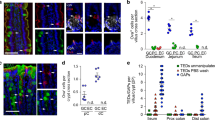
Abstract
The intestinal immune system samples luminal contents to induce adaptive immune responses that include tolerance in the steady state and protective immunity during infection. How luminal substances are delivered to the immune system has not been fully investigated. Goblet cells have an important role in this process by delivering luminal substances to the immune system through the formation of goblet cell–associated antigen passages (GAPs). Soluble antigens in the intestinal lumen are transported across the epithelium transcellularly through GAPs and delivered to dendritic cells for presentation to T cells and induction of immune responses. GAPs can be identified and quantified by using the ability of GAP-forming goblet cells to take up fluorescently labeled dextran. Here, we describe a method to visualize GAPs and other cells that have the capacity to take up luminal substances by intraluminal injection of fluorescent dextran in mice under anesthesia, tissue sectioning for slide preparation and imaging with fluorescence microscopy. In contrast to in vivo two-photon imaging previously used to identify GAPs, this technique is not limited by anatomical constraints and can be used to visualize GAP formation throughout the length of the intestine. In addition, this method can be combined with common immunohistochemistry protocols to visualize other cell types. This approach can be used to compare GAP formation following different treatments or changes to the luminal environment and to uncover how sampling of luminal substances is altered in pathophysiological conditions. This protocol requires 8 working hours over 2–3 d to be completed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012).
Knoop, K. A., McDonald, K. G., McCrate, S., McDole, J. R. & Newberry, R. D. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 8, 198–210 (2015).
Knoop, K. A. et al. Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes 8, 400–411 (2017).
Kulkarni, D. H. et al. Goblet cell associated antigen passages are inhibited during Salmonella typhimurium infection to prevent pathogen dissemination and limit responses to dietary antigens. Mucosal Immunol. 11, 1103–1113 (2018).
Knoop, K. A. et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol. 2, eaa01314 (2017).
Yu, Q. H. & Yang, Q. Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol. Int. 33, 78–82 (2009).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Heller, F. et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129, 550–564 (2005).
Chang, J. et al. Impaired intestinal permeability contributes to ongoing bowel symptoms in patients with inflammatory bowel disease and mucosal healing. Gastroenterology 153, 723–731.e1 (2017).
Michielan, A. & D’Incà, R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015, 628157 (2015).
Cukrowska, B. et al. Intestinal epithelium, intraepithelial lymphocytes and the gut microbiota—key players in the pathogenesis of celiac disease. World J. Gastroenterol. 23, 7505–7518 (2017).
Obrenovich, M. E. M. Leaky gut, leaky brain? Microorganisms 6, E107 (2018).
Hamilton, M. K. & Raybould, H. E. Bugs, guts and brains, and the regulation of food intake and body weight. Int. J. Obes. Suppl. 6, S8–S14 (2016).
Araújo, J. R., Tomas, J., Brenner, C. & Sansonetti, P. J. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 141, 97–106 (2017).
Shen, L., Weber, C. R., Raleigh, D. R., Yu, D. & Turner, J. R. Tight junction pore and leak pathways: a dynamic duo. Annu. Rev. Physiol. 73, 283–309 (2011).
Utzeri, E. & Usai, P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J. Gastroenterol. 23, 3954–3963 (2017).
Knoop, K. A., Miller, M. J. & Newberry, R. D. Transepithelial antigen delivery in the small intestine: different paths, different outcomes. Curr. Opin. Gastroenterol. 29, 112–118 (2013).
Volynets, V. et al. Assessment of the intestinal barrier with five different permeability tests in healthy C57BL/6J and BALB/cJ mice. Dig. Dis. Sci. 61, 737–746 (2016).
Wang, L. et al. Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods 421, 44–53 (2015).
Wu, L.-L. et al. Commensal bacterial endocytosis in epithelial cells is dependent on myosin light chain kinase-activated brush border fanning by interferon-γ. Am. J. Pathol. 184, 2260–2274 (2014).
Rock, K. L., Reits, E. & Neefjes, J. Present yourself! By MHC class I and MHC class II molecules. Trends Immunol. 37, 724–737 (2016).
Blum, J. S., Wearsch, P. A. & Cresswell, P. Pathways of antigen processing. Annu. Rev. Immunol. 31, 443–473 (2013).
Kang, S. K. et al. Identification of a peptide sequence that improves transport of macromolecules across the intestinal mucosal barrier targeting goblet cells. J. Biotechnol. 135, 210–216 (2008).
Jin, Y. et al. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials 33, 1573–1582 (2012).
Fan, T. et al. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur. J. Pharm. Biopharm. 88, 518–528 (2014).
Lee, J. Y. et al. Production of recombinant human growth hormone conjugated with a transcytotic peptide in Pichia pastoris for effective oral protein delivery. Mol. Biotechnol. 57, 430–438 (2015).
Xia, D. et al. Wheat germ agglutinin nanocage stabilized drug nanocrystals cross intestinal epithelium barrier via goblet cells. J. Control Release 213, e25– e26 (2015).
Xia, D. et al. Enhanced transport of nanocage stabilized pure nanodrug across intestinal epithelial barrier mimicking Listeria monocytogenes. Biomaterials 37, 320–332 (2015).
Kenngott, E. E. et al. Identification of targeting peptides for mucosal delivery in sheep and mice. Mol. Pharm. 13, 202–210 (2016).
Kiliaan, A. J. et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am. J. Physiol. 275, G1037– G1044 (1998).
Madara, J. L. & Trier, J. S. Structure and permeability of goblet cell tight junctions in rat small intestine. J. Membr. Biol. 66, 145–157 (1982).
Barbour, W. M. & Hopwood, D. Uptake of cationized ferritin by colonic epithelium. J. Pathol. 139, 167–178 (1983).
Colony, P. C. & Specian, R. D. Endocytosis and vesicular traffic in fetal and adult colonic goblet cells. Anat. Rec. 218, 365–372 (1987).
Knoop, K. A., McDonald, K. G., Kulkarni, D. H. & Newberry, R. D. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 65, 1100–1109 (2016).
Nagatake, T., Fujita, H., Minato, N. & Hamazaki, Y. Enteroendocrine cells are specifically marked by cell surface expression of claudin-4 in mouse small intestine. PLoS ONE 9, e90638 (2014).
Barbosa, F. L. et al. Goblet cells contribute to ocular surface immune tolerance-implications for dry eye disease. Int. J. Mol. Sci. 18, E978 (2017).
Mammoto, A. et al. Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics. Nat. Commun. 4, 1759 (2013).
Ko, B. Y., Xiao, Y., Barbosa, F. L., de Paiva, C. S. & Pflugfelder, S. C. Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight 3, 98222 (2018).
Weiner, M. L. Intestinal transport of some macromolecules in food. Food Chem. Toxicol. 26, 867–880 (1988).
Specian, R. D. & Neutra, M. R. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J. Cell Biol. 85, 626–640 (1980).
Neutra, M. R., O’Malley, L. J. & Specian, R. D. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am. J. Physiol. 242, G380– G387 (1982).
Pickett, J. A. & Edwardson, J. M. Compound exocytosis: mechanisms and functional significance. Traffic 7, 109–116 (2006).
Acknowledgements
This work was supported by grants AI136515, AI140755, AI12626, AI131342 and DK097317 (to R.D.N.); grants DK052574, DK09789 and AI095542 (to K.A.K.); Swedish Research Council International Postdoc Award 2014-00366 (to J.K.G.); and Crohn’s and Colitis Foundation 610605 (to D.H.K.).
Author information
Authors and Affiliations
Contributions
All authors conceived the protocol and reviewed and discussed the manuscript. K.A.K. and D.H.K. prepared the figures. K.A.K., J.E.D. and A.N.F. prepared the supplementary videos.
Corresponding author
Ethics declarations
Competing interests
R.D.N., K.A.K. and K.G.M. are inventors on the US Non-provisional Application Serial No. 15/880,658: Compositions and methods for modulation of dietary and microbial exposure.
Supplementary information
Supplementary Video 1
Mouse surgery and intraluminal dextran administration
Supplementary Video 2
Embedding and freezing of tissue
Supplementary Video 3
Sectioning of tissue for slide preparation
Rights and permissions
About this article
Cite this article
Knoop, K.A., Kulkarni, D.H., McDonald, K.G. et al. In vivo labeling of epithelial cell–associated antigen passages in the murine intestine. Lab Anim 49, 79–88 (2020). https://doi.org/10.1038/s41684-019-0438-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-019-0438-z
This article is cited by
-
The role of goblet cells and mucus in intestinal homeostasis
Nature Reviews Gastroenterology & Hepatology (2022)