibody levels following the primary immunization series (day 70) had waned over time, but the late booster dose increased levels beyond those achieved by primary series (Supplementary Fig. 11). Homologous vaccine boosted reactivity for PAMVAC- and ID1-ID2a_M1010-induced antibodies, and the heterologous vaccine boosted reactivity of PRIMVAC-induced antibodies as well. Indeed, the PAMVAC boost of the PRIMVAC-vaccinated group substantially increased the levels of antibodies against all three PMVs. Of note, the monomer and the conjugated vaccines similarly increased ELISA reactivity at D + 14 and D + 56 post-booster dose (Mann–Whitney test, P > 0.05 for all comparisons).
a–c, ELISA titer of IgG against PAMVAC (Pf-FCR3) (a), PRIMVAC (Pf-3D7) (b) and ID1-ID2a-M1010 (c) antigens were assessed in monkeys that received the conjugated antigens (solid-line, PAMVACcVLP or ID1-ID2a-M1010-EPA) and those who received the monomer antigens (dashed line). Samples collected before vaccination (pre-bleed) and those collected at D14 and D56 post-vaccination were analyzed. d–g, Plasma samples collected at the same time points were also analyzed for their surface reactivity to CS2 (d) and NF54 (e) IEs, as well as their ability to block those parasites binding to CSA (f and g). In a–g, PAMVAC-immunized (n = 4) and PRIMVAC-immunized (n = 3) monkeys received PAMVACcVLP; PAMVAC-immunized (n = 3) and PRIMVAC-immunized (n = 3) monkeys received the PAMVAC, while ID1-ID2a_M1010-immunized received the nanoparticle ID1-ID2a-M1010-EPA (n = 2) or monomer ID1-ID2a_M1010 (n = 3). For each group of vaccinated monkeys, the geometric mean and 95% confidence interval of the antibody activity measured by flow cytometry are shown (d and e). For ELISA and BIA, the mean and s.e.m. are shown (a–c, f and g).
Antibody reactivity to native VAR2CSA expressed by CS2 and NF54 isolates showed no change between pre-bleed and D + 56 time points in groups of monkeys initially vaccinated with PRIMVAC and ID1-ID2a_M1010 for both isolates (Fig. 6d,e), while PAMVAC-vaccinated monkeys showed an increase in reactivity to homologous CS2-IE (Mann–Whitney test, P = 0.05 for PAMVACcVLP boost group and P > 0.05 for PAMVAC boost group, both at D + 14 and D + 56 post-booster dose). The nanoparticles did not significantly increase the plasma binding inhibition activity against CS2 and NF54 at D + 14 for any group (Fig. 6f,g) (Mann–Whitney test, P > 0.05 for PAMVAC, PRIMVAC and ID1-ID2a_M1010 groups against CS2 and NF54), albeit serum activity of PRIMVAC-immunized animals was higher against both parasites after the booster dose, but still modest (less than 40%).
Discussion
Ideally, PMV will be administered to women before their first pregnancy to induce protective antibodies similar to those observed in PM-resistant multigravid women and will be boosted on exposure to infection during pregnancy6,10,36. Several lines of evidence support VAR2CSA as the leading PMV candidate to protect pregnant women from PM-related adverse pregnancy outcomes10,13,15,17,19,37,38,39,40,41. Recently published first-in-human trials of VAR2CSA-based vaccines established that adjuvanted PAMVAC and PRIMVAC are safe and immunogenic in malaria-naive and malaria-exposed women25,26. Although both PAMVAC and PRIMVAC vaccines adjuvanted with Alhydrogel induced high human antibody titers, functional activity was generally modest and limited to homologous variants of the vaccines25,26,42. The present study demonstrates the ability of the A. nancymaae model to predict the human immune response to VAR2CSA-based vaccination.
An NHP model susceptible to P. falciparum placental infection will be useful to study PM pathogenesis and evaluate vaccine activity (reviewed in ref. 43). The establishment of an Aotus model that displays key features of PM pathogenesis, such as placental sequestration and the acquisition of functional antibodies following exposure to placenta-binding parasites35, represents a major advance to support PMV development. The current data demonstrate that Alhydrogel-formulated PAMVAC, PRIMVAC and the ID1-ID2a construct of isolate M1010 were safe in Aotus monkeys and induced high levels of anti-VAR2CSA antibodies against the corresponding vaccine antigen, with some cross-reactivity against heterologous PMV antigens by ELISA. These findings are consistent with previous reports from PAMVAC and PRIMVAC preclinical studies in small animals16,21,24 and human clinical trials25,26, highlighting the safety and immunogenicity of VAR2CSA-based subunit vaccines in both soluble and particle-based forms. Cross-reactivity of PMV-induced antibodies against the vaccine antigens indicates that the induction of antibodies targeting epitopes shared between different recombinant PMV variants is not dependent on the host species.
However, cross-reactivity by ELISA did not correspond to broadly functional antibodies. Contrary to rodent studies of many VAR2CSA candidates including PRIMVAC and PAMVAC15,16,17,18,19,20,21,22,23, VAR2CSA antisera from Aotus poorly reacted to native VAR2CSA expressed on the surface of heterologous parasite strains while reacting strongly to homologous parasites. Similarly, Aotus antisera blocked CSA binding of homologous parasite strains, with up to 66.7% homologous inhibition activity in the PAMVAC group and 75.0% in the PRIMVAC group, but not heterologous parasites. This lack of cross-recognition and cross-inhibition activity substantiates the functional pattern of antibodies induced by both vaccines using the same dosage and adjuvant in women25,26. Although a higher vaccine dose and a more potent adjuvant might yield higher activity, in humans 100 µg of PRIMVAC adjuvanted with Alhydrogel or GLA-SE yielded limited cross-recognition against the FCR3-CSA and 7G8-CSA VAR2CSA-expressing parasites26. Overall, these observations clearly demonstrate that antibody activity induced by PMV antigens in humans aligns better to that induced in Aotus than in small animal models. The overlapping profile of antigens recognized by antibodies from human and Aotus after malaria infection34 could explain the high similarity in VAR2CSA-specific antibody response between these two hosts.
Antibodies to VAR2CSA are thought to be boosted through repeated exposure to placenta-binding parasites over successive pregnancies10,44,45. By exploring whether a malaria infection in pregnant Aotus monkeys immunized with PMV antigens will enhance the functional properties of the induced VAR2CSA-specific antibodies, we showed that a single week-long infection with CS2 did not boost antibody function at 4 weeks postpartum. Similarly, CS2 infection in pregnant monkeys vaccinated with Pfs25 did not induce antibodies to recombinant VAR2CSA or CSA-binding parasites. Since the PfCS2 inocula used for infection were predominantly binding to CD36 receptor, these observations suggest that few IEs had CSA-binding phenotype, and therefore animals might have experienced an infection too short to mount a significant antibody response to VAR2CSA. As most malaria infections in pregnant women are subpatent and asymptomatic46, it is possible that a longer duration of malaria infection may be needed to induce/enhance functional properties of VAR2CSA antibodies. To that end, a chronic model of PM in Aotus is currently under development and will be used to investigate whether chronic malaria infection in pregnant monkeys can induce/enhance functional activity of VAR2CSA antibodies.
Although no significant boost of PMV-induced antibodies occurred after CS2 inoculation during pregnancy, the high level of PRIMVAC-induced homologous functional antibodies persisted from D70 to 4 weeks postpartum in Aotus (up to 619 days), reproducing the observed long-term seroconversion in women after the last PRIMVAC immunization26. This finding indicates that VAR2CSA-specific antibodies induced by vaccination may be durable, and their functional activity could be expected to be sustained across pregnancies. This result is particularly important since the targeted population will be nulligravid women, and first pregnancy could occur long after the vaccination. Naturally acquired human VAR2CSA antibody responses are similarly durable, with antibody half-life estimated to be years in duration45,47.
The antibodies naturally acquired by multigravidae have strain-transcending functional activity against placenta-binding IE6, and VAR2CSA is thought to be the target of these antibodies (reviewed in ref. 48). Overall, in Aotus, the pattern of cross-inhibitory activity (measured by BIA) differed from cross-recognition of native VAR2CSA expressed by CS2 and NF54 (measured by flow cytometry), as many monkeys with IE surface cross-recognition had no cross-inhibitory activity. Transient cross-inhibition activity (for two monkeys in PAMVAC and one monkey in ID1-ID2a_M1010 groups) was detected at the time of CS2 infection, while a number of monkeys acquired cross-recognition activity of antibodies between their post-primary vaccination time point (D70) and 4 weeks postpartum. A shift in the antibody repertoire following CS2 infection might possibly explain the loss of cross-inhibition activity in these monkeys at 4 weeks postpartum. Since the durability of VAR2CSA-specific inhibitory antibodies are unclear, future investigations of the dynamics of inhibitory antibody generation in this Aotus model will be of interest. Because immunological memory specific for VAR2CSA can be maintained for many years without antigen re-exposure49, it is conceivable that affinity maturation yielding antibody with higher avidity may play a key role in the inhibitory and strain-transcending functional activities of VAR2CSA-induced antibodies50,51. Future studies might examine the levels of VAR2CSA-specific memory B cells before and after a P. falciparum infection in pregnancy to explore the dynamic of these cells in relation to PM.
Efforts to optimize malaria vaccines (including PMV) include use of cVLP and EPA nanoparticle platforms to enhance immunogenicity25,52,53. Here, PAMVAC and ID1-ID2a_M1010 nanoparticles generated in cVLP and EPA platforms, respectively, boosted antibody levels measured by ELISA above those seen after the primary vaccine series. However, it appeared that monomeric antigens boosted antibody responses equally well, suggesting this boosted response is not improved by nanoparticle presentation. Notably, heterologous PAMVAC boost of PRIMVAC-vaccinated Aotus monkeys substantially enhanced levels of antibodies against all three PMV antigens. However, the increase in ELISA titers induced by PMV (whether nanoparticle or monomer) did not correspond to an increase in functional activity assessed by flow cytometry and BIA, suggesting qualitative differences in VAR2CSA antibody responses. A head-to-head comparison of the cVLP-based and monomer vaccines for priming versus boosting in future Aotus studies will provide a useful indication for how a nanoparticle-based vaccine might behave in humans.
This study had limitations. One practical limitation of the Aotus model is the variable and sometimes long time window between vaccination and onset of pregnancy, unlike rodent models that more reliably reproduce. However, the Aotus is similar to what can be expected in humans, in whom timing of pregnancy is also variable. Therefore, this Aotus model may require extended time periods to assess vaccine boosting and efficacy to pregnancy infection. However, this may also be a model to understand immunological memory, as is also important in humans. As another limitation, the current study had limited power to assess placental parasite burden: because the Aotus practices placentophagy, a C-section must be performed to secure the placental sample. In this study, only few paired peripheral and placental parasitemia were obtained, and this weakened statistical power to determine any significant patterns, although general trend suggests a higher parasite burden in the placenta compared to peripheral blood irrespective of the immunization groups (Supplementary Fig. 12). The Aotus model has now been improved to define optimal timing for C-section (18 weeks of gestation), which will allow systematic collection of placental samples in future studies. In addition to the model of chronic PM in development, these improvements should strengthen the use of the Aotus model as a predictive model for vaccine-induced PM protection in pregnant women. Finally, due to logistical and regulatory requirements for the use of NHP models, accessibility of this Aotus model of PM may be limited to centers with the necessary infrastructure.
In conclusion, we demonstrated that the Aotus model is a suitable model to assess immunogenicity of VAR2CSA-derived vaccines, in contrast to small animal models. Current PMV candidates induce mainly homologous and little heterologous functional activity in humans and Aotus, suggesting that improvements to the immunogens and/or the adjuvants are needed to enhance protective antibody responses, as are studies that evaluate the potential for natural infection to boost vaccine antibody in women. The Aotus model further suggests that a brief PM episode is not sufficient to boost PMV functional activity, and models of chronic or recrudescent PM episodes in monkeys should be explored for this outcome. PMV nanoparticles are not superior to monomers as immunogens for boosting VAR2CSA-induced antibody in this model. Further work with the Aotus model should assess placental parasite burden as an endpoint for interventional studies, as well as the ability of chronic PM episodes to boost VAR2CSA functional antibodies. The Aotus PM model may be useful to assess second-generation PMVs seeking to increase strain-transcending activity and prioritize these for further clinical development.
Methods
Study design
A blinded, multi-phase study was designed to test three PMV candidates (Fig. 1a) using a newly established Aotus monkey model for PM35. In the first phase of this study, 40 naive female A. nancymaae were randomized to receive the VAR2CSA-based vaccine candidates PAMVAC (n = 9), PRIMVAC (n = 9) and ID1-ID2a_M1010 (n = 9) as well as a transmission-blocking vaccine candidate Pfs25 (n = 13) to serve as a control. Animals were immunized in 2 cohorts of 20 monkeys per cohort with ~5-month interval between both cohorts. Animals were randomized in permuted blocks of size 4 or 5 to reach a final randomization ratio of 1:1:1:1.4, yielding a power of approximately 90% to find a difference between any experimental group and control of at least 1.5× standard deviation. While the total sample size was dictated by logistical constraints, this randomization ratio was chosen to balance the increased size of the common control group with the information it would provide on each experimental vaccine and optimized statistical power. Vaccines were formulated on Alhydrogel, and each monkey received three immunizations at 4-week intervals (day (D) 0, D28 and D56), composed of 50 µg of vaccine by intramuscular injection in the thigh, alternating legs. Blood samples were collected at D0 and D70 to assess induction of functional antibodies following vaccination.
In the second phase of the study, 23 immunized monkeys (6 with PAMVAC, 5 with PRIMVAC, 4 with ID1-ID2a_M1010 and 8 with Pfs25) that became pregnant were infected with P. falciparum CS2 clone (number of IEs ranging from ~1.2 × 107 to 2.5 × 107). For this infection in pregnant monkeys, stocks of CS2 inoculum for this study were obtained after subpassing CS2 parasite (in human blood) into a male monkey to collect infected blood for CS2 culture in Aotus blood. Parasites were then maintained in culture over 4–6 weeks, and stocks of CS2 inoculum were made at 1% parasite in Aotus blood and frozen. Two lots of CS2 inoculums with similar parasite binding phenotype (predominantly binding to CD36 receptor) were used in this work (Supplementary Table 1). CS2 infections in pregnant monkeys were performed with thawed parasites inoculated in the saphenous vein. Infected animals were observed twice a day and monitored daily with parasitemia data collected from day 3 until animals were cured. P. falciparum infection was confirmed by PCR detection on peripheral blood 3 days after CS2 inoculation (for detailed parasitological data and other clinically relevant information, see Supplementary Data File 2). The infected pregnant monkeys were asymptomatic during this brief CS2 infection, and all animals were cured with mefloquine after end of pregnancy. As full normal gestation is ~19 weeks54, infection was initiated at ~17 weeks to allow for 7 days infection, and C-section performed at ~18 weeks to ensure placental sample collection. Samples were collected on the day of CS2 inoculation and 4 weeks postpartum to investigate whether vaccine-induced antibodies can be boosted or enhanced by a malaria episode during pregnancy. Although the study was not designed to compare parasite densities between groups, thin blood smear was prepared from peripheral and placental blood at delivery where available. Of note, 14 monkeys completed pregnancy with scheduled C-section whereas 9 animals delivered naturally and 2 had stillbirth before C-section, precluding collection of placenta samples.
The third phase of the study assessed PRIMVAC and PAMVAC vaccine-induced antibodies following a booster dose of cVLP modified to present the PAMVAC antigen or monomer PAMVAC. The vaccine antigen was coupled to preformed cVLP through a split protein covalent interaction to form PAMVACcVLP nanoparticle as previously described55,56. Similar assessment was conducted in monkeys immunized with the ID1-ID2a_M1010 candidate using the ExoProtein A (EPA) chemically conjugated with ID1-ID2a_M1010 to generate ID1-ID2a_M1010EPA nanoparticle as previously described57. Both PAMVACcVLP and ID1-ID2a_M1010EPA form nanoparticles. For this phase, 13 monkeys immunized with either PAMVAC (n = 7) or PRIMVAC (n = 6) received either the nanoparticle or monomer forms of PAMVAC, while 5 monkeys immunized with ID1-ID2a_M1010 (n = 5) received either the nanoparticle or monomer forms of ID1-ID2a_M1010. Thus, only PRIMVAC-immunized monkeys received a heterologous vaccine (PAMVAC). Pre-bleed blood samples as well as those collected 14 and 56 days post-booster dose injection were used to investigate whether a boost with PMV nanoparticles can enhance functional activity of PMV vaccine-induced antibodies.
The collected monkey sera from the different study time points were assayed for antibody reactivity to PMV and Pfs25 antigens by ELISA. Plasma antibodies were tested for IEs surface reactivity by flow cytometry and CSA-binding inhibition activity using CS2 and NF54 isolates. A clonal M1010 isolate to approximate the composite VAR2CSA sequence of ID1-ID2a_M1010 was not available.
Monkeys
Female A. nancymaae monkeys aged 2–13 years old were obtained from Keeling center (University of Texas MD Anderson Cancer Center, Michale E. Keeling Center for Comparative Medicine and Research). Average age of the monkeys was 6 years 7 months, 5 years 5 months, 4 years 10 months and 5 years 6 months, for PAMVAC, PRIMVAC. ID1-IG2a_M1010 and Pfs25 vaccine groups, respectively. No significant difference in the animal age (Supplementary Fig. 13, Mann–Whitney test, P > 0.05 for all comparisons) was detected across the different vaccination groups. All animals were of breeding age, and we have no evidence suggesting age might have affected the likelihood of pregnancy or subsequent outcomes based on the data reported in Supplementary Data File 2. Animals were housed in stainless steel 6.0 square-foot cages with PVC nesting boxes and wood perches, with a 12 h/12 h dark/light photoperiod cycle and room temperature (RT) at 24 °C. Animals were pair-housed male/female. Standard husbandry procedures included feeding Teklad New World Primate Diet, Zupreem Primate Diet Canned, diet supplements, and water ad libitum. The monkeys were housed and cared for according to the ‘Guide for the Care and Use of Laboratory Animals’ (ILAR, 2011), Animal Welfare Act and Animal Welfare Regulations (AWA, 2013; AWR, 2013) under approved protocol Laboratory of Malaria Immunology and Vaccinology (LMIV) 8E. Clinical assessments of the monkeys were performed by a staff of veterinary technicians and veterinarians. All animals that are suitable are recycled to other studies after completion of the trial. All animals were enrolled under the Institutional Animal Care and Use Committee-approved malaria candidate vaccine study. The LMIV, as part of the Public Health Service, Department of Health and Human Services, NIH Intramural Research Program, is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and holds a PHS Assurance on file with the National Institute of Health, Office of Laboratory Animal Welfare as required by the US Public Health Service Policy on Human Care and Use of Laboratory Animals.
Vaccines and formulations
Vaccines were based on the N-terminal constructs of VAR2CSA from three variants of P. falciparum (Fig. 1a). The PAMVAC vaccine candidate was generated from the ID1–DBL2–ID2a subunits of the FCR3 allele of VAR2CSA21. The PRIMVAC vaccine was designed to encompass the DBL1–DBL2 fragment of the 3D7 allele of VAR2CSA16. A composite VAR2CSA sequence derived from Illumina sequence pileups from the polyclonal maternal isolate PfM1010 was used to generate a vaccine protein from the ID1-ID2a fragment. Briefly, baculovirus expression clone and high-titer virus stock was produced by Protein Expression Laboratory (NCI-Leidos) using a synthetic codon optimized gene encoding ID1-ID2a_M1010 (GenBank Accession number KU665625). Recombinant ID1-ID2a_M1010 has a non-native amino-terminal alanine. All five putative N-linked glycosylation sites were mutated from NxT/S to NxA at positions T440, S507, S518, T694 and T732 relative to VAR2CSAFCR3. An in-frame His-tag was included on the carboxyl terminal end to facilitate purification. Bench-scale production was done in 10 liters bioreactor glass connected to Biostat BDCU controlling system (Sartorius-Stedim). The cells grew in SFX-insect medium (Hyclone) to a concentration of 2 × 106 per milliliter and were infected with 3 multiplicities of infection of the recombinant virus. The process was conducted at 27 °C, and the dissolved oxygen level was kept at 30%. After 48 h, the culture was collected, and the supernatants were concentrated and dialyzed into phosphate-buffered saline (PBS) pH 7.4 before purification on a Nickel-Sepharose FF column followed by size exclusion chromatography on a Superdex 200 column. The purity and integrity of ID1-ID2a_M1010 was evaluated by SDS–PAGE as well as by reversed-phase HPLC, and their identity confirmed by N-terminal sequence analysis using Edman degradation. Additionally, the theoretical masses were verified by electrospray ionization–mass spectrometry (Research Technologies Branch, NIH). Solution mass and aggregation profile was assessed by analytical size exclusion chromatography with in-line multiangle light scattering as shown in Supplementary Fig. 14.
In addition to these three VAR2CSA-based vaccine candidates, the Pichia pastoris-expressed recombinant Pfs25 (a transmission blocking vaccine candidate)58,59 was used as a non-VAR2CSA-based control vaccine. Further details of vaccine design are included in Supplementary Information.
Vaccines used in the primary series of vaccination and the booster dose were formulated on Alhydrogel platform. Details of the different formulations are presented in Supplementary Table 2. Primary vaccine formulations contain the stated doses (antigen content in a volume of 0.5 ml) (Supplementary Table 2a). Nanoparticles PAMVACcVLP at 12.5 µg/0.5 ml (amount of target antigen) and ID1-ID2a_M1010EPA at 50 µg/0.5 ml as well as the corresponding monomer antigens (PAMVAC and ID1-ID2a_M1010) at 50 µg/0.5 ml doses on Alhydrogel were injected to the PMV-immunized monkeys (Supplementary Table 2b). Vaccines for groups 1A and 2A as well as groups 1B and 2B were formulated by mixing 2× stocks of antigen in their appropriate buffers with 2× stocks of Alhydrogel in water for injection on the days of immunization. Vaccines for groups 3A and 3B were adsorbed on aluminum at least 24 h before immunization in a final aluminum concentration of 0.404 mg/0.5 ml (=0.808 mg/ml).
ELISA titers of PMV-induced antibody in Aotus monkeys
Antibody titers induced by PMV candidates as well as the control antigen (Pfs25) were measured by ELISA as previously described60,61. Briefly, flat-bottom 96-well ELISA plates (Immunolon 4; Dynex Technology Inc.) were coated with 100 ng per well with PAMVAC, PRIMVAC, ID1-ID2a_M1010 and Pfs25 antigens diluted in a carbonate buffer (15 mM sodium carbonate and 35 mM sodium bicarbonate). Plates were incubated at 4 °C overnight and blocked with 200 µl per well of the blocking buffer (5% skim milk powder in 1× Tris-buffered saline) for 2 h at RT. Aotus serum samples were diluted at 1:500 in blocking buffer, added to duplicated antigen-coated wells (100 liters per well) and incubated for 2 h at RT. Plates were washed and incubated with 1:3,000 dilution of the alkaline phosphatase conjugated anti-Human IgG (H + L) secondary antibody (#0751-1006, KPL Inc.) for 2 h at RT. After washing the plates, the substrate (0.1 mg per well of p-nitrophenyl phosphate, Sigma 104 substrate; Sigma) was added to the wells for 20 min incubation in dark at RT, and the absorbance was read at 405 nm using a Spectramax 340PC microplate reader (Molecular Devices Co.) and reported as OD.
Flow cytometry measurement of antiserum reactivity to IE surface
The ability of sera from vaccinated Aotus to bind the native antigen expressed on the surface of IE was assessed by flow cytometry. Enriched, mature trophozoite/schizont stages of IE were resuspended in the running buffer (2% of fetal bovine serum in 1× PBS) and 100 µl of the cell suspension was dispensed in each well (4 × 105 cells per well). Cells were incubated with 1:20 dilution of the Aotus plasma samples for 30 min at 4 °C. After unbound antibodies were washed, IE were labeled with 0.1% Sybr Green (Life Technologies) and IgG-bound IE were stained with phycoerythrine-conjugated goat (Fc γ-specific) anti-human IgG (#12-4998-82, eBioscience) for 30 min at 4 °C, then washed. Data were acquired by LSRII (BD Bioscience) and analyzed in FlowJo 10 software (Tree Star Inc.). For each test sample, the rMFI was obtained by normalizing the MFI value of the samples with the MFI of the pool of pre-bleed (D0) plasma samples. For qualitative analysis, a ratio greater than 1.2 was considered positive for parasite surface staining20. Pooled plasma from multigravidae and a VAR2CSA-specific human monoclonal antibody were included in each assay to confirm surface expression of VAR2CSA by the isolates.
Binding inhibition activity
The CSA binding inhibition capacity of sera from vaccinated Aotus was assessed by a static BIA on immobilized CSA receptor. Briefly, spots of Decorin (Sigma) at 2 µg/ml in 1× PBS were coated in a 100 × 15 mm Petri dish (Falcon 351029) and incubated overnight at 4 °C in a humid chamber. Spots were then blocked with 3% bovine serum albumin–1× PBS at 37 °C for 30 min. Enriched, mature trophozoite/schizont stages of CS2 and NF54 IE were adjusted to 20% parasite density at 0.5% hematocrit. IE were blocked in 3% bovine serum albumin–RPMI for 30 min at RT and incubated with Aotus plasma at 1:5 dilution for 30 min at 37 °C. IE cells were then added to duplicated spots and allowed to settle for 30 min at RT. Unbound IE were washed and adherent IE were immediately fixed with 1.5% glutaraldehyde for 10 min, stained with 5% Giemsa for 5 min and quantified by microscopy. A multigravid plasma pool and a D0 pre-bleed plasma pool from the Aotus monkeys were included in each plate as positive and negative controls, respectively. The percentage of inhibition was determined relative to the well with the D0 pre-bleed pool. For a given test sample, the percentage of inhibition was calculated as follows: %inhibition = 100 − (Bound-IEtestsample/Bound-IED0-pool) × 100.
Statistical analysis
A computer-generated allocation table was used to randomize Aotus monkeys into each vaccination group by an independent party. Analysis of the immunological data was executed using GraphPad Prism 8. The activity of antibodies raised against each antigen measured by ELISA, flow and BIA was compared using the Mann–Whitney test. Wilcoxon matched-pairs signed-rank test was used to evaluate cross-reactivity of PMV-induced antibodies to heterologous antigens and isolates, as well as antibody-boosting activity by CS2 inoculation during pregnancy and PMV-conjugated antigens. Spearman’s correlation coefficient (ρ) was used to analyze the relationship between ELISA antibody titers and their ability to recognize the native VAR2CSA and the blockade of the parasite binding to CSA. P values <0.05 were considered significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are present in the paper or Supplementary Information and are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature 415, 673–679 (2002).
Brabin, B. J. An analysis of malaria in pregnancy in Africa. Bull. World Health Organ. 61, 1005–1016 (1983).
Rogerson, S. J. et al. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect. Dis. 18, e107–e118 (2018).
Rogerson, S. J., Hviid, L., Duffy, P. E., Leke, R. F. & Taylor, D. W. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 7, 105–117 (2007).
Desai, M. et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7, 93–104 (2007).
Fried, M., Nosten, F., Brockman, A., Brabin, B. J. & Duffy, P. E. Maternal antibodies block malaria. Nature 395, 851–852 (1998).
Ricke, C. H. et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165, 3309–3316 (2000).
Staalsoe, T. et al. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363, 283–289 (2004).
Duffy, P. E. & Fried, M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71, 6620–6623 (2003).
Ndam, N. T. et al. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg. Infect. Dis. 21, 813–823 (2015).
Baruch, D. I. et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82, 77–87 (1995).
Fried, M. & Duffy, P. E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272, 1502–1504 (1996).
Salanti, A. et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49, 179–191 (2003).
Viebig, N. K. et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep. 6, 775–781 (2005).
Bigey, P. et al. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J. Infect. Dis. 204, 1125–1133 (2011).
Chene, A. et al. Down-selection of the VAR2CSA DBL1-2 expressed in E. coli as a lead antigen for placental malaria vaccine development. NPJ Vaccines 3, 28 (2018).
Clausen, T. M. et al. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J. Biol. Chem. 287, 23332–23345 (2012).
Doritchamou, J. et al. Differential adhesion-inhibitory patterns of antibodies raised against two major variants of the NTS-DBL2X region of VAR2CSA. Vaccine 31, 4516–4522 (2013).
Fried, M. et al. Multilaboratory approach to preclinical evaluation of vaccine immunogens for placental malaria. Infect. Immun. 81, 487–495 (2013).
Magistrado, P. A. et al. High efficacy of anti DBL4varepsilon-VAR2CSA antibodies in inhibition of CSA-binding Plasmodium falciparum-infected erythrocytes from pregnant women. Vaccine 29, 437–443 (2011).
Nielsen, M. A. et al. The influence of sub-unit composition and expression system on the functional antibody response in the development of a VAR2CSA based Plasmodium falciparum placental malaria vaccine. PLoS ONE 10, e0135406 (2015).
Obiakor, H. et al. Identification of VAR2CSA domain-specific inhibitory antibodies of the Plasmodium falciparum erythrocyte membrane protein 1 using a novel flow cytometry assay. Clin. Vaccine Immunol. 20, 433–442 (2013).
Srivastava, A. et al. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS ONE 6, e20270 (2011).
Chene, A. et al. Preclinical immunogenicity and safety of the cGMP-grade placental malaria vaccine PRIMVAC. EBioMedicine 42, 145–156 (2019).
Mordmuller, B. et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin. Infect. Dis. 69, 1509–1516 (2019).
Sirima, S. B. et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect. Dis. https://doi.org/10.1016/s1473-3099(19)30739-x (2020).
Ataide, R., Mwapasa, V., Molyneux, M. E., Meshnick, S. R. & Rogerson, S. J. Antibodies that induce phagocytosis of malaria infected erythrocytes: effect of HIV infection and correlation with clinical outcomes. PLoS ONE 6, e22491 (2011).
Jaworowski, A. et al. Relationship between human immunodeficiency virus type 1 coinfection, anemia, and levels and function of antibodies to variant surface antigens in pregnancy-associated malaria. Clin. Vaccine Immunol. 16, 312–319 (2009).
Aitken, E. H. et al. Developing a multivariate prediction model of antibody features associated with protection of malaria-infected pregnant women from placental malaria. eLife https://doi.org/10.7554/eLife.65776 (2021).
Tutterrow, Y. L. et al. High avidity antibodies to full-length VAR2CSA correlate with absence of placental malaria. PLoS ONE 7, e40049 (2012).
Vanda, K. et al. The development, fine specificity, and importance of high-avidity antibodies to VAR2CSA in pregnant Cameroonian women living in Yaounde, an urban city. Front. Immunol. 12, 610108 (2021).
Jones, T. R., Obaldia, N. III, Gramzinski, R. A. & Hoffman, S. L. Repeated infection of Aotus monkeys with Plasmodium falciparum induces protection against subsequent challenge with homologous and heterologous strains of parasite. Am. J. Trop. Med. Hyg. 62, 675–680 (2000).
Stowers, A. W. et al. Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect. Immun. 70, 6961–6967 (2002).
Taghavian, O. et al. Antibody profiling by proteome microarray with multiplex isotype detection reveals overlap between human and Aotus nancymaae controlled malaria infections. Proteomics 18, e1870115 (2018).
Sharma, A. et al. Plasmodium falciparum in Aotus nancymaae: a new model for placental malaria. J. Infect. Dis. https://doi.org/10.1093/infdis/jiac096 (2022).
Duffy, P. E. & Fried, M. Plasmodium falciparum adhesion in the placenta. Curr. Opin. Microbiol. 6, 371–376 (2003).
Viebig, N. K. et al. Disruption of var2csa gene impairs placental malaria associated adhesion phenotype. PLoS ONE 2, e910 (2007).
Tuikue Ndam, N. G. et al. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis. 192, 331–335 (2005).
Tuikue Ndam, N. G. et al. Variable adhesion abilities and overlapping antigenic properties in placental Plasmodium falciparum isolates. J. Infect. Dis. 190, 2001–2009 (2004).
Dechavanne, S. et al. Parity-dependent recognition of DBL1X-3X suggests an important role of the VAR2CSA high-affinity CSA-binding region in the development of the humoral response against placental malaria. Infect. Immun. 83, 2466–2474 (2015).
Brolin, K. J., Persson, K. E., Wahlgren, M., Rogerson, S. J. & Chen, Q. Differential recognition of P. falciparum VAR2CSA domains by naturally acquired antibodies in pregnant women from a malaria endemic area. PLoS ONE 5, e9230 (2010).
Gamain, B., Chene, A., Viebig, N. K., Tuikue Ndam, N. & Nielsen, M. A. Progress and insights toward an effective placental malaria vaccine. Front. Immunol. 12, 634508 (2021).
Doritchamou, J., Teo, A., Fried, M. & Duffy, P. E. Malaria in pregnancy: the relevance of animal models for vaccine development. Lab Anim. 46, 388–398 (2017).
Salanti, A. et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200, 1197–1203 (2004).
Fowkes, F. J. et al. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J. Infect. Dis. 206, 1612–1621 (2012).
Carmona-Fonseca, J. & Arango, E. M. Asymptomatic plasmodial infection in pregnant women: a global scenario. J. Vector Borne Dis. 54, 201–206 (2017).
Fried, M. et al. Antibody levels to recombinant VAR2CSA domains vary with Plasmodium falciparum parasitaemia, gestational age, and gravidity, but do not predict pregnancy outcomes. Malar. J. 17, 106 (2018).
Fried, M. & Duffy, P. E. Designing a VAR2CSA-based vaccine to prevent placental malaria. Vaccine 33, 7483–7488 (2015).
Ampomah, P., Stevenson, L., Ofori, M. F., Barfod, L. & Hviid, L. B-cell responses to pregnancy-restricted and -unrestricted Plasmodium falciparum erythrocyte membrane protein 1 antigens in Ghanaian women naturally exposed to malaria parasites. Infect. Immun. 82, 1860–1871 (2014).
Foote, J. & Milstein, C. Kinetic maturation of an immune response. Nature 352, 530–532 (1991).
Babakhanyan, A. et al. Comparison of the specificity of antibodies to VAR2CSA in Cameroonian multigravidae with and without placental malaria: a retrospective case–control study. Malar. J. 14, 480 (2015).
Thrane, S. et al. A novel virus-like particle based vaccine platform displaying the placental malaria antigen VAR2CSA. PLoS ONE 10, e0143071 (2015).
Shimp, R. L. Jr. et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 31, 2954–2962 (2013).
Gozalo, A. & Montoya, E. Reproduction of the owl monkey (Aotus nancymai) (primates: Cebidae) in captivity. Am. J. Primatol. 21, 61–68 (1990).
Thrane, S. et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J. Nanobiotechnol. 14, 30 (2016).
Janitzek, C. M. et al. A proof-of-concept study for the design of a VLP-based combinatorial HPV and placental malaria vaccine. Sci. Rep. 9, 5260 (2019).
Scaria, P. V. et al. Protein–protein conjugate nanoparticles for malaria antigen delivery and enhanced immunogenicity. PLoS ONE 12, e0190312 (2017).
Talaat, K. R. et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel(R), a transmission blocking vaccine against Plasmodium falciparum: an open label study in malaria naive adults. PLoS ONE 11, e0163144 (2016).
Tsai, C. W., Duggan, P. F., Shimp, R. L. Jr., Miller, L. H. & Narum, D. L. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J. Biotechnol. 121, 458–470 (2006).
Doritchamou, J. Y. et al. VAR2CSA domain-specific analysis of naturally acquired functional antibodies to Plasmodium falciparum placental malaria. J Infect Dis 214, 577–586 (2016).
Miura, K. et al. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 26, 193–200 (2008).
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health; the Danish Research Councils, The Eurostars Programme, the European Union in the Seventh program Framework Programme (FP7-HEALTH-2012-INNOVATION; under grant agreement 304815), the Danish Advanced Technology Foundation (under grant number 005-2011-1), the Bill and Melinda Gates Foundation (grant numbers 42387 and OPP1055855 to S.G.R.); the French National Research Agency (ANR-16-CE11-0014-01), grants from Laboratory of Excellence GR-Ex, reference ANR-11-LABX-0051 and the French Parasitology consortium ParaFrap (ANR-11-LABX0024). The labex GR-Ex is funded by the program ‘Investissements d’avenir’ of the French National Research Agency, reference ANR-11-IDEX-0005-02; a grant from the Bundesministerium für Bildung und Forschung (BMBF), Germany through Kreditanstalt für Wiederaufbau (KfW) (reference no. 202060457) and through funding from Irish Aid, Department of Foreign Affairs and Trade, Ireland. The funding sources of this study were not involved in study design, nor in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. We thank J. P. Gorres for editing the manuscript. We are grateful to S. Wong-Madden, J. Kwan, M. Cowles, R. Stevens, R. Morrison and J. Neal for their technical assistance and advice, and F. A. Somé, M. del Mar Castro Noriega and M. Niangaly for project management assistance.
Author information
Authors and Affiliations
Contributions
J.D., M.A.N., N.K.V., S.H., M.F., T.G.T., B.G. and P.E.D. conceived the study, designed the studies, and interpreted data. L.E.L., M.C.N., A. Salanti, C.A., K.R., D.L.N., P.V.S. and K.M.R. contributed to study design and data interpretation. J.D., L.E.L., A.C., A.F.S., J.-P.S., S.O.-G., C.M.J., A.J.S., S.B.C., M.S.-S., E.K.B., J.S., B.B.C., S.N., K.H., T.O., S.C., A. Sharma, H.T., B.B. and P.V.S. performed study experiments. M.L.T. provided medical and surgical care to the animals. J.D., M.F. and P.E.D. analyzed data. J.D. and P.E.D. wrote the manuscript with input from all authors and approval for the final version.
Corresponding author
Ethics declarations
Competing interests
T.G.T. and A. Salanti are named on a patent owned by University of Copenhagen for the use of VAR2CSA as a malaria vaccine. M.A.N., A.F.S., C.M.J., A. Salanti and T.G.T. are named on a patent to use capsid particles in vaccine development. The other authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14 and Tables 1 and 2.
Supplementary Data File 1
Local and systemic reactogenicity.
Supplementary Data File 2
Timing of immunization, pregnancy and infection, and parasitological data.
Source data
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Fig. 5
Source data.
Source Data Fig. 6
Source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doritchamou, J., Nielsen, M.A., Chêne, A. et al. Aotus nancymaae model predicts human immune response to the placental malaria vaccine candidate VAR2CSA. Lab Anim 52, 315–323 (2023). https://doi.org/10.1038/s41684-023-01274-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-023-01274-2