Abstract
Indigenous health has posted complex challenges worldwide, particularly due to historical economic, territorial, social and environmental processes, which may lead to emergence and reemergence of pathogens. In addition to few Coxiella burnetii serosurveys in vulnerable populations, especially in developing tropical countries, no comprehensive One Health approach has focused on human-animal infection along with potential environmental determinants. Accordingly, this study aimed to assess the seroprevalence of anti-C. burnetii antibodies in indigenous populations and their dogs from 10 indigenous communities distributed in southern and southeastern Brazil, along with the correspondent healthcare professionals. In overall, 8/893 (0.90%; 95% CI 0.45–1.76) indigenous and 1/406 (0.25%) dog samples were seropositive, with 7/343 (2.04%) individuals the 1/144 (0.69%) dog from the Ocoy community, located in the city of São Miguel do Iguaçu, bordering Argentina at south, and far 10 km at west from Paraguay. All 84 healthcare professionals tested seronegative.
Similar content being viewed by others
Introduction
Indigenous health has posted complex challenges worldwide and particularly in Brazil due to historical economic, territorial, social and environmental processes which may lead to emergence and reemergence of pathogens1. An overall 1,693,535 indigenous persons, accounting for 0.83% of the nationwide population, was recently estimated by the 2022 Brazilian Census and included 305 ethnic groups speaking more than 274 languages2. As these populations mostly live in remote areas, under basic infrastructure and struggling to maintain their primitive ancestral culture, native indigenous communities have been considered among the highest social vulnerability in Brazil1. In such a scenario, the close human-animal-environmental interaction may also impact determinants of indigenous health, particularly with expansion of livestock production and clandestine mining, pushing these populations even closer to endemic and novel pathogens, under absence of basic health conditions1.
Q fever has been described as a worldwide underreported and underdiagnosed zoonosis, mostly due to initial asymptomatic infection leading to nonspecific symptoms, making difficulty prompt and proper diagnosis. However, disease may progress and trigger cardiac and neurological problems, particularly in patients with persistent infection3.
The Q fever causative agent, Coxiella burnetii, exposure has been reported in 85/887 (9.6%) indigenous Orang Asli population of Malaysia4, and in 11/267 (4.1%) indigenous persons of James Bay native communities in Canada3. Although with not fully established epidemiology, these studies have associated Coxiella burnetii seropositivity with geographical and demographic factors, and with wildlife contact4. In addition, Australian aboriginal dogs were 2.8-fold more likely infected than purebred, domestic and shelter dogs5. Finally, a single study in South America has found no exposure out of 73 individuals tested in 2014 and 2015 at the indigenous Halite-Paresi community, located in the central-western Brazil6.
Although considered underdiagnosed and with little occurrence data in Latin America3, C. burnetii infection has reportedly affected cattle and small ruminant populations in southern and southeastern Brazil, causing animal abortions and exposing human populations up to 30 km away from the infected farms7,8,9. Although febrile illness has been commonly associated with several other diseases, C. burnetii may persist in the body, causing chronic complications such as endocarditis, hepatitis, and meningitis10,11. As underestimated disease, C. burnetii impact may be even higher in indigenous communities, with no confirmed cases or serological exposure evidence to date in Brazil.
In addition to few C. burnetii serosurveys in vulnerable populations, particularly in developing tropical countries, no comprehensive One Health approach to date has surveyed the C. burnetii infection in indigenous populations, characterized by their close relationship with natural forest areas and wildlife animals. Accordingly, this study aimed to assess the seroprevalence of anti-C. burnetii antibodies in indigenous persons, their dogs and healthcare professionals from 10 indigenous communities distributed in southern and southeastern Brazil.
Results
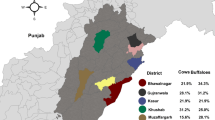
A total of 893 human and 406 dog serum samples were obtained from the 10 indigenous communities, including 343 individuals and 144 dogs at Ocoy community, 144 and 55 at Kopenoty, 82 and 34 at Araça’í, 79 and 44 at Ekeruá, 78 and 34 at Nimuendajú, 48 and 33 at Tereguá, 39 and 8 at Guaviraty, 33 and 18 at Tupã Nhe’e Kretã, 24 and 21 at Kuaray Haxa, and 23 and 15 at Pidoty. In addition, the 84 healthcare professionals were sampled during the incursions to the indigenous communities (Fig. 1).
Sampling locations and distribution of C. burnetti exposure in indigenous communities of Paraná and São Paulo states, size distance invariance hypothesis (SDIH) of national catching areas. All components of the map were open source and open data-direct link to the source of icons and symbols used: https://github.com/qgis/QGIS/tree/master/images/svg; direct link of the boundaries from the officially public data of the Brazilian government, used as background base layer: https://geoftp.ibge.gov.br/cartas_e_mapas/bases_cartograficas_continuas/bc250/versao2021/post_gis/bc250_2021_11_18.zip; public indigenous areas and SDIH areas for shading and location: https://geoserver.funai.gov.br/geoserver/web/. Map produced by the authors using QGIS 3.18.
Overall, 8/893 (0.90%; 95% CI 0.45–1.76) indigenous and 1/406 (0.25%) dog samples presented anti-C. burnetii antibodies, with 7/343 (2.04%) individuals and the 1/144 (0.69%) dog from the Ocoy community, located in the city of São Miguel do Iguaçu, bordering Argentina at south, and far 10 km at west from Paraguay. All 84 healthcare professionals tested negative. Among the positive human samples, six presented 1:128 titers, one 1:64 and one 1:256, while the dog titers were 1:128. Separating by community, seven of the positive human samples and the positive dog samples were from the Ocoy community, Paraná. Human seropositivity in the Ocoy community was 2.04% (7/343, 95% CI 1.00–4.15) and dog seropositivity was 0.70% (1/144, 95% CI 0.12–3.83). The other positive human sample was from Kopenoty community, São Paulo state. Table 1 shows the results of the analysis of risk factors associated with C. burnetii positivity in indigenous communities. It was observed a statistical significance between the seropositivity for C. brunetti and the habit of hunting.
Discussion
This is the first report of anti-C. burnetii antibodies in indigenous communities of Brazil. Ocoy was the most seropositive indigenous community, presenting 7/8 human and the single dog cases. As specific characteristics, Ocoy community was the closest to bordering countries, with frequent international crossing to visit other indigenous communities located nearby in Argentina and Paraguay. Also, this community was the most inserted into wildness, historically transferred from their original area (which was submerged) and living at the artificial lakeshore of the Itaipu Company, effective in 1982 and the biggest electric powerplant dam in Brazil since, currently the third worldwide in electricity production. The other only seropositive human sample was detected at the Kopenoty community, São Paulo state, likely exposed outside the indigenous area.
Vulnerable populations worldwide have been reportedly more susceptible to infections in general, due to factors such as social inequality, housing conditions, lack of basic sanitation and basic hygiene habits9. In Brazil, descendants of former black slaves have shown 44/200 (22.0%) seropositivity to anti-C. burnetii antibodies, indicating a higher risk of exposure of such vulnerable population when compared to general population. Nonetheless, geographical isolated location, cultural habits and other associated factors may have prevented Brazilian indigenous populations from their exposure to C. burnetii, as only 8/893 (0.90%) seropositive samples were found herein. In addition, no previous detection was made out of 73 indigenous individuals in the Amazon of central-western Brazil, serosurveyed in 2 consecutive years6.
The Ocoy community may present unique geographical and social characteristics among the indigenous communities surveyed herein. As already mentioned, the original Ocoy indigenous area was confiscated and later submerged by the Itaipu electric power plant dam, a binational company assembled on the Paraná river at Iguassu city, between Brazil and Paraguay, nearby the Argentinian border12. The Iguassu city was created to house the 20,000 employees at the time, currently the second biggest Brazilian city throughout the 15,735 km (9778 miles) terrestrial border, with 285,500 habitants12. Thus, the growth and significant changes may have influenced the wildlife relationship and predispose circulation of Coxiella and other infectious agents in such formerly isolated indigenous communities.
Despite the indigenous habits obtained herein were not statistically associated as risk factors for Q fever, low seropositivity have impaired proper analysis. Moreover, the habits of the Ocoy community were similar to those of other surveyed communities. Nonetheless, wildlife contact during hunting and trapping was statistically associated with leptospirosis but not with Q fever due to < 5% seroprevalence in 267 James Bay residents4, while < 1% prevalence was observed in 917 blood samples from Nunavik residents13, both near Quebec, Canada. Finally, two indigenous Orang Asli communities closer to forest areas in Malaysia were significantly more likely seropositive than the other surveyed villages, with an overall 9.6% (85/887) seroprevalence4.
In contrast, human populations living outside indigenous communities of southern and southeastern Brazil have shown higher C. burnetii seroprevalence, including 129/604 (21.5%) urban febrile individuals in São Paulo9 and 21/437 (4.8%) in Minas Gerais states7, and 44/200 (22.0%) rural descendants of former black slaves in the Paraná state14. The absence of exposure in indigenous communities within the same areas may be explained by the considerable distance between such populations and cattle and sheep farms, livestock species frequently associated with C. burnetii infection and environmental contamination in Brazil8,15,16. Likewise, absence in the Kuaray Haxa, Pidoty and Guaviraty indigenous communities, all located at environmentally preserved seashore and oceanic island areas, have also indicated that livestock distance may be an associated protective factor for C. burnetii exposure.
Although Q fever transmission may occur by contact with dog vaginal fluids after giving birth, the role of dogs in the human cycle remains unclear6. The only case reported to date has described a Q fever outbreak affecting three family members with pneumonia, following exposure to an infected parturient dog and death of all puppies17. In addition, a single study comparing dogs from native communities with domestic and shelter dogs has shown that Aboriginal dogs in Australia were 2.8 times more likely infected than other dog groups5. Such pattern was not observed herein in dogs of Brazilian indigenous communities, with only 1/144 (0.69%) seropositive dog. Thus, further studies should be conducted to fully establish the zoonotic potential and associated risk factors of Q fever between dogs and humans. Noteworthy, the study herein was also the first assessment in indigenous dog populations to the C. burnetii infection in the Americas.
As pre-Columbian Brazilian indigenous communities (estimated of 1,000 different ethnicities with 5–13 million habitants in 1500) have never domesticated any native wildlife species, exotic domestic animals brought from Portuguese and other European settlers including companion, livestock, and pest species had a disastrous impact in the original indigenous lifestyle18. The presence of domestic dogs (and cats) has been historically increased nationwide in almost all Brazilian indigenous communities, even in communities deeply isolated into the Amazon region as the Yanomamis, currently aggravated by pet abandonment in surrounding areas (by illegal settlers, miners, and hunters) associated to uncontrolled pet reproduction, and lack of veterinarian assistance including vaccination, deworming and tick control19. As a serious historical consequence, dogs have played an important role in several zoonotic diseases in Brazilian indigenous communities including toxocariasis, toxoplasmosis, tungiasis, strongyloidiasis, rabies, leptospirosis, leishmaniasis, Chagas disease and rickettsiosis19. Thus, the gap in Q fever occurrence and impact in these communities has led to the pioneer study herein.
Herein, although the odds ratio estimation was not statistically significant considering the 95% CI (lower limit was close to 1.0), the seropositivity for C. brunetii was associated with the habit of hunting. A relevant risk of exposure to tick-borne pathogens, including C. brunetii, has been considered for hunters during hunting activities20. Therefore, it is possible to argue that the indigenous population evaluated herein are prone to be exposed to tick bite and becoming infected by C. burnetii while hunting.
As limitations, although no cattle, horses, sheep, or swine were allowed (or observed) in the indigenous communities, the present study has not mapped or surveyed livestock animals in the surroundings areas as associated risk factors. Particularly in the Ocoy indigenous community, livestock raising and production in nearby farms may be a potential source for the C. burnetii exposure found herein, and should be further investigated.
Finally, future studies should be conducted in nearby Paraguayan and Argentinean indigenous communities, to pinpoint and fully establish the source of C. burnetii infection, and potential spreading by terrestrial international crossing of indigenous individuals. In addition, future pneumonia patients from the Ocoy community should be surveyed for Q fever as rule out diagnosis, as a guideline model for surveillance and prevention activities in Brazil and other endemic countries.
This is the first report of anti-C. burnetii antibodies in indigenous communities of Brazil, mostly at the Ocoy indigenous community, with frequent international border crossing to visit other indigenous communities located nearby in Argentina and Paraguay, serving as an alert for international spreading of disease. Future surveys should be conducted in Paraguayan and Argentinian indigenous communities cross the border, to fully establish such infection risk and transmission.
Material and methods
Ethics statement
This study was approved by the Human Health Ethics Committee at the Brazilian Ministry of Health (protocol 52.039.021.9.0000.0102). Before participating in the present study, informed consent was obtained from all participants and/or their legal guardians. All procedures were performed in accordance with relevant guidelines and regulations.
Indigenous communities
The serosurvey herein was carried out in the ethnic groups of Guarani, Terena and Kaingang from 10 different indigenous communities, located in the Paraná state (six communities) at southern and São Paulo state (four communities) at southeastern Brazil. All six indigenous communities in the Paraná state were situated within the Atlantic Forest biome at seashore (3), countryside (2), and far-west (1) areas, while all four communities in São Paulo state were situated nearby each other, within the Cerrado biome.
The six indigenous communities of Paraná state included Tupã Nhe’e Kretã, Araça’í, Pidoty, Kuaray Haxa, Guaviraty, and Ocoy. These communities were characterized by living on natural resources for survival such as fishing and agriculture, in close contact with wildlife, and with poor basic hygiene infrastructure. The Kuaray Haxa, Pidoty, and Guaviraty communities were located on seashore and oceanic islands, where biodiversity and traditional finishing culture have been well-preserved, while Tupã Nhe’e Kretã and Araça’í were situated in preserved areas of the metropolitan area of Curitiba, state capital and the eighth biggest Brazilian city with 1.8 million habitants. Finally, the far-west Ocoy community was marked by traditional indigenous countryside culture, high people movements through Paraguayan and Argentinean borders, and majority of individuals speaking only Guarani (and not the official Portuguese language).
The four indigenous communities of São Paulo state were located nearby and included Kopenoty, Tereguá, Ekeruá and Nimuendajú. These communities were less traditional in indigenous culture than those in the Paraná state and had adequate basic sanitation, living mostly by agriculture as the main family activity income. Many indigenous people worked outside the communities, in nearby towns or on rural properties.
Healthcare professionals
Healthcare professionals working in the indigenous communities through the National Unified Health System (SUS) were also invited to voluntarily participate in the serosurvey. Blood samples were collected at the headquarters—Special Department of Indigenous Health (SDIH) Seashore South (Fig. 2). The health professionals were grouped according to their level of contact with the population (high: doctors, nurses, drivers, and teachers; medium: multidisciplinary teams; low: administrators), their role in the communities and the frequency of their visits.
The 34 Special District of Indigenous Health (SDIH) centers and correspondent areas within the geographical map of Brazil, including the SDIH—Seashore South (number 13). All components of the map were open source and open data—direct link to the source of icons and symbols used: https://github.com/qgis/QGIS/tree/master/images/svg; direct link of the boundaries from the officially public data of the Brazilian government, used as background base layer: https://geoftp.ibge.gov.br/cartas_e_mapas/bases_cartograficas_continuas/bc250/versao2021/post_gis/bc250_2021_11_18.zip; public indigenous areas and SDIH areas for shading and location: https://geoserver.funai.gov.br/geoserver/web/. Map produced by the authors using QGIS 3.18.
Study design and sample collection
After signing an individual voluntary consent, human blood samples were collected from indigenous people and health professionals by cephalic venipuncture performed by certified nurses, and dog blood samples by jugular venipuncture performed by certified veterinarians. All blood samples were centrifuged at 800g for 5 min, serum placed in cryotubes and kept at − 20 °C until processing. Epidemiological data was collected using individual questionnaires, mostly helped by an indigenous translator, to obtain information including on age, gender, and hunting habits. All indigenous people living in these locations were included in the study, with no age limit. Dog owners also answered epidemiological questionnaires with information on their dogs including age, gender, race, and hunting habits.
Serological analysis
For human and dog serodiagnosis, anti-C. burnetii antibodies were detected by immunofluorescence with a previously established protocol21, using C. burnetii strain At12-infected Vero cells as antigen. Human-specific and dog-specific IgG fluorescein isothiocyanate (FITC) antibodies were used. Positive and negative controls were obtained from human and dog serum samples, previously tested in the herein laboratory, with positive and negative titers of 1:16. Phosphate buffered saline (PBS) with a pH of 7.2 was used to create sequential dilutions at ratios of 1:16, 1:64, 1;128, 1:256, 1:512, 1:1024, and 1:4096. Samples were considered positive when antibody titers were equal or greater than 64. For seropositive samples, final titers were determined as the last dilution in which ≥ 50% of bacterial fluorescence remained apparent. As this in-house test was not designed to differentiate antibody phases, the study herein has not identified acute from chronic seropositive patients. However, the aim of the present study was to assess serological prevalence of anti-C. burnetii antibodies in healthy vulnerable populations21. In addition, such diagnostic tests have proven to be as effective at detecting anti-phase II antibodies as anti-phase I antibodies, which could lead to the possibility of detecting chronic patients in further studies21.
Statistical analysis
The association between anti-C. burnetii antibodies (IgG) and risk factors (gender, age, and hunting habit of indigenous) was evaluated by the Fisher exact test (univariate analysis) due to the low number of seropositive cases (7/690). The odds ratio (OR) was calculated with 95% confidence intervals (95% CI) by median biased estimation. All statistical analysis was conducted in R software v. 4.2.3 (R core team). A P value of < 0.05 was considered significant.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Scalco, N. & Louvison, M. Indigenous health: struggles and resistance in the construction of knowledge. Correspondência https://doi.org/10.1590/S0104-12902020000003 (2020).
Censo 2022|IBGE. https://www.ibge.gov.br/estatisticas/sociais/trabalho/22827-censo-demografico-2022.html.
de França, D. A. et al. Overview of Q fever in Brazil: An underestimated zoonosis. Rev. Inst. Med. Trop. Sao Paulo 65, e39 (2023).
Khor, C.-S. et al. Seroprevalence of Q {Fever} among the indigenous {People} ({Orang} Asli}) of {Peninsular {Malaysia}. Vector Borne Zoonotic Dis. 18, 131–137 (2018).
Shapiro, A. J. et al. Seroprevalence of Coxiella burnetii in Australian dogs. Zoonoses Public Health 63, 458–466 (2016).
Vivi-Oliveira, V. et al. Seroprevalence and risk factors of Q fever in an indigenous community in the Brazilian Legal Amazonia/ Soroprevalência e fatores de risco da febre q em uma comunidade indígena da amazônia legal brasileira (2023).
Meurer, I. R. et al. Seroprevalence estimate and risk factors for Coxiella burnetii infections among humans in a highly urbanised Brazilian state. Trans. R. Soc. Trop. Med. Hyg. 116, 261–269 (2022).
Mioni, M. de S. R. et al. Coxiella burnetii in slaughterhouses in Brazil: A public health concern. PLoS One 15, e0241246 (2020).
de França, D. A. et al. Seropositivity for Coxiella burnetii in suspected patients with dengue in São Paulo state, Brazil. PLoS Negl. Trop. Dis. 16, e0010392 (2022).
Miller, H. K., Priestley, R. A. & Kersh, G. J. Q Fever: A troubling disease and a challenging diagnosis. Clin. Microbiol. Newsl. 43, 109–118 (2021).
Wery, F., Delaere, B., Schraverus, P. & Higny, J. Q fever endocarditis. Acta Cardiol. 76, 668–669 (2021).
Grief, S. N. & Miller, J. P. Infectious disease issues in underserved populations. Prim. Care 44, 67–85 (2017).
AVÁ-GUARANI: A construção de Itaipu e os direitos territoriais.
de França, D. A. et al. Serosurvey of Coxiella burnetii in descendants of former black slaves (Quilombola Communities) of Southern Brazil. Microorganisms. https://doi.org/10.3390/microorganisms12010092 (2024).
Messier, V. et al. Seroprevalence of seven zoonotic infections in Nunavik, Quebec (Canada). Zoonoses Public Health 59, 107–117 (2012).
Mioni, M. de S. R. et al. Molecular detection of Coxiella burnetii in aborted bovine fetuses in Brazil. Acta Trop. 227, 106258 (2022).
de França, D. A. et al. Serosurvey of Coxiella burnetii in police officers and working dogs in Brazil: Case report and One Health Implications. Trop. Med. Infect. Disease. https://doi.org/10.3390/TROPICALMED9040078 (2024).
Pettan-Brewer, C. et al. From the approach to the concept: One Health in Latin America-experiences and perspectives in Brazil, Chile, and Colombia. Front. Public Heal. 9, 687110 (2021).
Linardi, P. M. et al. Occurrence of the off-host life stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann. Trop. Med. Parasitol. 104, 337–345 (2010).
Sgroi, G. et al. Tick exposure and risk of tick-borne pathogens infection in hunters and hunting dogs: a citizen science approach. Transbound. Emerg. Dis. 69, e386–e393 (2022).
de França, D. A. et al. Comparison of three serologic tests for the detection of anti-Coxiella burnetii antibodies in patients with Q Fever. Pathog. (Basel, Switzerland) 12, 873 (2023).
Acknowledgements
To the Special Indigenous Health District (DSEI), the Special Secretariat for Indigenous Health (SESAI), and the indigenous community leaders, namely Andreia de Fátima Fernandes, Dionísio Rodrigues, Josias Castro da Silva, Regiane Rodrigues, and Rivelino Gabriel de Castro, for collaborating with our actions and collection.
Funding
The present research was funded through the Brazilian National Council for Scientific and Technological Development-CNPq (404687/2021–0 and 401302/2022–9).
Author information
Authors and Affiliations
Contributions
A.W.B. made substantial contributions to the conception, A.W.B. design of the work; J.H.F., D.A.F., M.C.S., R.G., V.A.S., L.M.B. and A.W.B. made the analysis, J.H.F., D.A.F., M.C.S., L.M.B., M.B.L., R.G., V.A.S., L.B.K. and A.W.B. made the interpretation of data; D.A.F., L.B.K., A.W.B. have drafted the work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farinhas, J.H., de França, D.A., Serpa, M.C. et al. One Health approach to Coxiella burnetii in Brazilian indigenous communities. Sci Rep 14, 10142 (2024). https://doi.org/10.1038/s41598-024-60850-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60850-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.