Abstract
Leishmaniasis is a disease caused by a protozoan of the genus Leishmania, affecting millions of people, mainly in tropical countries, due to poor social conditions and low economic development. First-line chemotherapeutic agents involve highly toxic pentavalent antimonials, while treatment failure is mainly due to the emergence of drug-resistant strains. Leishmania arginase (ARG) enzyme is vital in pathogenicity and contributes to a higher infection rate, thus representing a potential drug target. This study helps in designing ARG inhibitors for the treatment of leishmaniasis. Py-CoMFA (3D-QSAR) models were constructed using 34 inhibitors from different chemical classes against ARG from L. (L.) amazonensis (LaARG). The 3D-QSAR predictions showed an excellent correlation between experimental and calculated pIC50 values. The molecular docking study identified the favorable hydrophobicity contribution of phenyl and cyclohexyl groups as substituents in the enzyme allosteric site. Molecular dynamics simulations of selected protein–ligand complexes were conducted to understand derivatives’ interaction modes and affinity in both active and allosteric sites. Two cinnamide compounds, 7g and 7k, were identified, with similar structures to the reference 4h allosteric site inhibitor. These compounds can guide the development of more effective arginase inhibitors as potential antileishmanial drugs.
Similar content being viewed by others
Introduction
Human leishmaniasis are a group of vector-borne diseases, transmitted by the bite of infected female phlebotomine sandflies, caused by protozoan parasites comprising more than 20 species of the genus Leishmania1. According to the clinical manifestations, there are three main forms of leishmaniasis (ICD-11 code: 1F54): (i) visceral leishmaniasis (VL), which is fatal if left untreated in over 95% of cases; (ii) cutaneous leishmaniasis (CL), which is the most common form; and (iii) mucosal/mucocutaneous leishmaniasis (ML or MCL)2. Globally, leishmaniasis is among the top 10 neglected tropical diseases (NTDs), it is endemic in 99 tropical and subtropical countries, with more than 12 million people infected3. Over 90% of new cases have been reported in Brazil, Ethiopia, Somalia, Sudan, and India in the past 4 years4. This disease is primarily associated with poor social conditions and low economic development since poverty dramatically impacts human health5.
To date, there is no licensed vaccine against human leishmaniasis6. The treatment against all Leishmania forms still involves using chemotherapeutic agents, mainly administered parenterally (intravenous, intramuscular or intralesional), including pentavalent antimony (SbV) compounds as first-line drugs, such as meglumine antimoniate and sodium stibogluconate, which are highly toxic, and non-antimony-based compounds as second-line drugs, such as amphotericin B and pentamidine, which also have severe side effects7. Miltefosine, another example of a non-antimony-based compound, despite being the only drug for oral use against leishmaniasis, presents teratogenic risk8,9,10. The failures of current drug therapy are partially due to the emergence of drug resistant Leishmania strains11. Therefore, it is crucial to discover new antileishmanial chemotherapeutic agents, more effective, and selective. According to the Drugs for Neglected Diseases initiative (DNDi)12, the target product profile (TPP) for new antileishmanial chemotherapeutic agents includes activity against all species of Leishmania, efficacy in treatment of less than two weeks, preferably a once-daily oral drug, and safer than available treatment9.
In the pursuit of discovering novel drug candidates, molecular targets that are crucial for the survival of parasites have been thoroughly investigated to identify compounds that are both selective and less toxic13. One noteworthy target from this research is the arginase (ARG) enzyme, also known as L-arginine aminohydrolase (EC 3.5.3.1), which has shown promise as a potential target in developing new antitrypanosomal drugs4,14,15,16.
ARG is a manganese-dependent metalloenzyme that catalyzes the hydrolyzes of L-arginine into L-ornithine and urea, while the L-ornithine product is a precursor of polyamines necessary to produce trypanothione, a vital antioxidant agent that controls reactive oxygen species (ROS)17,18,19,20,21. This makes L-ornithine a crucial component of the redox defense mechanism of parasites. The expression and activity of ARG in Leishmania species contribute to a higher infection rate and play a vital role in the pathogenicity of the parasite4,14. An increase in ARG activity reduces L-arginine availability to the nitric oxide synthase (NOS) enzyme4. This, in turn, diminishes the formation of nitric oxide (NO), which lowers the host’s defensive capacity and increases the parasite’s infectivity. Simply put, the ARG enzyme can negatively regulate the levels of NO produced in the body by consuming the NOS enzyme substrate. It is worth noting that scientific studies have shown that ARG inhibitors can reduce the ability of Leishmania spp. to establish an infection in macrophages14.
Computer-aided drug design (CADD) techniques, including ligand-based drug design (LBDD) and structure-based drug design (SBDD) approaches, are widely applied in the drug discovery process22. The field of quantitative structure–activity relationships (QSAR) modeling, whether through regression or classification methods, aims to correlate chemical structures with their biological responses, and it is mostly applied as an LBDD approach23,24. However, the traditional approach of using topological (1D and/or 2D) molecular descriptors alone does not consider the tridimensional (3D) geometric features of molecules, which can lead to difficulties adequately describing interactions between ligands and receptors25,26. Therefore, a tridimensional QSAR (3D-QSAR) method can be applied to overcome these limitations for better results.
Comparative Molecular Field Analysis (CoMFA)27 is a 3D-QSAR ligand-based and alignment-dependent method developed by Cramer et al.27 that utilizes statistical techniques to correlate molecular (steric and electrostatic) interaction fields (MIFs), which are interaction energies calculated with virtual probes in a 3D space, such as in the GRID method developed by Goodford28 with biological response endpoints 29. Ragno et al. (2020) developed a Python implementation of the CoMFA method, named Py-CoMFA, which is publicly available through the www.3d-qsar.com web portal to build 3D-QSAR models30,31. The resulting accuracy data can help understand the (quantitative) structure–activity relationships (Q)SAR that are most relevant for developing antileishmanial agents32,33.
On the other hand, a SBDD approach, such as the ligand-receptor molecular docking34 and molecular dynamics (MD) simulations methods35, combined with a LBDD approach, such as the CoMFA method (3D-QSAR)36,37, have paved the way for significant advancements in drug design. These computational tools have enabled researchers to study bioactive substances’ molecular mechanisms of action and have become a crucial strategy for identifying new hits38. In addition, molecular docking is necessary to understand the structural requirements and to analyze the interactions between ligands and receptors39, and in addition, to derive ligand-receptor complexes for further studies, such as molecular dynamics (MD) simulations, which may provide information about the stability of promising compounds analyzing the ligand-receptor complexes in the aqueous system40.
Furthermore, it is worth highlighting that this study aims to apply the Py-CoMFA (3D-QSAR) modeling method to a set of 34 compounds recently reported in the literature from 2018 to 2023 as inhibitors of the Leishmania (L.) amazonensis arginase (LaARG) enzyme, a relevant target of the parasite life cycle. During the cited period, there was a shortage of literature publications that incorporated a complete in silico study with multiple techniques such as 3D-QSAR (especially Py-CoMFA), molecular docking, and MD simulations for investigating arginase inhibitors, principally those focused targeting arginase from L. (L.) amazonensis, which has a greater ability to resist macrophages, neutrophils, and leishmanicidal drugs than other species of Leishmania41,42. After that, structure–activity relationship studies helped us to analyze the influence of aliphatic groups, with or without heteroatoms, and the presence of the cyclohexyl, pyrrole, and indole rings as side chains. Finally, the putative binding modes of the most active inhibitors were investigated by molecular docking on the LaARG enzyme, while the most relevant protein–ligand complexes were submitted to molecular dynamics simulations to verify the dynamic behavior of the putative interaction modes and binding affinities of those derivatives.
Results and discussion
Py-CoMFA (3D-QSAR) modeling
Py-CoMFA (3D-QSAR) models were constructed with 34 inhibitors reported in the literature between 2018 and 2023 against arginase from L. (L) amazonensis (LaARG). These 34 inhibitors belong to the chemical classes of chromones (1a–1d)43, pyrazolo-pyrimidines (2a–2f)44, phenylhydrazines or phenylamide (3a–3f)45, cinnamides (cinnamic acid amides or cinnamides) (4a–4k)4, and esters (5a–5f)46 derived from 3,4-dihydroxycinnamic acid (caffeic acid)47 (6) (Fig. 1).
Considering a traditional qualitative SAR analysis, it has been observed that cinnamic acid (6) and its derivatives, cinnamides (4a–k) and cinnamic esters (5a–f), are the most promising compounds that act as arginase inhibitors. Their IC50 values range from 1.3 to 200 µM. Among these compounds, cinnamic acid itself (6) and disubstituted cinnamide derivatives with acetylated groups (4i, 4g) or hydroxy or dihydroxybenzene (4a–4f) have been shown to have the strongest inhibitory activity (Fig. 1). Another group of inhibitors that exhibit exceptional inhibitory activity are chromones (1a–1d). These are also substituted with dihydroxybenzene with an IC50 range of 1.4 to 9 µM. It is evident that among these compounds, β-carbonyl hydroxylation significantly reduces the inhibitory activity of chromones 1b–d by up to six times. The non-β-carbonyl hydroxylated derivative 1a, on the other hand, exhibits the highest inhibitory potency (IC50 = 1.4 µM) (Fig. 1). Nitrogen derivatives like pyrimidines (2a–f), phenylhydrazines (3a–e), and phenylamide (3f) exhibit moderate inhibitory activity with IC50 values ranging from 12 to 100 µM. However, the biological activity appears to improve when the phenylhydrazine nucleus is included in the structural framework, with values ranging from 12 to 38 µM (Fig. 1).
It is important to keep in mind that Py-CoMFA models, as with other 3D-QSAR methods, can be affected by various factors that can impact their accuracy. Some of these factors include the conformational analysis method, putative bioactive conformation selection, and alignment rule, which is unfavorable to noncovalent molecular interactions48. Therefore, ensuring that the molecular target being evaluated in the model is carefully studied is crucial the probe atom variations need to be assessed49, and the set of molecules should be well-defined to obtain reliable results. It is important to note that hydrophobicity is not well quantified50, and thus, structures with high hydrophobicity can lead to misinterpretations. Additionally, the use of CoMFA is appropriate only with in vitro data.
In this way, we first performed in the 3D-QSAR server (https://www.3d-qsar.com/), a conformational analysis of all inhibitors using the RDkit method with the UFF force field through the Py-ConfSearch module. The most extended conformation of each inhibitor was selected as the putative bioactive conformation, since in several crystal structures of L. mexicana arginase (LmARG), a series of boronic amino-acid inhibitors (PDB ID: 5HJ9, 5HJA, 4IU0, 4IU4) adopts an extended conformation. The same occurs for a substrate analog amino-acid inhibitor (PDB ID: 4IU1) and the L-ornithine product (PDB ID: 4IU5). Then, we aligned the inhibitors (Fig. 2A) using the Py-Align module, applying the RDkit method using the most extended conformation of the most active inhibitor 4h (IC50 = 1.3 µM) as an alignment template (Fig. 2B).
(A) Superimposed structures of all inhibitors under study. Compounds are shown in ball-and-stick model, and the atoms are color coded as follows carbon (grey), oxygen (red), nitrogen (blue), fluorine (green), and sulfur (yellow), while hydrogen atoms were omitted for clarity. (B) Longest conformation of 4 h used as alignment template. Figure generated with Py-Align and Py-ConfSearch, respectively.
Once the dataset compounds are aligned, we utilize two methods to split the dataset into training and test sets. While most QSAR studies, including CoMFA, generally apply random splitting, we also wanted to investigate the potential differences when implementing a supervised approach, such as the Kennard-Stone method 51. Twenty-five compounds were used as the training set for deriving the QSAR models, and nine compounds were used as the test set for external validation (Tables 1, 2).
Compared to the random training and test sets splitting method, we found that the Kennard-Stone51 splitting method provided the compounds that show high dissimilarity in relation to the independent variables (descriptors) that were separated as a test set and the best parameters for our models. We explored three different fields, namely steric (STE), electrostatic (ELE), and the combination of both (STE + ELE) (see Table S1). However, we found that the models based on steric fields (STE) provided the best results (as shown in Table 1), and hence, we conducted all further analyses based on these models.
We also tested the models with different probe atoms and charges (Csp3, + 1; Osp3, − 1; and H, + 1) and found that all models performed well, showing statistical parameters indicating that the coefficient of determination (r2) was more significant than 0.9 for all models. In contrast, the r2 from the leave-one-out cross-validation (q2) was greater than 0.5 for models from the Kennard-Stone splitting method, considering Csp3 (+ 1) and Osp3 (− 1) as probe atoms, in addition to a Pearson correlation coefficient with positive value (r ≅ 1), i.e., variables are directly correlated52. However, the best model was for Csp3 (+ 1) as a probe atom, with higher r2 = 0.999 and q2 = 0.573 (Table 1). We also performed a Y-randomization test, which showed low values of q2, indicating that our developed 3D-QSAR models with original data are robust and not inferred by chance (Table 1).
Alignment dependence and conformational sensitivity are problems that need to be considered when using Py-CoMFA (3D-QSAR)50. In this way, since the Kennard-Stone splitting method using Csp3 (+ 1) as a probe and STE field produced the most favorable statistical results, we decided to assess the model using docking conformations (Figure S1) in the alignment for QSAR modeling. Despite the model being based on the STE + ELE fields combination presenting great results (Table S2, S3, and Figure S2), the Kennard-Stone splitting method, with Csp3 (+ 1) as the probe and STE field, continues to provide the most satisfactory outcomes for all statistical parameters evaluated.
In this way, Fig. 3 shows the plots of the experimental (pIC50Exp) and predicted (pIC50Pred) biological response values for the training and test sets compounds, according to the Py-CoMFA models built using the STE field, 2 Å grid spacing, and the Kennard-Stone splitting method, showing the best performance for the probe atoms Csp3 (+ 1) and Osp3 (− 1) compared to the H (+ 1) probe atom. In fact, the Py-CoMFA models had a squared linear correlation coefficient (R2) of 0.861 to Csp3 (+ 1) (Fig. 3A; y = 0.9086x + 0.467; R2 = 0.8608), 0.843 to Osp3 (− 1) (Fig. 3B; y = 0.8995x + 0.508; R2 = 0.8436), and 0.712 to H (+ 1) (Fig. 3C; y = 0.8144x + 0.9211; R2 = 0.7123) as probe atoms.
Experimental versus predicted pIC50 values for the training and test set compounds according to the Py-CoMFA (3D-QSAR) models built using steric field, 2 Å grid spacing, and the Kennard-Stone training and test sets splitting method, by probe atoms and charges: (A) Csp3 (+ 1) (y = 0.9086x + 0.467; R2 = 0.8608); (B) Osp3 (− 1) (y = 0.8995x + 0.508; R2 = 0.8436); and (C) H (+ 1) (y = 0.8144x + 0.9211; R2 = 0.7123).
Table 2 presents the predicted pIC50 and residual (Res = pIC50Exp − pIC50Pred) values calculated with the Py-CoMFA models, considering the Kennard-Stone splitting method, for the training and test set inhibitors during the internal (leave-one-out cross-validation, LOO-cv) and external validations, respectively. Inhibitors of the training and test sets were considered outliers if showing residual values exceeding twice the standard deviation of estimate model (Csp3, 2*SD = 0.888; Osp3, 2*SD = 0.948, H, 2*SD = 0.962). Based on this criterion, the Py-CoMFA models did not identify outliers in the training and test sets compounds to Csp3 and Osp3 models and just one outlier (5f) in the test set of H model (Table 2). The lowest residual values between experimental and predicted pIC50 were for the models from Csp3 (+ 1) and Osp3 (− 1) and the higher ones for the H (+ 1) atom probe (Table 2). Although the H (+ 1) probe atom model presented the worst performance, the three models are highly correlated, since the residuals of all three models showed a strong Pearson correlation with Csp3 versus Osp3 (r = 0.96), Csp3 versus H (r = 0.92), and Osp3 versus H (r = 0.92).
The Py-CoMFA Csp3 (+ 1) contour maps of the steric MIF of the most active compound 4h showed favorable (green) fields on the phenyl and amide group, indicating that bulk substituents on those positions could enhance the protein–ligand interactions. However, it is essential to note that substitution in the nitrogen atom of the amide group has unfavorable (yellow) fields, particularly for bulk substituents such as phenyl group (Fig. 4).
Based on the information obtained from the contour maps of the best Py-CoMFA predictive model, 23 new compounds (7a–w) were designed, maintaining the general cinnamide structure (Fig. 5). In the para-position of the phenyl ring, R1, we tested substituent groups (R1 = H, CH3, OH, NH2, OCH3, F, and O(CO)CH3) with different degrees of inductive and/or resonance effect as electron donor or acceptor. As a substituent at the nitrogen atom of the amide group, we tested H, aliphatic groups, with or without heteroatoms, and with cyclohexyl, pyrrole, and indole rings (Fig. 5).
The Py-CoMFA (Csp3, + 1) model was used to predict the pIC50 values of the newly designed compounds (7a–w). Out of the 23 compounds analyzed, we selected six that had predicted pIC50 values greater than 5.00 M based on the activity of the reference compound 4h (pIC50 = 5.886 M). Although all compounds did not present a predicted biological activity, value greater than the reference compound, interestingly, the highest pIC50 values were observed at 7g (pIC50Pred = 5.169 M) and 7k (pIC50Pred = 5.169 M) with H at R1 position, and indole and cyclohexyl substituents in the R2 position, respectively (Table 3), while the pyrrole ring did not contribute to the activity. Among the aliphatic substituents, the best four compounds were 7o, 7p, 7q, and 7b with acetyl, methyl(thio)ethyl, methoxy-ethyl, and amino groups, respectively. Aliphatic side chains with no heteroatoms or hydroxyl groups did not contribute to the activity as well as para-position substitution of the phenyl ring in R1, with electron donor or acceptor groups, since pIC50 values were lower than unsubstituted derivatives (Table 3).
Applicability domain
To assess the applicability domain, we employed two methods: k-nearest neighbors (kNN) using Euclidean distances as metric and Leverage53,54,55. The kNN methodology is centered on assessing data points’ similarity through their feature vectors, with similarity being defined by the distance in the feature space, often computed using the Euclidean distance metric. It specifically recognizes the k-nearest neighbors of a query data point and employs them for predictive purposes or data classification53.
In contrast, the leverage technique appraises the impact of individual data points on the dataset’s overall comprehension. It computes leverage metrics for each data point, signifying its influence on the model’s analyses or predictions53.
Whereas kNN underscores similarity and neighborhood associations, the leverage approach underscores the significance and sway of individual data points within the dataset. Consequently, while kNN aids in clustering akin data points, the leverage method assists in pinpointing influential or anomalous data points that could considerably impact model performance or comprehension.
Upon evaluating the limits established by kNN (3.02) and Leverage (0.24) for the training set, we found no outlier compounds in the external set, indicating that its chemical space aligns closely with the training set’s (Fig. 6)56,57.
Molecular docking
The constructed Py-CoMFA (3D-QSAR) model was based on compound 4h as an alignment reference. This compound has been reported in the literature for its mixed-type inhibition4, which means it can bind to allosteric sites both to the free enzyme or enzyme–substrate complex. On the other hand, compound 4e is reported as a competitive inhibitor 4 binding to the active site of the free enzyme. As compounds 7g and 7k share a similar structure with the reference compound and were chosen due to their predicted pIC50 activity, we aimed to investigate their behavior in both the potential allosteric sites and the active site of the LaARG protein. Our goal was to compare their behavior with the reference inhibitors 4e and 4h, respectively.
The FTMap58 server was used to detect hot spot residues and identify potential drug-binding sites, including the active site, resulting in two clusters of interest (for more details, please refer to the Supporting Information). The first cluster corresponds to the active site region (amino acid residues detected as hot spots: His102, His127, Asp125, Asp129, His142, Asp231, and Asp233), including two Mn(II) cations, which are presented in a zoom view within a green color (Fig. 6). The second cluster corresponds to an allosteric site region (amino acid residues detected as hot spots: Lys1, Lys2, Met3, Ser4, Trp40, Phe94, Leu96, Leu269, Val270, and Met302), which is presented in a zoom view within an ochre color (Fig. 7). This allosteric site corresponds to a cavity composed of residues from one of the monomers close to the protein–protein interface and in a region opposite the catalytic site.
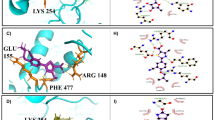
In the first instance, we investigated the potential interaction of derivatives 7g and 7k at the enzyme’s active site by molecular docking. As previously mentioned, compound 4e is described as a competitive inhibitor 4, so both compounds, 7g and 7k, were docked in the first cluster corresponding to the active site of the LaARG model (Fig. 8). In the best docking pose of 7g within the LaARG active site (Fig. 8A), a strong charge-assisted H-bond interaction is observed between the NH group (neutral donor) of the indole ring of 7g and the carboxylate group (ionized acceptor) of Glu185, while this aromatic indole ring also performs a pi-pi T-shaped interaction with the aromatic imidazole ring of His127. Meanwhile a pi-alkyl interaction is observed between the phenyl ring of the cinnamide moiety of 7g and Pro246. Likewise, in the best docking pose of 7k within the LaARG active site (Fig. 8B), an H-bond interaction is observed between the cinnamide NH group and the N3 atom of the imidazole ring of His127, while the cinnamide phenyl ring performs a pi–pi interaction with the imidazole ring of His142, as well as cyclohexyl methyl substituent at the R2 position performs an alkyl-alkyl interaction with Pro246 (Fig. 8B). The inhibitor 4e had a p-OH substituent at the R1 position. This allowed for a H-bond interaction with Glu185. Two more interactions were observed with the amino acids His127 and Gly244. Similar to compounds 7g and 7k, there was also an interaction between Pro246 and the 3,4-dihydroxyphenyl ring (Fig. 8C).
Best molecular docking poses of cinnamide derivatives 7g (A) and 7k (B) and reference 4e (C) within the active site of the LaARG model and the overlapped poses of 7g, 7k and 4e (D). The dashed black lines represent the H-bond interactions with amino acids (represented by spheres). Residues are represented by sticks (green color) interacting with pi-alkyl (dashed yellow lines), pi-pi T-shaped (dashed orange lines), or alkyl-alkyl (dashed blue lines).
Regardless of the types of interactions of these compounds, by the rings as substituents or with the cinnamide moiety, it is worth highlighting that compounds 7g and 7k showed the best-predicted pIC50 values of 5.169 M and 5.151 M, respectively. These compounds occupy the same region within the active site at approximately 6.6 (7g) and 7.8 Å (7k) from Mn2+ ions, interacting mainly with the same His127 and Pro246 residues, presenting only an inverted head-to-tail orientation in relation to each other, in addition to a similar binding mode compared to reference inhibitor 4e (Fig. 8D). Those interactions were important for the ligand binding on the enzyme’s active site, which may reflect in the inhibition of the arginase enzyme.
Afterward, we investigated the potential interaction of compounds 4h, 7g, and 7k into the second cluster of LaARG, corresponding to an allosteric site, by molecular docking (Fig. 9). In the best docking pose of 4h within the LaARG allosteric site (Fig. 9A), H-bond interactions are observed between the cinnamide NH group and the carbonyl oxygen atom of the Ala264 main chain, while the cinnamide phenyl ring performs pi-alkyl, and pi-sulfur interactions with the side chain groups of Leu269 and Met303, respectively. In addition, the 3,4-dihydroxyphenyl ring of 4h performs H-bond interactions with the oxygen atom of Glu265 (Fig. 9A).
Best molecular docking poses of cinnamide derivatives 4h (A), 7g (B), and 7k (C) within an allosteric site of the LaARG model and the overlapped poses of 4h, 7g, and 7k (D). The dashed black lines represent the H-bond interactions with amino acids (represented by spheres). Residues represented by sticks (ochre color) interacting by pi-alkyl (dashed yellow lines), pi-sulfur (dashed purple lines), cation-pi (dashed green lines), or amide-pi stacked (dashed pink lines).
In the best docking pose of 7g (Fig. 9B), an H-bond interaction is observed between the cinnamide NH group and the carbonyl oxygen atom of the Ala264 main chain likewise 4h. The cinnamide phenyl ring performs cation-pi, pi-alkyl, and pi-sulfur interactions with the side chain groups of Lys1, Leu269, and Met303, respectively. In addition, the indole ring of 7g performs two interactions, an amide-pi stacking with Glu265 and a pi-alkyl with Ala264 (Fig. 9B).
In the best docking pose of 7k (Fig. 9C) there is an H-bond interaction between the cinnamide NH group and Leu269, while the cinnamide phenyl ring performs two pi-alkyl interactions with the side chains of Ala89 and Val270 (Fig. 9C). In general, compounds 4h and 7g bound similarly (Fig. 9D) to the allosteric site, while 7k bound closer to the protein–protein interface of the enzyme trimer, i.e., between two monomers.
Molecular dynamics simulations
Subsequently, we conducted classical molecular dynamics (MD) simulations in aqueous systems with the best docking poses of 4h within the allosteric site, 4e within active site, and 7g and 7k within both active and allosteric sites of the LaARG model to verify the dynamic behavior of the putative interaction modes and binding affinities of those derivatives during 200 ns, using the GROMACS software59 with Charmm36 force field60.
Molecular dynamics simulations in the active site
The stability and persistence of interactions were evaluated by RMSD (root-mean-square deviations) of the ligand atoms and Cα atoms of the amino acids in the protein–ligand complexes throughout the MD simulations (Fig. 10A–C). During the RMSD ligand atoms analysis of simulation conducted on the active site, it was observed the reference inhibitor 4e moved out of the active site within the first nanoseconds of the MD (Fig. 10A). It then exhibited movement between the 75 and 125 ns, before returning to its initial position and binding to the active site in the final phase of the simulation. This resulted in a high value of RMSD and standard deviation (SD) of 23.30 ± 16.7 Å. Compound 7g, on the other hand, showed a tendency to move away after 10 ns, bound to the surface of the allosteric site from 50 to 100 ns before leaving again (Fig. 10B). The mean RMSD value was 28.4 ± 10.6 Å and similar to 4e. Compound 7k was observed to leave the active site at the beginning of the simulation, presenting the highest RMSD and SD values of 30.4 ± 19.7 Å (Fig. 10C). We analyzed the Cα-RMSD of the amino acids from the active site of the LaARG-ligand complexes. To ligands 4e, 7g and 7k bonded in the active site, Cα-RMSD values and SD were low, 0.56 ± 0.11 Å, 0.54 ± 0.12 Å and 0.67 ± 0.25 Å, respectively (Fig. 10D–F).
RMSD (Å) analysis relative to the atoms of 4e (A), 7g (B), and 7k (C) and Cα-RMSD analysis of the amino acid relative to complex with 4e (D), 7g (E), and 7k (F) in the LaARG model by molecular dynamics simulations in aqueous systems (200 ns), considering the initial ligand positions within the active site. The red arrow highlights the ligand movement.
The root-mean-square fluctuation (RMSF) during the 200 ns of simulation was used to evaluate the residue’s mobility. The LaARG model residues with the highest observed RMSF values were grouped into four main groups (Fig. 11D). The first group comprises the residues Gln14-Tyr25 (colored in yellow, Fig. 11A–D), which are located around the active site (in green, Fig. 11D), approximately 10 Å from the two Mn(II) cations, and presented RMSF values of 2.3 to 3.0 Å. The second group comprises the residues Gly50-Ala75 (in red, Fig. 11A–D), which are close to the protein surface and their RMSF values varied from 3.72 to 4.9 Å. The third group comprises the residues Pro225-Gly250 (in blue, Fig. 11A,B,D), which are located next to the active site and present RMSF values between 2.0 and 2.9 Å. The fourth group is related to residues Val275-His287 (in purple, Fig. 11A and D), which is below the active site, with considerable RMSF values of around 3.0 Å. It was evident that the greatest fluctuations contribution was from the 4e inhibitor, despite the planned compounds 7g and 7k also showing similar values in the surrounding residues near the active site.
Cα-RMSF analysis relative to the molecular dynamics simulations in aqueous systems (200 ns) of the LaARG-ligand complexes of 4e (A), 7g (B) and 7k (C) in the active site; (D) 3D-structure of the LaARG model highlighting the active site (green) and residues groups purple, yellow, blue, and red with the highest RMSF values.
The analysis of inhibitor 4e’s H-bond interactions revealed that it has the potential to inhibit the target protein by interacting with specific residues such as Asp129, Glu185, Leu269, and Met202 present in the active site (Fig. 12A). The lifetime of these interactions ranged from 9.18% to 34.6% (Fig. 12A), although the RMSD analysis pointed out a lack of interactions between the period of 75 to 125 ns. The H-bond analysis in the active site of the LaARG-7g and LaARG-7k complexes revealed that these intermolecular interactions involving the ligands and certain residues, such as Gly66 and Thr310 (as shown in Fig. 12B) and Arg262 (Fig. 12C), had a short lifetime (5% to 15.9%) and were located away from the initial binding poses (active site), as pointed out by RMSD analysis.
We calculated the binding free energy (ΔGbind), using the MM-PBSA method, for ligands that remained bound to the active site of the LaARG model during the MD simulations. The ΔGbind values reflect that 4e (− 17.8 kcal/mol) and 7g (− 20.6 kcal/mol) presented similar affinity for the active site (Table 4). Upon analysis, we also observed that the van der Waals and solvent-accessible surface values for cinnamates 7g and 7k are quite similar. However, we noticed that the difference in ΔGbind values can be attributed to the higher solvation cost of 7k, which has substantially higher values of 15.1 kcal/mol when compared to 7 g with its lower value of 5.14 kcal/mol. This clearly indicates the higher ΔGbind value observed (− 7.49 kcal/mol).
Molecular dynamics simulations in allosteric site
Subsequently, we conducted MD simulations in aqueous systems with the best docking poses of 4h, 7g, and 7k within the allosteric site of the LaARG model. During the simulation, the compound 4 h displayed notable movement in the allosteric site after 10 ns and maintained its position throughout the rest of the simulation, with an RMSD value of 12.2 ± 2.07 Å, indicating a significant shift in its conformation (Fig. 13A).
RMSD (Å) analysis relative to the atoms of 4h (A), 7g (B), and 7k (C) and Cα-RMSD analysis of the amino acid relative to complex with 4h (D), 7g (E), and 7k (F) in the LaARG model by molecular dynamics simulations in aqueous systems (200 ns), considering the initial ligand positions within the allosteric (black) or active (red) site. The red arrow highlights the ligand movement.
On the other hand, RMSD analysis of 7g (Fig. 13B) indicated the persistence on the binding site until 125 ns of the simulation, showing slight movement between 50 and 125 ns inside the cavity before eventually dissociating with RMSD = 17.28 ± 14.13 Å. Like reference 4h, ligand 7k was found to have a strong affinity for the allosteric site (Fig. 13C) since the binding persisted almost throughout the entire period of the simulations, with only two slight movements observed between 80 and 112 ns and 185 ns, while the RMSD and SD values were low (4.34 ± 0.76 Å).
When ligands 4h, 7g, and 7k were bonded on the allosteric site, the Cα-RMSD to the active site were lower, 0.57 ± 0.13, 0.51 ± 0.14, 0.45 ± 0.10, Å, respectively (in red, Fig. 13D–F). Analysis of the Cα-RMSD for the allosteric site also indicated low variation of 0.54 ± 0.11 Å (4h), 0.43 ± 0.09 Å (7g), and 0.49 ± 0.12 Å (7k), suggesting no significant shift compared to the docking complex (in black, Fig. 13D–F). The structures were stabilized after the first nanoseconds of simulation with a standard deviation below 0.14 Å (Fig. 13D–F).
As observed in the Cα-RMSF analysis of the compounds in the active site, their presence in the allosteric site also caused fluctuations in the regions, mainly of protein loops, to residues Gln14-Tyr25 (colored in yellow, Fig. 14A,B,D) and Gly50-Ala75 (in red, Fig. 14A–D) closed to the protein surface, and Pro225-Gly250 next to allosteric site (in purple, Fig. 14B–D), ranging from 2.5 to 4.2 Å. In addition to these, two more regions presented high RMSF values, belonging to residues Leu150-Pro170 (in blue, Fig. 14A–D) and Val275-Arg300 (in orange, Fig. 14C–D) about 3.0 Å.
Cα-RMSF analysis relative to the molecular dynamics simulations in aqueous systems (200 ns) of the LaARG-ligand complexes of 4h (A), 7g (B) and 7k (C) in the allosteric site; (D) 3D-structure of the LaARG model highlighting the allosteric site (ochre) and residues groups purple, yellow, blue, orange, and red with the highest RMSF values.
About H-bond interactions observed in the allosteric site, the reference inhibitor 4h showed persistent interaction with residues Leu269 and Val270 with high lifetime values of 79.2% (and 8.27%) and 82.8%, respectively. In addition, also performs H-bond with Thr233 (24.4%) continually throughout the MD simulation (Fig. 15A). The derivative 7g interacted with residues Ala264 and Glu265 within the first 50 ns and a 5–19% lifetime. It also interacted with Gly267 from 50 to 125 ns, with a lifetime of 14%, before disconnecting from the enzyme (Fig. 15B). Notably, in arginase from Leishmania mexicana, a salt-bridge cluster (Glu277-Arg216) is known to stabilize the formation of the trimer, which is not present in the human arginase I61. In the LaARG, this salt-bridge is conserved by Glu265-Arg204 and could lead to a possible selectivity for the parasite enzyme. Despite their short lifetimes, these interactions at the allosteric site were equally persistent than those observed at the active site, suggesting that 7g may have affinity for both sites. On the other hand, 7k showed high affinity for LaARG’s allosteric site, presenting lifetime values of up to 76% throughout the MD simulation, mainly with residue Leu269, as reference inhibitor 4h, and others, such as Lys1 and Val270 (Fig. 15C).
Finally, we calculated ΔGbind for ligands that remained bound to the allosteric (4h, 7g, and 7h) site of the LaARG model during the MD simulations (Table 5). It is more evident that cinnamide derivative 7k is promising for inhibition of LaARG in the allosteric site since it presented an equivalent ΔGbind value of − 16.6 ± 1.76 kcal/mol as reference inhibitor 4h (− 17.0 ± 2.14 kcal/mol), while derivative 7g showed lower ΔGbind = − 13.4 ± 1.95 kcal/mol.
Based on our previous discussions, the binding free energy values also indicate the favorable hydrophobicity contribution of phenyl and cyclohexyl groups as substituents, probably due to the characteristic of several residues on the LaARG allosteric site, such as valine, leucine, phenylalanine, serine, methionine, and tryptophan. As a result, the 7k derivative with phenyl and cyclohexyl have stronger interactions within this pocket also due to molecular volume very similar to 4h than the indole-substituted 7g, which is larger.
Finally, the highest energy cost of desolvation of the binding site was observed for cinnamides 4h (20.1 kcal/mol) and 7k (17.3 kcal/mol) due to their substituents characteristics when compared to the indole-substituted 7g (5.62 kcal/mol), in addition to highest energy values of the van der Waals (4h, − 27.9 kcal/mol, and 7k, − 27.7 kcal/mol) and electrostatic terms (4h, − 6.00 kcal/mol, and 7k, − 2.89 kcal/mol) required for a favorable interaction and resulting in their best ΔGbind values (Table 5). It was noted that ΔGbind analysis of the ligands was consistent, as previously emphasized by RMSD, RMSF, and intermolecular interactions via H-bonding, demonstrating the proposed cinnamide 7k promising as a mix or uncompetitive LaARG inhibitor.
In conclusion, it is worth noting that the H-bond interactions between the LaARG model and cinnamide derivatives (4e, 4h, 7g, 7k) occur through the amide group, which acts as a pharmacophore feature. However, the phenyl and cyclohexyl rings of proposed cinnamide 7k are crucial for interaction in the allosteric site, which is mostly made up of residues with aliphatic side chains and is more hydrophobic compared to the active site formed by aspartate and histidine residues and more adaptable to interactions with indole ring of the 7g. It is crucial to emphasize that these compounds should be synthesized and studied in vitro against the arginase molecular target. This approach is necessary to obtain precise and confirmatory details about their inhibitory mechanism of action.
Drug-likeness (oral bioavailability) and ADMET (absorption, distribution, metabolism, excretion, and toxicity) in silico predictions
After analyzing the interactions evidenced by the molecular docking and molecular dynamics results, the two promising compounds, 7g and 7k, had some of their properties related to drug-likeness (oral bioavailability) and ADMET (absorption, distribution, metabolism, excretion, and toxicity) profile predicted in silico (Table 6).
In our in silico study, according to the drug-likeness predicted properties (MW ≤ 500; LogP ≤ 5; HBA ≤ 10; HBD ≤ 5; TPSA ≤ 140) (Table 6), we have found that 7g and 7k do not violate any of the Lipinski and Veber rules. Moreover, both compounds have the potential to be promising as they show predicted high absorption from the gastrointestinal (GI) tract (Table 6). This indicates that they can cross the cell barriers and enter the bloodstream to reach their biological target62. However, it is important to note that these compounds also have the potential to cross the blood–brain barrier (BBB), which may cause toxic side effects62 (Table 6).
Compounds 7g and 7k were predicted as non-substrate of P-glycoprotein (P-gp) (Table 6), an efflux pump protein that impacts drug pharmacokinetics by carrying substances out of cells63. Therefore, 7g and 7k should not affect P-gp function, avoiding overdoses in combined therapies (Table 6).
Cytochrome P450 (CYP) enzymes are fundamental in the metabolism of drugs and xenobiotics64. According to our in silico predictions, 7g and 7k may have the ability to inhibit CYP1A2 and CYPC19. Additionally, 7g may also inhibit CYP2D6 and CYP3A4. Since the CYP1 enzyme family is responsible for metabolizing carcinogenic substances, we should be cautious about administering any substances that inhibit this enzyme65. In addition, the two compounds showed probability of having short half-life (Table 6).
Regarding toxicity predictions, the two compounds did not show any evidence of mutagenic, tumorigenic, reproductive, or irritant properties. They also did not demonstrate a risk of hepatotoxicity. Additionally, there was no indication that they would be active as hERG blockers, which could potentially induce lethal arrhythmia66 (Table 6).
Materials and methods
Data collection and chemical structure construction
A set of 34 inhibitors of the Leishmania (L.) amazonensis arginase (LaARG) from different chemical classes and the corresponding half-maximal inhibitory concentration (IC50, μM) values were collected from data published in the literature covering the years from 2018 to 2023 and selecting active compounds with no reported in silico studies. These 34 inhibitors belong to the chemical classes of chromones (1a–1d)67,68, pyrazolo-pyrimidines (2a–2f)44, phenylhydrazines (3a–3f)45, cinnamides (cinnamic acid amides or cinnamides) (4a–4k)4, and esters (5a–5f)46 derived from 3,4-dihydroxycinnamic acid (caffeic acid)47 (6) (Fig. 1). The 2D and 3D structures of each compound were sketched and constructed in the Marvin Sketch software (https://chemaxon.com/marvin), and geometry optimization was performed using the MMFF94 force field in the same software (ChemAxon, https://chemaxon.com/products/marvin).
Py-CoMFA (3D-QSAR) modeling
A general workflow for developing a 3D-QSAR model, using the 3D-QSAR server (https://www.3d-qsar.com/), comprises uploading a dataset with the Py-MolEdit module (chemical structures and biological activity values), aligning the dataset with the Py-Align module (or optionally using a previously aligned dataset), and then build a model with the Py-CoMFA module.
The (IC50, μM) values of all inhibitors were converted into pIC50 (− LogIC50, M) values using the Py-MolEdit module in the 3D-QSAR server (https://www.3d-qsar.com/). The 34 selected compounds were divided randomly or by the Kennard-Stone method into two groups: 25 as training set and 9 as test set. The pIC50 values were used as the dependent variable (y = biological activity response), while the Py-CoMFA molecular interaction fields (MIFs) were taken as independent variables (x = descriptors). The MIFs are calculated at each grid point of a virtual cubic box by means of a predefined probe atom as steric (STE) and electrostatic (ELE) interaction fields by means of the Lennard–Jones and Coulomb potentials, respectively, and also as a combined steric and electrostatic (STE + ELE) field.
We analyzed the conformations of all inhibitors using the RDkit method applying UFF force field, max attempts of 1000, prune RMS threshold = 0.1, cluster method by RMSD considering 2.0 Å and 50 iterations, through the Py-ConfSearch platform (https://v2.3d-qsar.com/v2/pyconfsearch). We selected the most extended conformation of each inhibitor. The compound with the highest pIC50 (i.e., lowest IC50) value among all the inhibitors was selected as a template for the conformer alignment of other compounds, using Py-Align platform (https://v2.3d-qsar.com/v2/pyalign) applying RDkit method using the most extended conformation of the most active inhibitor. The Py-CoMFA steric (STE), electrostatic (ELE), and combined (STE + ELE) molecular interaction fields were calculated through the 3D-QSAR server (https://v2.3d-qsar.com/v2/pycomfa/) using the following parameters: probes atoms and charges = Csp3 (+ 1), Osp3 (− 1), and H (+ 1); grid spacing = 2.0 Å; grid extension = 5.0 Å; cutoff energy = 30 kcal/mol; minimum sigma = 2 Å; Gasteiger charge model; and a maximum number (N) of principal components (PC) = 15. The efficiency of the Py-CoMFA (3D-QSAR) model was determined by the partial least square (PLS) regression technique.
Internal validation was performed using the leave-one-out cross-validation (LOO-cv) method and applying the Y-randomization test. The cross-validation (q2) and quadratic linear correlation coefficient (r2) analysis were used to select the best model. Statistical analyses of Pearson’s correlation and F-test between models were performed using the GraphPad software (version 8.00) (GraphPad Software, Inc., USA, 6.00) – ANOVA.
To assess the applicability domain, we employed two methods: k-nearest neighbors (kNN) using Euclidean distances as metric and Leverage53,54,55.
Designing of new compounds
Based on the information obtained from the contour maps of the best predictive Py-CoMFA model, 23 new compounds (Fig. 5) were designed by substitution of specific substituent groups (CH3, OH, NH2, OCH3, F and OCO2CH3) with different degrees of inductive and/or resonance effects as electron donor or acceptor in the R1-position of the phenyl ring, and in the R2-position we tested the presence of the cyclohexyl, pyrrole and indole rings. The pIC50 values of the newly designed compounds were predicted using the best predictive constructed Py-CoMFA model.
Molecular docking
We used the structural 3D model of the Leishmania (L.) amazonensis arginase (LaARG) (UniProt accession number: O96394) built previously by Camargo et al.69 using comparative (homology) modeling and as a template the crystal structure of the Leishmania mexicana arginase enzyme in complex with the catalytic product (L-ornithine) (PDB ID: 4IU5, Resolution: 1.95 Å). The model is available in ModelArchive at https://doi.org/10.5452/ma-a8uzk. Leishmania (L.) amazonensis is a member of the Leishmania mexicana complex, which can cause all types of leishmaniasis infections. The molecular docking was performed using the GOLD (v. 2022.3) (Genetic Optimization for Ligand Docking) program, applying the ChemPLP function score70.
The FTMap family of web servers (https://ftmap.bu.edu/) was used to detect and characterize hot spot amino acid residues as potential binding sites58, revealing two regions for the LaARG protein model to be evaluated by molecular docking: the active site and an allosteric site. The LaARG active site region, including the detected hot spot residues and two Mn(II) cations, was centered on the Cartesian coordinates (x = 15.5865, y = − 14.5855, z = − 3.9095) in the middle of Mn(II)(1) and Mn(II)(2) cations. The LaARG allosteric site region was centered on the Cartesian coordinates (x = 12.0600, y = − 5.7110, z = − 16.3440) of the center of mass of the detected hot spot residues. The radius (r) of both binding sites was set to 15 Å. The ligands were subjected to 10 iterative docking runs. We performed clustering analysis to identify probable best-scored pose results. The analysis of intermolecular interactions was carried out using the Discovery Studio Visualizer program (Dassault Systèmes BIOVIA, Discovery Studio Modeling Environment, Release 2017, San Diego: Dassault Systèmes, 2016) and the figures were constructed using the Visual Molecular Dynamics (VMD) program (http://www.ks.uiuc.edu/Research/vmd/)71.
Molecular dynamics simulations
The molecular dynamics simulations were performed using the GROMACS version 5.1.4 and 2019 package72,73, applying the CHARMM36 all-atom (AA) force field60 and TIP3P water model with the protein–ligand poses (complexes) from molecular docking. The atoms order was corrected by sort_mol2_bonds.pl Python script and ligand topology acquired through the CHARMM General Force Field for organic molecules (CGenFF) server (https://cgenff.umaryland.edu/initguess/).
The ionization states of the protein’s residues were adjusted to pH 7.4 using the pdb2gmx Python script. Each complex was centered inside a cubic periodic box (4.374 × 3.702 × 3.504 nm, volume = 453.95 nm3), solvated with water TIP3P type, and neutralized with Na+ ions. The residues Asp125, Asp129, Asp231, Asp233, His102, His127, and His142 coordinated with metal ions and had their distance restraints and constraints included in protein topology.
Systems were submitted to energy minimization steps using a convergence criterion of 1000 kJ mol−1. nm−1 followed by gradient conjugate until 100 kJ mol−1.nm−1. The minimized systems were submitted to two equilibration steps, considering 300 K of temperature (V-rescale thermostat) and 1 bar of pressure (Parrinello-Rahman barostat), realized during 1 ns and position restraint for enzyme and ligand. First, considering volume, and temperature constant (NVT ensemble), and then considering the system as isothermal-isobaric (NPT ensemble). Hydrogen atoms in the system were frozen using the LINCS algorithm, and the long-distance electrostatic interactions using the PME algorithm with a cut-off radius of 1 nm were applied to the van der Waals and Coulomb interactions. After the thermalization step, the molecular dynamics (MD) simulations were performed during 200 ns, using 2 fs integration time and 20 Å of cut-off radius to the long-distance interactions.
The LaARG-ligand complexes in aqueous systems were evaluated by the root mean square deviations (RMSD) and root mean square fluctuation (RMSF), the hydrogen bonding (H-bond) intermolecular interactions were computed considering the cut-off until 0.50 nm by the package of GROMACS 2019. The H-bonds frequency was calculated using the hbmap2grace package74, and the graphs were plotted using the XMGRACE 5.1.19 program75.
The binding free energy (ΔGbind) between the ligands and the LaARG model was calculated using the molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) method through the module added to the GROMACS 5.1.4 program package. The contribution of residues to the binding energy was computed by MmPbSaDecomp.py Python script. Figures were constructed using the Visual Molecular Dynamics (VMD) program (http://www.ks.uiuc.edu/Research/vmd/).
In silico predictions of pharmacokinetic and toxicity parameters (ADMET)
In silico pharmacokinetic (absorption, distribution, metabolism, and excretion, ADME) parameters related to oral bioavailability (drug-likeness) of the compounds were assessed by rule-based filters from Lipinski76 and Veber using ADMETlab 2.0 platform (https://admetmesh.scbdd.com/). In silico toxicity predictions were performed using the OSIRIS Property Explorer (https://www.organic-chemistry.org/prog/peo/) platform.
Conclusions
The study utilized 3D-QSAR (Py-CoMFA) and other molecular modeling techniques, such as molecular docking and molecular dynamics simulations, to analyze how arginase inhibitors from L. (L.) amazonensis interact and their affinity. The pIC50 values calculated with the best Py-CoMFA model were in excellent correlation with the experimental values. The molecular docking study identified the favorable hydrophobicity contribution of phenyl and cyclohexyl groups as substituents in the cinnamide compounds. These groups can act as pharmacophoric groups in designing new inhibitors. The study showed that derivatives interacted more effectively with the allosteric site of the protein rather than the active site. The results identified two compounds which can guide the development of more effective arginase inhibitors for the treatment against leishmaniasis.
Data availability
The authors confirm all data generated and analyzed during this study are available in the article and in the supplementary information.
References
World Health Organization (WHO). Leishmaniasis. https://www.who.int/health-topics/leishmaniasis (2024).
Grimaldi, G. & Tesh, R. B. Leishmaniases of the New World: Current concepts and implications for future research. Clin. Microbiol. Rev. 6, 230–250 (1993).
Pan American Health Organization. Leishmaniasis. https://www.paho.org/en/topics/leishmaniasis (2024).
Da Silva, E. R. et al. Cinnamides target leishmania amazonensis arginase selectively. Molecules 25, 5271 (2020).
Nagle, A. S. et al. Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem. Rev. 114, 11305–11347 (2014).
de Carvalho, M., Clímaco, L. K. & Fujiwara, R. T. Vaccine development for human leishmaniasis. In Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges: Focus on Leprosy, Leishmaniasis, Melioidosis and Tuberculosis (ed. Christodoulides, M.) (Springer International Publishing, 2023). https://doi.org/10.1007/978-3-031-24355-4_14.
Sundar, S. & Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 16, 237–252 (2015).
Pradhan, S., Schwartz, R. A., Patil, A., Grabbe, S. & Goldust, M. Treatment options for leishmaniasis. Clin. Exp. Dermatol. 47, 516–521 (2022).
Uliana, S. R. B., Trinconi, C. T. & Coelho, A. C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 145, 464–480 (2018).
Pan American Health Organization. Guideline for the Treatment of Leishmaniasis in the Americas 2nd edn. (Pan American Health Organization, 2022). https://doi.org/10.37774/9789275125038.
Wijnant, G.-J. et al. Tackling drug resistance and other causes of treatment failure in leishmaniasis. Front. Trop. Dis. 3, 837460 (2022).
Drugs for Neglected Diseases. Leishmaniasis. http://www.dndi.org/diseases-projects/leishmaniasis (2024).
Jones, N. G., Catta-Preta, C. M. C., Lima, A. P. C. A. & Mottram, J. C. Genetically validated drug targets in leishmania: Current knowledge and future prospects. ACS Infect. Dis. 4, 467–477 (2018).
Stempin, C. C., Tanos, T. B., Coso, O. A. & Cerbán, F. M. Arginase induction promotes Trypanosoma cruzi intracellular replication of Cruzipain-treated J774 cells through the activation of multiple signaling pathways. Eur. J. Immunol. 34, 200–209 (2004).
Roberts, S. C. et al. Arginase plays a pivotal role in polyamine precursor metabolism in leishmania. J. Biol. Chem. 279, 23668–23678 (2004).
Pessenda, G. & da Silva, J. S. Arginase and its mechanisms in Leishmania persistence. Parasite Immunol. 42, e12722 (2020).
Colotti, G. & Ilari, A. Polyamine metabolism in Leishmania: From arginine to trypanothione. Amino Acids 40, 269–285 (2011).
Ilari, A., Fiorillo, A., Genovese, I. & Colotti, G. Polyamine-trypanothione pathway: An update. Future Med. Chem. 9, 61–77 (2017).
Phillips, M. A. Polyamines in protozoan pathogens. J. Biol. Chem. 293, 18746–18756 (2018).
Aoki, J. I., Laranjeira-Silva, M. F., Muxel, S. M. & Floeter-Winter, L. M. The impact of arginase activity on virulence factors of Leishmania amazonensis. Curr. Opin. Microbiol. 52, 110–115 (2019).
Santiago-Silva, K. M., Camargo, P. G. & Bispo, M. L. F. Promising molecular targets related to polyamine biosynthesis in drug discovery against leishmaniasis. Med. Chem. 19, 2–9 (2023).
Hassan Baig, M. et al. Computer aided drug design: Success and limitations. Curr. Pharm. Des. 22, 572–581 (2016).
Cherkasov, A. et al. QSAR modeling: where have you been? Where are you going to?. J. Med. Chem. 57, 4977–5010 (2014).
Muratov, E. N. et al. QSAR without borders. Chem. Soc. Rev. 49, 3525–3564 (2020).
Jagiello, K. et al. Advantages and limitations of classic and 3D QSAR approaches in nano-QSAR studies based on biological activity of fullerene derivatives. J. Nanoparticle Res. 18, 256 (2016).
Aouidate, A. et al. Furanone derivatives as new inhibitors of CDC7 kinase: Development of structure activity relationship model using 3D QSAR, molecular docking, and in silico ADMET. Struct. Chem. 29, 1031–1043 (2018).
Cramer, R. D., Patterson, D. E. & Bunce, J. D. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J. Am. Chem. Soc. 110, 5959–5967 (1988).
Goodford, P. J. A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J. Med. Chem. 28, 849–857 (1985).
Silakari, O. & Singh, P. K. QSAR: Descriptor calculations, model generation, validation and their application. in Concepts and Experimental Protocols of Modelling and Informatics in Drug Design 29–63 (Elsevier, 2021). https://doi.org/10.1016/B978-0-12-820546-4.00002-7.
Ragno, R. et al. Teaching and learning computational drug design: Student investigations of 3D quantitative structure-activity relationships through web applications. J. Chem. Educ. 97, 1922–1930 (2020).
Ragno, R. www.3d-qsar.com: A web portal that brings 3-D QSAR to all electronic devices—the Py-CoMFA web application as tool to build models from pre-aligned datasets. J. Comput. Aided. Mol. Des. 33, 855–864 (2019).
Halder, A. K. & Dias Soeiro Cordeiro, M. N. Advanced in silico methods for the development of anti- leishmaniasis and anti-trypanosomiasis agents. Curr. Med. Chem. 27, 697–718 (2020).
de Sousa, N. F. et al. Selene-Ethylenelacticamides and N-aryl-propanamides as broad-spectrum leishmanicidal agents. Pathogens 12, 136 (2023).
Torres, P. H. M., Sodero, A. C. R., Jofily, P. & Silva-Jr, F. P. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 20, 4574 (2019).
AlRawashdeh, S. & Barakat, K. H. Applications of molecular dynamics simulations in drug discovery. In Computational Drug Discovery and Design (eds Gore, M. & Jagtap, U. B.) 127–141 (Springer, 2024). https://doi.org/10.1007/978-1-0716-3441-7_7.
Aouidate, A. et al. Combined 3D-QSAR and molecular docking study on 7,8-dialkyl-1,3-diaminopyrrolo-[3,2-f] Quinazoline series compounds to understand the binding mechanism of DHFR inhibitors. J. Mol. Struct. 1139, 319–327 (2017).
Nour, H. et al. Combined computational approaches for developing new anti-Alzheimer drug candidates: 3D-QSAR, molecular docking and molecular dynamics studies of liquiritigenin derivatives. Heliyon 8, e11991 (2022).
Challapa-Mamani, M. R. et al. Molecular docking and molecular dynamics simulations in related to leishmania donovani: An update and literature review. Trop. Med. Infect. Dis. 8, 457 (2023).
Daoui, O. et al. 3D-QSAR, ADME-Tox, and molecular docking of semisynthetic triterpene derivatives as antibacterial and insecticide agents. Struct. Chem. 33, 1063–1084 (2022).
Daoui, O., Elkhattabi, S. & Chtita, S. Rational identification of small molecules derived from 9,10-dihydrophenanthrene as potential inhibitors of 3CLpro enzyme for COVID-19 therapy: A computer-aided drug design approach. Struct. Chem. 33, 1667–1690 (2022).
Martinez, P. A. & Petersen, C. A. Chronic infection by Leishmania amazonensis mediated through MAPK ERK mechanisms. Immunol. Res. 59, 153–165 (2014).
Henard, C. A., Carlsen, E. D., Hay, C., Kima, P. E. & Soong, L. Leishmania amazonensis amastigotes highly express a tryparedoxin peroxidase isoform that increases parasite resistance to macrophage antimicrobial defenses and fosters parasite virulence. PLoS Negl. Trop. Dis. 8, e3000 (2014).
Manjolin, L. C., dos Reis, M. B. G., do Maquiaveli, C. C., Santos-Filho, O. A. & da Silva, E. R. Dietary flavonoids fisetin, luteolin and their derived compounds inhibit arginase, a central enzyme in Leishmania (Leishmania) amazonensis infection. Food Chem. 141, 2253–2262 (2013).
Feitosa, L. M. et al. New pyrazolopyrimidine derivatives as Leishmania amazonensis arginase inhibitors. Bioorganic Med. Chem. 27, 3061–3069 (2019).
Crizanto de Lima, E. et al. Phenylhydrazides as inhibitors of Leishmania amazonensis arginase and antileishmanial activity. Bioorg. Med. Chem. 27, 3853–3859 (2019).
de Come, S. A. et al. In vitro and in silico analyses of new cinnamid and rosmarinic acid-derived compounds biosynthesized in Escherichia coli as Leishmania amazonensis arginase inhibitors. Pathogens 11, 1020 (2022).
da Silva, E. R. et al. Cinnamic acids derived compounds with antileishmanial activity target Leishmania amazonensis arginase. Chem. Biol. Drug Des. 93, 139–146 (2019).
Madhavan, T. A review of 3D-QSAR in drug design. J. Chosun Nat. Sci. 5, 1–5 (2012).
Roy, K., Kar, S. & Das, R. N. Introduction to 3D-QSAR. In Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment 291–317 (Elsevier, 2015). https://doi.org/10.1016/C2014-0-00286-9.
Verma, J., Khedkar, V. & Coutinho, E. 3D-QSAR in drug design—A review. Curr. Top. Med. Chem. 10, 95–115 (2010).
Puzyn, T., Mostrag-Szlichtyng, A., Gajewicz, A., Skrzyński, M. & Worth, A. P. Investigating the influence of data splitting on the predictive ability of QSAR/QSPR models. Struct. Chem. 22, 795–804 (2011).
Schober, P. & Schwarte, L. A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 126, 1763–1768 (2018).
Sahigara, F. et al. Comparison of different approaches to define the applicability domain of QSAR models. Molecules 17, 4791–4810 (2012).
Roy, K., Kar, S. & Ambure, P. On a simple approach for determining applicability domain of QSAR models. Chemom. Intell. Lab. Syst. 145, 22–29 (2015).
Hanser, T., Barber, C., Marchaland, J. F. & Werner, S. Applicability domain: Towards a more formal definition. SAR QSAR Environ. Res. 27, 865–881 (2016).
Dias-Silva, J. R., Oliveira, V. M., Sanches-Neto, F. O., Wilhelms, R. Z. & Queiroz Júnior, L. H. K. SpectraFP: A new spectra-based descriptor to aid in cheminformatics, molecular characterization and search algorithm applications. Phys. Chem. Chem. Phys. 25, 18038–18047 (2023).
Santana, M. V. qsar_ad (Applicability domain for QSAR models). GitHub repository https://github.com/marcossantanaioc/qsar_ad (2022).
Kozakov, D. et al. The FTMap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nat. Protoc. 10, 733–755 (2015).
Van Der Spoel, D. et al. GROMACS: Fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Huang, J. & MacKerell, A. D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
D’Antonio, E. L. et al. Crystal structure of arginase from Leishmania mexicana and implications for the inhibition of polyamine biosynthesis in parasitic infections. Arch. Biochem. Biophys. 535, 163–176 (2013).
Bagchi, S. et al. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Devel. Ther. 13, 3591–3605 (2019).
Vilar, S., Sobarzo-Sánchez, E. & Uriarte, E. In silico prediction of P-glycoprotein binding: Insights from molecular docking studies. Curr. Med. Chem. 26, 1746–1760 (2019).
McDonnell, A. M. & Dang, C. H. Basic review of the cytochrome P450 system. J. Adv. Pract. Oncol. 4, 263–268 (2013).
Nelson, D. R. et al. P450 superfamily: Update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6, 1–42 (1996).
Du, L., Li, M. & You, Q. The interactions between hERG potassium channel and blockers. Curr. Top. Med. Chem. 9, 330–338 (2009).
Garcia, A. R. et al. Leishmania infantum arginase: Biochemical characterization and inhibition by naturally occurring phenolic substances. J. Enzyme Inhib. Med. Chem. 34, 1100–1109 (2019).
Da Silva, E. R. et al. Dietary polyphenols rutin, taxifolin and quercetin related compounds target: Leishmania amazonensis arginase. Food Funct. 10, 3172–3180 (2019).
Camargo, P. G. et al. Thiohydantoins as anti-leishmanial agents: In vitro biological evaluation and multi-target investigation by molecular docking studies. J. Biomol. Struct. Dyn. 40, 1–10 (2020).
Korb, O., Stützle, T. & Exner, T. E. An ant colony optimization approach to flexible protein–ligand docking. Swarm Intell. 1, 115–134 (2007).
Humphrey, W., Dalke, A. & Schulten, K. V. M. D. Visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Abraham, M. J. et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Berendsen, H. J. C., van der Spoel, D. & van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995).
Gomes, D. E. B., da Silva, A. W., Lins, R. D., Pascutti, P. G. & A., S. HbMap2Grace. Software for mapping the hydrogen bond frequency. http://lmdm.biof.ufrj.br/software/ (2024).
Turner, P. J. XMGRACE, Version 5.1.19. Center for coastal and land-margin research, Oregon Graduate Institute of Science and Technology, Beaverton, OR (2005).
Lipinski, C. A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 1, 337–341 (2004).
Acknowledgements
The authors thank the “Centro Nacional de Processamento de Alto Desempenho em São Paulo” (CENAPAD-SP) for the computational resources used for the molecular dynamics’ simulations.
Funding
This research was funded by the Brazilian governmental agencies: FAPERJ (“Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro”) (E-26/205.966/2022, E-26/205.967/2022, SEI-260003/019723/2022), CAPES (“Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”), and CNPq (“Conselho Nacional de Desenvolvimento Científico e Tecnológico”).
Author information
Authors and Affiliations
Contributions
P.G.C. Conceptualization, methodology, formal analysis, investigation, writing. C.R.S. Methodology, formal analysis. M. G. A. Review and editing, supervision. C. R. R. Review and editing, project administration, supervision, software. C. H. S. L. Review and editing, project administration, supervision, software.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Camargo, P.G., dos Santos, C.R., Girão Albuquerque, M. et al. Py-CoMFA, docking, and molecular dynamics simulations of Leishmania (L.) amazonensis arginase inhibitors. Sci Rep 14, 11575 (2024). https://doi.org/10.1038/s41598-024-62520-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62520-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.