Abstract
Gut microbiota impact host physiology, though simultaneous investigations in ectothermic vertebrates are rare. Particularly, amphibians may exhibit more complex interactions between host physiology and the effects of gut microbiota due to the combination of seasonal changes and complex life histories. In this study, we assessed the relationships among food resources, gut bacterial communities, and host physiology in frogs (Phelophylax nigromaculatus), taking into account seasonal and life history variations. We found that food sources were not correlated with physiological parameters but had some relationships with the gut bacterial community. Variations in gut bacterial community and host physiology were influenced by the combined effects of seasonal differences and life history, though mostly driven by seasonal differences. An increase in Firmicutes was associated with higher fat content, reflecting potential fat storage in frogs during the non-breeding season. The increase in Bacteroidetes resulted in lower fat content in adult frogs and decreased immunity in juvenile frogs during the breeding season, demonstrating a direct link. Our results suggest that the gut microbiome may act as a link between food conditions and physiological status, and that the combined effect of seasons and life history could reinforce the relationship between gut microbiota and physiological status in ectothermic animals. While food sources may influence the gut microbiota of ectotherms, we contend that temperature-correlated seasonal variation, which predominately influences most ectotherms, is a significant factor.
Similar content being viewed by others
Introduction
The direct impact of gut microbiota on animal physiology is recognized through symbiosis and co-evolution with the host1,2. Endothermic vertebrates, including humans, have been the main focus of gut microbiota research. Compared to ectothermic vertebrates, studies on their physiological processes or response mechanisms are more developed3,4. There has been a recent increase in studies on ectothermic animals' gut microbiota, but still, this area needs more investigation due to the relative lack of research compared to endothermic creatures. The knowledge gap is pronounced and understanding gut microbiota in ectothermic animals can be a complex task. Ectothermic animals' body temperature is determined by external factors, and that affect the gut microbiota5,6. Most research involving these creatures has focused on comparing microbial communities in relation to health status, field investigation, and exposure to environmental factors7,8,9. However, our understanding of the direct relationship between an ectothermic host's physiological state and its gut microbiota remains incomplete. Numerous studies have indicated potential impacts of gut microbiota changes on aspects such as immunity, digestive efficiency, and energy metabolism. Further research is necessary to quantify these interactions and elucidate the mechanisms of response. Through such studies, we can achieve a more detailed insight into the response of animal health to these complex interactions, thus advancing our knowledge of the field.
Frogs may undergo more drastic environmental changes that can affect their gut microbiota and physiology compared to other vertebrates. As most frog species alternate between terrestrial and aquatic habitats throughout their life cycles, they experience a variety of environmental combinations10,11. Specifically, the physiological changes during metamorphosis from tadpoles to adults and the seasonal patterns of reproduction in temperate regions represent extreme environmental shifts. One of the most significant changes during metamorphosis is the transition from an herbivorous to a carnivorous diet, which accompanies substantial modifications in visceral organs12. These changes in gastric or intestinal morphology and diet during metamorphosis profoundly alter the diversity and community composition of the gut microbiota13. The physiological effects of life history are important because they can affect not only a single life history, but also later life stages14,15.
As a result, we can expect variations between juvenile frogs, who are in a post-metamorphosis growth phase, and adults, whose growth is complete. These differences in gut microbiota homeostasis and physiological stability may stem from the complexities of amphibian life history and physical growth factors, such as body surface area and weight. It is also important to note that their biology, including food source, reproduction, physiology, and metabolism, are subject to seasonal changes, especially temperature variations in temperate regions16. Winter hibernation halts feeding activities, modifies physiology, and significantly reduces metabolic rate, affecting processes such as digestion and glucose metabolism17,18. Additionally, hormonal levels and energy metabolism may shift during the breeding season post-hibernation19,20 and in non-breeding seasons in preparation for hibernation21,22. Thus, the varied experiences of life history and seasonal changes can trigger more complex responses when combined as opposed to when they occur individually.
In this study, we aimed to ascertain whether a combination of life history and seasonal changes affects the physiological state and relationship of black-spotted pond frogs (Phelophylax nigromaculatus), encompassing factors such as dietary composition, gut bacterial community, body composition, and innate immunity. Previous research revealed that shifts in insect fauna, encompassed by seasonal variations, lead to alterations in the food source, as evidenced by gut contents, thereby influencing the gut microbiota23. However, these changes in food sources might also be integrated as part of the overall seasonal transformation.
Furthermore, although changes in the gut microbiota may vary between host species and in the intensity of their response, these alterations are not merely short-term phenomena. Thus, using techniques like stable isotope analysis, which reflects food sources over longer terms (weeks or months), and aligning the response timescales of both the microbiota and the food source can enhance the accuracy of our inferences24. Carbon stable isotope ratios enable the identification of the origin of consumed food sources, while nitrogen stable isotope ratios offer insights into an individual's trophic level25,26. By combining these two isotope ratios, one can estimate both food resource availability and prey contribution27,28.
To evaluate the frogs' physiological state, we analyzed body composition, an indicator of energy storage and metabolism29,30, and innate immunity, which can signify resilience against diseases31,32. Food sources can directly influence amphibian physiology, such as body composition and immunity29,33, and potentially alter physiology through interactions with gut microbiota. Notably, fat serves as a primary energy source for amphibians, from reproduction to successful hibernation17,34, making the understanding of its interaction with gut microbiota, particularly in a seasonal context, essential. Additionally, immunity plays a crucial role in population sustainability and presents significant challenges for amphibians, with disease infection being a primary factor in population decline35. To enhance the conservation and ecological understanding of amphibians, it is crucial to explore the dynamics of immune responses across seasonal and life cycle changes, particularly during periods of instability. We anticipated that adult and juvenile frogs would exhibit distinct physiological variation (immunity and fat ratio) by seasonal difference, with even more pronounced differences of host physiology and gut microbiota observed in juvenile frogs. Conversely, adaptation to life history in adult frogs may be more stable compared to juvenile frogs, even though energy-intensive breeding behaviors like spawning and advertisement calling during the breeding season represent significant energy expenditure throughout their life history20,36. Though identifying and accurately interpreting physiological responses to large and intricate factors, a blend of life history and seasonal responses, can be an immensely challenging task, it is a crucial step toward understanding these phenomena in real-world contexts. Ultimately, we elucidated a relationship between food source, gut bacterial community, and physiology, furthering our understanding of these interactions in ectothermic animals.
Results
Comparison of stable isotope ratio
We compared the food sources among adult breeding (AB), adult non-breeding (AN), juvenile breeding (JB), and juvenile non-breeding (JN) frogs. There was no significant difference (H = 3.26, p = 0.35) in the carbon stable isotope ratio (δ13C) was observed, whereas the nitrogen stable isotope ratio (δ15N) showed a slight difference (H = 8.57, p = 0.04) (Fig. 1a–c). The difference in δ15N between adult and juvenile frogs in both the breeding and non-breeding seasons was not observed (Dunn’s post hoc, p > 0.05). In adult frogs, the δ15N values did not differ significantly by season (Dunn’s post hoc, p > 0.05), whereas juvenile frogs had higher δ15N ratios during the breeding season compared to the non-breeding season (Dunn’s post hoc test, p < 0.05). The sample size-corrected standard ellipse area (SEAc), which indicates food resource availability estimated by using δ13C and δ15N, was highest in adult frogs during the breeding season (SEAc = 9.90) and lowest during the non-breeding season (SEAc = 4.25). In juvenile frogs, a higher SEAc was observed in the non-breeding season (SEAc = 8.90) than in the breeding season (SEAc = 7.18), which was in contrast to the pattern observed in adult frogs (Fig. 1d).
Food resource availability and δ13C and δ15N values of adult breeding (AB), adult non-breeding (AN), juvenile breeding (JB), and juvenile non-breeding frogs (JN). (a) Standard ellipse areas and convex hull, which estimate food resource availability, among the four frog groups, (b) Comparison of δ15N values among the four groups, (c) Comparison of δ13C values among the four groups, (d) Comparison of food resource availability (standard ellipse area) among the four groups. Significant differences (p < 0.05) were determined using a Dunn’s post hoc test following a Kruskal–Wallis test, and are indicated by lowercase letters in panels (b) and (c).
Diversity of gut bacterial community
We compared the alpha diversity, and found that the number of observed species (H = 3.69, p = 0.30) and chao1 index (H = 3.69, p = 0.30) did not exhibit significant differences among the four groups (Fig. 2a–b). The Shannon index, based on species heterogeneity (H = 25.28, p < 0.01), and the PD (phylogenetic diversity) whole tree (H = 12.72, p < 0.01) showed significant differences among the four groups (Fig. 2c–d). In the comparison of the Shannon index, no significant difference was found between adult and juvenile frogs (Dunn's post hoc, p > 0.05); however, seasonal differences in diversity were observed. The Shannon index in the frogs from the non-breeding season was higher than that in the frogs from the breeding season (Dunn's post hoc, p < 0.05). In the comparison of the PD whole tree diversity, significant differences were observed only between adult frogs in the non-breeding season and juvenile frogs in the breeding season (Dunn's post hoc, p < 0.05), attributed to the combined effects of life history and seasonal variations.
Alpha and beta diversity of gut bacterial communities of frogs by season and life history: (a) Observed species, (b) Chao1 index, (c) Shannon index, (d) phylogenetic diversity (PD) whole tree, (e) scatter plot of principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity, and (f) dissimilarities of gut bacterial communities among the four groups. Significant differences (p < 0.05) were determined using a Dunn’s post hoc test following a Kruskal–Wallis test, and are indicated by lowercase letters in panels (a), (b), (c), (d), and (f).
Beta diversity also explains the strong difference of composition in gut bacterial community between breeding and non-breeding seasons, rather than differences between adult and juvenile frogs. PERMANOVA analysis revealed significant differences in the bacterial community composition among the four groups (R2 = 0.18, F = 5.71, p < 0.05). PCo1 and PCo2 contributed to only 22.7% of the variance (Fig. 2e); nonetheless, differences of composition in bacterial community among groups were significant (Bray–Curtis dissimilarity, p < 0.05). PCoA accounted for differences of composition in the bacterial community during the breeding season. In comparison with the Bray–Curtis dissimilarity, the composition of the bacterial community was significantly divided by the breeding season (Dunn's post hoc, p < 0.05). Furthermore, within the breeding season, there was a significant division in bacterial community composition between adult and juvenile frogs (Dunn's post hoc, p < 0.05) (Fig. 2f).
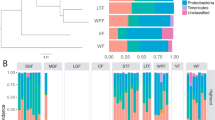
Composition of bacterial community and functional group
At the phylum and genus levels, the relative abundance of the gut bacterial composition significantly differed in frogs between the breeding season and the non-breeding season, although not between adult and juvenile frogs. At the phylum level, among the four groups, the gut bacterial communities of frogs were dominated by Firmicutes, Bacteroidetes, and Proteobacteria (Fig. 3a). In particular, Firmicutes (H = 24.416, p < 0.001) and Bacteroidetes (H = 32.434, p < 0.001) had remarkable differences in the four groups (Fig. 3b). The relative abundance of Firmicutes in frogs the breeding season (adult; 49.24%, juvenile; 44.05%) was lower (Dunn’s post hoc, p < 0.05) than that in the non-breeding season (adult; 62.72%, juvenile; 66.41%). Contrastingly, Bacteroidetes showed higher (Dunn’s post hoc, p < 0.05) relative abundances in frogs from the breeding season (adult; 31.59%, juvenile; 39.94%) than those from the non-breeding season (adult; 11.46%, juvenile; 15.01%). At the genus level, Bacteroides dominated most of the gut bacterial composition in the breeding season both adult (24.96%) and juvenile frogs (32.14%), whereas in the non-breeding season, Faecalicatena, Bacteroides, Clostridium, and Pseudoflavonifractor dominated evenly (Fig. 3c). In Venn diagram, there was less overlap of gut bacterial Operational Taxonomic Units (OTUs) in frogs from the breeding season and frogs from the non-breeding season than that in adult and juvenile frogs, resulting in clear differences in composition of the gut bacterial community in seasons (Fig. 3d). The number of unique OTUs was 270 in adult frogs during the breeding season, 312 in adult frogs during the non-breeding season, 276 in juvenile frogs during the breeding season, and 389 in juvenile frogs during the non-breeding season. The overlapping OTUs in adult and juvenile frogs from the breeding and non-breeding seasons were 17 and 51, respectively. Contrastingly, the overlapping OTUs of adult and juvenile frogs in the breeding and non-breeding seasons were very high at 280 and 331, respectively.
Relative abundance and Venn diagram illustrating the overlap of gut bacterial taxa in frogs, segmented by season and life history: (a) The composition of the bacterial community at the phylum level across the four groups, (b) A comparison of the relative abundance of bacterial taxa at the phylum level, where significant differences are marked with asterisks (* for p < 0.05, *** for p < 0.001) and non-significant differences are noted as 'ns'. Significant differences were identified using a Kruskal–Wallis test, (c) The composition of the bacterial community at the genus level across the four groups, (d) A Venn diagram depicting the overlap of gut bacterial operational taxonomic units (OTUs) among the four groups.
The abundance of functional groups in the gut bacterial composition also differed by the breeding season. Metabolism was the function with the highest relative abundance in the four groups, and environmental and genetic information processing were the bacterial functional groups with the next highest relative abundance (Fig. 4a). During the breeding season, both adult and juvenile frogs exhibited a higher relative abundance of functional groups related to metabolism and a lower relative abundance of functional groups involved in environmental information processing compared to their counterparts in the non-breeding season. At the second enrichment level, the relative abundance of metabolism in functional groups was mainly related to carbohydrate and amino acid metabolism, and energy metabolism. During the breeding season, frogs exhibited relatively high carbohydrate metabolism, but low amino acid and energy metabolisms. The relative abundance of functional groups involved in environmental information processing, specifically membrane transport and signal transduction, was found to be lower during the breeding season compared to the non-breeding season (Fig. 4b). At the third enrichment level, the functional groups of ABC transporters, amino sugar and nucleotide sugar metabolism, methane metabolism, and starch and sucrose metabolism were those with prominent differences. During the breeding season, frogs exhibited a lower relative abundance of ABC transporters and methane metabolism, and a higher relative abundance of amino sugar and nucleotide sugar metabolism (Fig. 4c).
Difference of immunity and body composition
Body length and weight did not significantly differ between seasons for adult frogs (Dunn's post hoc, p > 0.05). In contrast, juvenile frogs exhibited significantly lower body length and weight during the breeding season compared to the non-breeding season (Dunn's post hoc, p < 0.05) (Table 1). Bacterial killing ability (BKA), which represents the innate immunity of complement systems, fat content ratio, and lean body content ratio, had the significant difference estimated by combination in life history and seasonal difference, among the four groups (Table 1). Juvenile frogs in breeding season had a lower BKA than that of the other groups, and only adult frogs in the non-breeding period had a higher fat content and lower lean body mass than that of the other groups.
Relationship of food resource, gut bacterial community, and physiological conditions
Spearman's correlation analysis of the food source contents, gut bacterial diversity, relative abundance of Firmicutes and Bacteroidetes, and physiological conditions showed a correlation among some contents (Fig. 5). Observed species, Chao1 index, and Shannon index, which are metrics of alpha diversity, did not show significant correlations with food sources or physiological conditions (p > 0.05). A significant correlation (Spearman's R = 0.35, p < 0.05) between δ13C and δ15N was observed, and particularly, δ15N significantly correlated with the PD whole tree (Spearman's R = 0.24, p = 0.04) and the relative abundance of Firmicutes (Spearman's R = − 0.23, p = 0.04) and Bacteroidetes (Spearman's R = 0.25, p = 0.02), explaining the relationship between food source and gut bacterial community. However, δ15N had no direct correlation (p > 0.05) with items representing the physiological state. Contrastingly, the PD whole tree and relative abundance of Firmicutes and Bacteroidetes significantly correlated with physiological conditions. Increases in BKA correlated with decreases in PD whole tree (Spearman's R = − 0.30, p = 0.01), increased relative abundance of Firmicutes (Spearman's R = 0.28, p = 0.01), and decreased relative abundance of Bacteroidetes (Spearman's R = − 0.28, p = 0.01). An increase in fat content explained an increase in the relative abundance of Firmicutes (Spearman's R = 0.23, p = 0.04) and a decrease in the relative abundance of Bacteroidetes (Spearman's R = − 0.27, p = 0.01), whereas an increase in lean body content explained an increase in the relative abundance of Bacteroidetes (Spearman's R = 0.23, p = 0.03).
Correlation among food sources (δ13C and δ15N), gut bacterial diversity (observed species, Chao1 index, Shannon index, and phylogenic diversity whole tree; PD), relative abundance of the two dominant bacterial phyla in the gut (Firmicutes and Bacteroidetes), and physiological condition (bacterial killing ability; BKA, fat content, and lean body content) by Spearman correlation analysis. The color gradient of the correlation plot indicates the Spearman correlation coefficient, and gray boxes indicate significant correlations (p < 0.05).
Discussion
Phenological difference and relationship of food source and gut microbiota
In this study, we confirmed that differences in food sources, gut bacterial community, and host physiology are influenced by the combined effects of seasonal changes and life history. Seasonality significantly contributed to variations in food sources and the diversity and composition of the gut bacterial community. The physiology of the host appeared to reflect the combined influences of seasonal differences and life history. The gut bacterial community was particularly closely related to host physiology, with correlations also observed with certain food source components. However, no direct correlation was identified between host physiology and food sources.
The differences in δ15N and SEAc values, representing the trophic level and food availability, respectively, revealed seasonal difference. Particularly, δ15N was associated with certain aspects of gut bacterial diversity and composition, but not immunity or body composition. Generally, frogs are opportunistic predators, and their food sources are often determined by body size (including head size) and environmental resource availability37,38. However, adult frogs allocate less energy to somatic growth due to reproductive efforts; thus, the reduced food availability during the non-breeding season, as compared to the breeding season, may stem from seasonal variations in environmental resources rather than differences in body size. This is expected as the composition of prey insects in temperate regions greatly varies with the seasons39. Our findings suggest a combination of growth and seasonality determines the differences in trophic level and food availability in juvenile frogs. Unlike adults, juveniles allocate energy to somatic growth year-round instead of focusing on breeding.
We also observed correlations between the relative abundances of Firmicutes and Bacteroidetes and δ15N, including PD. This is likely because seasonal differences in food source and gut bacterial community strengthened the relationship between the two variables. The patterns of differences in certain indices of gut bacterial diversity mirrored the differences in δ15N. Differences in gut microbiota according to food resource availability, confirmed by gut contents according to seasonal changes, were reported earlier23. However, akin to our study, differences in food sources were ultimately incorporated into seasonal changes. As a result, the combined effects of seasons and life history can amplify this relationship. While food sources undoubtedly affect changes in the gut microbiome, we suggest that seasonality may have a stronger effect in ectothermic animals. To gain a better understanding, the strength of the direct correlation between food sources and gut microbiota needs to be validated through laboratory experiments.
However, physiological parameters did not show any correlation with food conditions. Therefore, we postulate that instead of a direct correlation between physiological parameters and food sources, the gut bacterial community could mediate the response between these two factors. To elucidate more detailed processes and clarify their interrelationships, future studies will need to explore manipulations of food sources under laboratory conditions.
Relationship of gut microbiome and host physiology strengthen by combined effect of seasons and life history
Seasonal differences strongly influence variation in gut bacterial diversity and composition, more so than life history. Some comparisons revealed the result of a combined effect of seasonal differences and life history. The PD whole tree showed statistical differences between adult frogs from the non-breeding season and juvenile frogs from the breeding season. Contrastingly, prior research has indicated that there is no significant gap in the diversity and composition of gut microbiota between adult and juvenile frogs8,40. These studies seemingly contradict the seasonal patterns of gut microbiota found in both endothermic and ectothermic animals41,42. For endothermic animals, the story is more complex. Changes in microbiota according to external temperature arise from metabolic alterations, all aimed at maintaining body temperature43. Ectothermic animals, on the other hand, see most alterations in their microbial community due to changes in body temperature itself, along with related physiological factors44. Gut microbial diversity may provide physiological stability by modulating resistance or resilience in different environments that hosts and populations may experience45. Our results support the fact that gut bacterial diversity strongly varies with seasonal change and additionally suggest that differences can be enhanced when combined with life history.
The ecological context shaped by combined effect of seasons and life history seems to significantly impact the variations of the bacterial phyla Firmicutes and Bacteroidetes. Firmicutes play a crucial role in carbohydrate fermentation and enhancing nutrient uptake efficiency46. In addition, the symbiotic relationship between Firmicutes and Bacteroidetes is notable, with the ratio of these two microbial populations being associated with conditions like obesity, characterized by high fat mass47. A high proportion of Firmicutes is likely to foster fat absorption and energy storage in the host. This process effectively boosts the efficiency of caloric intake48,49. In the non-breeding season, the relative abundance of Firmicutes surpassed that of the breeding season. This trend aligns well with the primary activities during the non-breeding season for frogs: feeding, digestion, and energy storage in anticipation of hibernation. Our observations further indicate that functional groups related to digestion predominantly exist in both adult and juvenile frogs during the non-breeding season. Typically, fat content in frogs tends to peak just before hibernation, in the period between summer and fall, preparing them for successful hibernation and the subsequent breeding season17,50. This relationship also appears to be linked to the increased prevalence of the genus Clostridium, which could be important for energy absorption during the non-breeding season51. The observed correlation between higher levels of Firmicutes and increased fat content suggests that this bacterial group may support fat storage at this time. This likely occurs through a close interaction with the host's physiology, illustrating a complex and fascinating interplay between the gut microbiota and the host's metabolic processes.
Members of the Bacteroidetes phylum aid in immune and intestinal microbalance by degrading polysaccharides, facilitating nutrient utilization, developing the immune system, and assisting in intestinal mucosal vascularization52. The high relative abundance of Bacteroidetes in the breeding season may play different physiological support roles in adult and juvenile frogs. Decreases in immunity were found only in juvenile frogs during the breeding season, while lower fat content and higher lean body content were observed exclusively in adult frogs during the non-breeding season. After hibernation, adult and juvenile frogs appear in paddy fields for breeding and growth, respectively12,53. Adult frogs experience explosive energy consumption for reproduction during the breeding season, and juvenile frogs must recover their immunity in a decreased immune state. Unlike juvenile frogs, adult frogs exhibit reproductive behavior rather than growth during the breeding season, showing a low fat content during the breeding season among the annual rhythms50. In the case of male frogs, energy consumption is considerable during the breeding season because they make advertisement calls, move to find females, and find and protect territories favorable for breeding54. This energy usually comes from glucose and stored fat55, and the abundance of Bacteroidetes in adult frogs during the breeding season suggests that this metabolism may be bacterial assisted. In fact, the functional groups related to glucose metabolism were dominant in adult frogs during the breeding season. Similar patterns are observed at the genus level. The genus Bacteroides, which increases during the breeding season, plays a role in sensing nutrient availability and facilitating energy utilization. The observed decrease in fat content alongside an increase in Bacteroides, a member of the phylum Bacteroidetes, may reflect a relationship strengthened by both seasonal changes and life history. This suggests that the gut microbiota, particularly Bacteroides, may support the reproductive activities of adult male frogs by aiding in metabolic processes.
Immunity can be lowered during hibernation or at low temperatures because the low temperatures increase the energy costs of immune cell production53. Decreased immunity requires time for recovery53. In our study, this was only found in juvenile frogs. As expected, adults appeared to have relatively high immunity stability with minimal seasonal fluctuations. However, juvenile frogs possess a more immature immune status compared to adult frogs; thus, the decreased immunity observed in juvenile frogs during winter appears to recover more slowly as the spring season progresses than in adult frogs. In this process, Bacteroidetes, which play a role in developing the immune system, may exist in a high state to support immune recovery in juvenile frogs during the breeding season52. Within the Bacteroidetes phylum, Bacteroides was notably abundant during the breeding season and is also associated with the host immune system and pathogen control56. The contrast in immune states and the high levels of Bacteroides in juvenile frogs between breeding and non-breeding seasons illustrate this association. The observed negative correlations between these two factors underscore a relationship strengthened by phenological changes. Furthermore, the distinct physiological responses observed in adult and juvenile frogs indicate that similar bacterial community shifts within the same season can support different physiological outcomes depending on the amphibian's life history.
From a physiological viewpoint, BKA may also be influenced by energy trade-offs57,58, suggesting that frogs with high fat, exhibiting a high-energy state, may also have increased innate immunity. Immune balance is influenced by several steroid hormones, notably those involved in energy metabolism and reproduction. An increase in corticosterone, which responds to stress, may reduce immunity59, while testosterone supplementation often boosts it in larger males60. Moreover, this regulation of physiological immunity is affected not just by a range of environmental factors but also by dietary control61. Additionally, in frogs, high fat content is positively associated with fertility, influencing the fertilization success in male frogs as well as egg and clutch sizes in female frogs62,63. High fat contents, characterized by a narrow prey spectrum and low activity in captive frogs, is considered detrimental for individual frogs64, but this does not seem to be the case in wild populations. We also demonstrated the frogs most vulnerable to disease or those with weak immune responses are likely to be juvenile frogs from winter to spring. Since the increased immunity of juvenile frogs during the non-breeding season reaches the same level as in adult frogs, a frog can be comparatively safe once it has passed through the spring season.
Conclusion
Seasonal differences mainly influenced food sources, gut bacterial diversity, and host physiology. Phenological elements like life history and seasonal shifts appear to exert a more profound impact on gut microbiome changes, relegating food sources to a lesser role (Fig. 6). In the end, seasonal factors, including temperature, heavily guide ectothermic animals, and their responses can be magnified with life history considerations.
A diagram for the conclusion of this study. Gray arrows indicate direct correlations, while dashed arrows indicate potential relationships that may be indirect. Seasonal variation cause to be strong differences in the three traits, and combined effect seasonal variation and life history strengthened the relationship between physiology and the gut bacterial community. Additionally, the gut bacterial community can serve as a bridge connecting the relationship between food source and physiology.
While innate immunity had high volatility in juvenile frogs, changes in fat content hinged solely on reproductive metabolism of adult frogs. Conversely, variations in gut microbiota, including aspects like bacterial diversity and composition, were consistent across life stages. We thus infer that juvenile frog may not be more susceptible to gut bacterial community shifts during seasonal transitions, such as from spring to summer, unlike immunity. However, since this conclusion may specifically apply to the species studied, future research across multiple species and taxa is necessary. Also, extreme conditions like hibernation might tell a different story.
Importantly, our research stands out in identifying a direct link between gut microbiota and the host's physiological state in ectotherms. Moreover, the correlations found between food sources and host physiology hint at the gut microbiota serving as an intermediary connection. These findings enrich our understanding of microbial communities and complex physiological responses in ectothermic animals. Still, the intricate nature of physiological processes, governed by various intertwined factors, calls for a more thorough investigation. The existing insights shed light on this multifaceted relationship but also highlight the need for continued exploration.
Methods and materials
Field investigation
Black-spotted pond frogs (P. nigromaculatus) are known to be highly philopatric, meaning they often remain in the same location throughout both breeding and non-breeding seasons, engaging in breeding and feeding behaviors65. Juvenile frogs, which do not reproduce, also appear at breeding sites after wintering and immediately begin feeding.
We collected both adult and juvenile frogs in May 2022, during the breeding season, from six paddy fields in Gongju City (Fig. 7). All adult frogs collected were males, identified by the presence of a vocal sac and nuptial pads on the first finger, which are secondary sexual characteristics. Juvenile frogs were distinguished as individuals with a smaller body size lacking male characteristics. In the field, individuals that did not clearly fit these distinctions were not collected. To minimize the potential effects of regional characteristics, topography, and cultivation practices, we selected three plain paddy fields and three terraced paddy fields for study. Although the water composition and altitude of these two types of paddy fields are similar, plain paddy fields are larger and accommodate mechanized farming. In contrast, terraced paddy fields have not been subject to land consolidation and are located on sloping hillsides, surrounded by forests. From each site, three to four frogs were collected, resulting in a total of 20 adult and 20 juvenile frogs collected during the breeding season. The same six sites were used to collect adult and juvenile frogs in August 2022, during the non-breeding season, with the same number of individuals per group, three to four adult and juvenile frogs per site. The study's total sample comprised 80 individuals: 20 adult breeding (AB), 20 adult non-breeding (AN), 20 juvenile breeding (JB), and 20 juvenile non-breeding frogs (JN).
Frogs were immediately transported to the Kongju University Animal Laboratory within one hour of collection on the day of capture. All experimental procedures involving animals were conducted in accordance with the regulations and with the approval of the Experimental Animal Ethics Committee of Kongju National University (KNU_2022-01).
Black-spotted pond frogs (Pelophylax nigromaculatus) sampling sites. This map illustrates six sampling sites for Black-spotted Pond Frogs. Three sites are located in plain paddy fields and three in terraced paddy fields, all indicated by red circles. The map was generated using QGIS software (version 3.28.0), available at https://qgis.org.
Blood extraction and immune assay
In response to acute stress, the bacterial killing ability (BKA), which represents innate immunity, may change within 15 h66,67. Therefore, blood from the frogs was extracted within 2 h of collection. To alleviate the pain from the syringe needle, we anesthetize the frogs using a 0.5 g/L MS-222 (tricaine methane sulfonate) solution. This was buffered with sodium bicarbonate, referring to the concentrations and methods found in previous studies68,69.
The procedure was precise: After laying down the frog to check for the loss of the righting reflex, we used a wet cotton swab to confirm the loss of the corneal reflex. Then, blood was drawn from the fully anesthetized individual through cardiac venipuncture with a sterile syringe. Blood samples were swiftly transferred to heparinized vacuum tubes (SST) and spun at 3000 × g for 10 min. We collected the supernatant to obtain a plasma sample for the bacterial killing assay, storing it at − 40 °C until analysis. Samples stored at − 40 °C were analyzed within 1–2 weeks. After completing the blood extraction, the snout-vent length was measured with digital calipers, and body mass was recorded using a digital balance. Next, we performed bacterial killing assays, adhering to protocols from an earlier study70. We started by diluting the plasma sample 20-fold with amphibian Ringer's solution. Then, we added a working solution of non-pathogenic Escherichia coli (Microbio-Logics #24 311-ATCC 8739, St Cloud, MN, USA) to all samples. Carefully prepared controls included both positive and negative configurations. Everything was incubated at 37 °C for an exact hour. After this incubation, 500 µL of tryptic soy broth (TSB) was carefully added to both the samples and controls using a pipette. The mixed bacterial suspension was then transferred to a 96-well microplate in duplicate. After a 2-h incubation, we embarked on a meticulous process of measuring the bacterial optical density, using a microplate spectrophotometer at a 600 nm wavelength, taking six readings at 1-h intervals. By taking six readings, we identified the growth phase of the bacteria, at which point the BKA was calculated. Finally, BKA was calculated according to a specific formula [1 − (optical density of the sample/optical density of the positive control)], providing an indication of the proportion of killed bacteria in the plasma samples relative to the positive control.
Analysis of gut bacterial community
To analyze the gut bacterial community of the frogs, each individual was euthanized by pithing after anesthesia prior to dissection. The dissection was performed immediately after measuring body length and weight. To prevent cross-contamination, frogs were dissected on a clean bench using sterilized equipment such as scissors and tweezers. The frog's peritoneum was opened, the starting point of the small intestine and end points of the large intestine were removed, and the sample was transferred to a tube containing beads provided by the Qiagen DNeasy PowerSoil Pro Kit (Qiagen, Hilden, Germany). Afterward, the tweezers and scissors were sequentially flame sterilized, alcohol sterilized, and washed with sterile water, and the intestinal samples were cut several times. Frogs from which the intestinal samples were extracted were stored in 99.5% ethanol for further analysis.
Extracting genomic DNA from the gut was done using the Qiagen DNeasy PowerSoil Pro Kit. After adding lysis buffer to the tube, we homogenized the sample using a TissueLyser II. The extraction process followed the manufacturer's instructions, and the genomic DNA was quantified using a Denovix-QFX fluorometer (DeNovix Inc., Wilmington, DE, USA) and a QFX dsDNA High Sensitivity Assay Kit. All samples were confirmed to have no abnormalities after electrophoresis was used to check the genomic DNA quality.
Bacterial genomic DNA extracted from frog intestinal samples was used to amplify the 16S ribosomal RNA gene (16S rRNA gene) region. Forward and reverse primer targeting the 16S rRNA gene V4 region containing the overhang adapter sequence provided by Illumina were used to amplify the target region: 515F: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCGCGGTAA-3′) and reverse primer (806R: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3′. We conducted PCR under the conditions outlined in 'Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System'71, utilizing the KAPA HiFi HotStart Ready Mix (Kapa Biosystems Inc., Wilmington, USA). After purifying the amplified PCR products with the Agencourt AMPure XP PCR purification system (Beckman Coulter, Brea, CA, USA), we conducted index PCR with the Nextera XT Index Kit (Illumina, San Diego, CA, USA). After secondary PCR, the products underwent another purification round using the same purification system. Concentration measurement of the purified index PCR product and electrophoresis confirmed the size and quality of the PCR products. The samples were pooled, diluted, and analyzed using an Illumina MiniSeq system (Illumina, San Diego, CA, USA).
Paired-end sequencing data in the FASTQ format were analyzed using QIIME2 (v.2022.2) and associated plugins. The DADA2 pipeline was employed for quality filtering, noise removal, merging denoised paired-end sequences, and feature-table construction. Trimming parameters were set based on Demux visualization. Taxonomic assignment of appropriate taxa was performed using the default settings of the feature-classifier plugin for QIIME2 against the EzBioCloud 16S reference database (https://www.ezbiocloud.net/, accessed on January 19, 2023). The dataset was filtered to contain only bacteria. The dataset was filtered to contain only bacteria. As a result, the dataset was classified into operational taxonomic units (OTUs) with 97% similarity. Functional group predictions were analyzed using the Tax4Fun functional classification database (http://tax4fun.gobics.de, accessed on July 23, 2023), which categorized OTUs based on sequence similarity72.
Body composition measurements
After euthanizing the frogs, we removed all internal organs, including gastrointestinal tract, heart, lungs, liver, kidney etc. to focus our attention on the body's tissues from our study. The process required meticulous removal of everything from the esophagus' starting point to the cloacal cavity's end point, applying the same procedure to each individual. We then measured the body composition, including both fat and lean body content, utilizing dual-energy X-ray absorptiometry (DEXA; Medikors InAlyzer, Seongnam, Korea).
The DEXA analysis computes the lean body content as the sum of water and muscle content, a principle applied in our study29,73. We made efforts to remove the maximum body water by preserving tissue samples in 99.5% ethanol for a minimum of three months, ensuring consistency in the retention period across all samples. As a result, the lean body content we measured was seen as the muscle mass, free of most body water. The examination of fat and lean body contents went beyond mere analysis; it served as a tool to compare physiological conditions and discover the correlation between the gut bacterial community's composition and food sources.
Stable isotope ratio
Following the measurements of body composition, we turned our attention to the hind limb muscles of the frogs, extracting them to explore the carbon (δ13C) and nitrogen (δ15N) stable isotope ratios. Since a previous study reported minimal changes in δ13C and δ15N for samples stored in ethanol for 8 weeks74, we considered our results to be stable as well. Transferred to 1.5 Ml microcentrifuge tubes, the muscles were lyophilized to rid them of moisture, then homogenized using a TissueLyser II (Qiagen, Hilden, Germany).
Lipid content can influence the carbon stable isotope ratio, as indicated by previously study75. To account for this, we performed a lipid removal procedure. The procedure involved the addition of a 2:1 (v/v) chloroform to 99.5% methanol mixture to the sample, followed by a 15-min sonication and centrifugation at 3000g for 5 min. This cycle was repeated three times, concluding with another round of lyophilization to remove residual solutions. However, for the nitrogen stable isotope ratio, we opted to use the homogenized samples without extracting lipids. This decision was guided by the risk of errors in stable isotope ratios when lipids are extracted76.
The subsequent step involved transferring 0.1–0.2 mg of the homogenized tissue samples into a 6 × 4 mm tin capsule using an electronic balance. Precision was key, so we used an electronic microbalance (ME204, Mettler Toledo, Japan) to ensure accuracy. The samples' stable isotope ratio was then analyzed with an isotope ratio mass spectrometer (EA-IRMS; EuroEA-Isoprime IRMS, GV Instruments, UK), employing the formula δX (‰) = ((R_sample/R_reference) − 1) × 1000, where "X" symbolizes either carbon or nitrogen, and "R" refers to the 12C/13C or 15N/14N ratio. We used atmospheric N2 as the standard reference material for nitrogen, and Vienna Pee Dee Belemnite (VPDB) for carbon.
Statistical analysis
The availability of food resources was ascertained using the SIAR (Stable Isotope Analysis in R) package77. This was performed within a Bayesian mixing model, utilizing R software version 4.2.0 (https://www.r-project.org/)78. From this analysis, two key metrics were obtained: the standard ellipse area (SEA) to infer food resource availability, and a sample size-corrected standard ellipse area (SEAc), aimed at reducing SEA bias.
We employed the Kruskal–Wallis test to compare the differences in various parameters including SVL, body weight, BKA, Fat, Lean, δ13C, δ15N, and the alpha diversity of bacterial communities among four groups (AB, AN, JB, and JN). When a significant difference emerged from this test, we proceeded with Dunn's post hoc test. GraphPad Prism software, version 8.00 (GraphPad Software, San Diego, CA, USA), facilitated our statistical analyses.
Analyses of alpha and beta diversities in the bacterial communities were carried out with the microeco package79 in R software version 4.2.0 (https://www.r-project.org/)78. We scrutinized differences in alpha diversity indices (observed species, Chao1 index, Shannon diversity index, and phylogenetic diversity; PD), Bray–Curtis dissimilarity, and the relative abundance of gut bacterial taxa among four groups (AB, AN, JB, and JN) using the Kruskal–Wallis test. Dunn's post hoc test was employed for comparison when significant differences emerged.
A permutational multivariate analysis of variance (PERMANOVA) was utilized to analyze differences in bacterial community composition, and the results were visualized through principal coordinate analysis (PCoA). For bacterial taxonomic data, we carried out a Hellinger transformation and compared it with a dissimilarity matrix, which was constructed using the Bray–Curtis distance metric.
We conducted a comprehensive correlation analysis among various factors, including food sources (δ13C and δ15N), gut bacterial diversity metrics (observed species, Chao1 index, Shannon index, and PD whole tree), the relative abundances of Firmicutes and Bacteroidetes (the two dominant OTUs in the gut bacterial community), and physiological conditions such as BKA, fat content, and lean body content. The method of choice for this phase was Spearman correlation analysis, and we set the threshold for statistical significance at p < 0.05.
ARRIVE guidelines
Animal experimental procedures and laboratory animal management were conducted with the approval of the Laboratory Animal Ethics Committee of the Kongju National University (KNU_2022-01). The study is performed in accordance with ARRIVE guidelines.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Shapira, M. Gut microbiotas and host evolution: Scaling up symbiosis. Trends Ecol. Evol. 31, 539–549 (2016).
Tremaroli, V. & Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012).
Sommer, F. & Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol 11, 227–238 (2013).
Groen, R. N., De Clercq, N. C., Nieuwdorp, M., Hoenders, H. R. & Groen, A. K. Gut microbiota, metabolism and psychopathology: A critical review and novel perspectives. Crit. Rev. Clin. Lab. Sci. 55, 283–293 (2018).
Fontaine, S. S., Novarro, A. J. & Kohl, K. D. Environmental temperature alters the digestive performance and gut microbiota of a terrestrial amphibian. J. Exp. Biol. 221, jeb187559 (2018).
Huyben, D. et al. Dietary live yeast and increased water temperature influence the gut microbiota of rainbow trout. J. Appl. Microbiol. 124, 1377–1392 (2018).
Knutie, S. A., Gabor, C. R., Kohl, K. D. & Rohr, J. R. Do host-associated gut microbiota mediate the effect of an herbicide on disease risk in frogs?. J. Anim. Ecol 87, 489–499 (2018).
Tong, Q. et al. Effects of captivity and season on the gut microbiota of the brown frog (Rana dybowskii). Front. Microbiol. 10, 1912 (2019).
Yang, Y., Song, X., Chen, A., Wang, H. & Chai, L. Exposure to copper altered the intestinal microbiota in Chinese brown frog (Rana chensinensis). Environ. Sci. Pollut. Res. 27, 13855–13865 (2020).
Wells, K. D. Complex life cycles and the ecology of amphibian metamorphosis. In The Ecology and Behavior of Amphibians (ed. Wells, K. D.) 612–657 (University of Chicago Press, 2010).
Wake, D. B. & Koo, M. S. Amphibians. Curr. Biol. 28, R1237–R1241 (2018).
Gosner, K. L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 (1960).
Kohl, K. D., Cary, T. L., Karasov, W. H. & Dearing, M. D. Restructuring of the amphibian gut microbiota through metamorphosis. Environ. Microbiol. Rep. 5, 899–903 (2013).
Relyea, R. A. The lasting effects of adaptive plasticity: Predator-induced tadpoles become long-legged frogs. Ecology 82, 1947–1955 (2001).
Crespi, E. J. & Warne, R. W. Environmental Conditions Experienced During the Tadpole Stage Alter Post-metamorphic Glucocorticoid Response to Stress in an Amphibian (Oxford University Press, 2013).
Wells, K. D. Temperature relations. In The Ecology and Behavior of Amphibians (ed. Wells, K. D.) 122–156 (University of Chicago Press, 2010).
Tattersall, G. J. & Ultsch, G. R. Physiological ecology of aquatic overwintering in ranid frogs. Biol. Rev. 83, 119–140 (2008).
Costanzo, J. P. Overwintering adaptations and extreme freeze tolerance in a subarctic population of the wood frog, Rana sylvatica. J. Comp. Physiol. B 189, 1–15 (2019).
Girgenrath, M. & Marsh, R. L. Season and testosterone affect contractile properties of fast calling muscles in the gray tree frog Hyla chrysoscelis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1513–R1520 (2003).
Moore, I. T. & Jessop, T. S. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm. Behav. 43, 39–47 (2003).
Jönsson, K. I. et al. Sexual patterns of prebreeding energy reserves in the common frog Rana temporaria along a latitudinal gradient. Ecography 32, 831–839 (2009).
Chen, W., Wang, X. & Fan, X. Do anurans living in higher altitudes have higher prehibernation energy storage? Investigations from a high-altitude frog. Herpetol. J. 23, 45–49 (2013).
Huang, C. & Liao, W. Seasonal variation in gut microbiota related to diet in Fejervarya limnocharis. Animals 11, 1393 (2021).
Bruestle, E. L. et al. Novel trophic interaction between lake sturgeon (Acipenser fulvescens) and non-native species in an altered food web. Can. J. Fish. Aquat. Sci. 76, 6–14 (2019).
Post, D. M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83, 703–718 (2002).
Rundel, P. W., Ehleringer, J. R. & Nagy, K. A. Stable Isotopes in Ecological Research, vol. 68 (Springer Science & Business Media, 2012).
Garcia, A. et al. Spatial diet overlap and food resource in two congeneric mullet species revealed by stable isotopes and stomach content analyses. Community Ecol. 19, 116–124 (2018).
Potapov, A. M., Tiunov, A. V. & Scheu, S. Uncovering trophic positions and food resources of soil animals using bulk natural stable isotope composition. Biol. Rev. 94, 37–59 (2019).
Park, J.-K. & Do, Y. Evaluating the physical condition of Hyla japonica using radiographic techniques. Sci. Total Environ. 726, 138596 (2020).
Park, J.-K., Kim, J. B. & Do, Y. Examination of physiological and morphological differences between farm-bred and wild black-apotted pond frogs (Pelophylax nigromaculatus). Life 11, 1089 (2021).
Demas, G. E., Zysling, D. A., Beechler, B. R., Muehlenbein, M. P. & French, S. S. Beyond phytohaemagglutinin: Assessing vertebrate immune function across ecological contexts. J. Anim. Ecol. 80, 710–730 (2011).
Hawley, D. M. & Altizer, S. M. Disease ecology meets ecological immunology: Understanding the links between organismal immunity and infection dynamics in natural populations. Funct. Ecol. 25, 48–60 (2011).
Venesky, M. D. et al. Dietary protein restriction impairs growth, immunity, and disease resistance in southern leopard frog tadpoles. Oecologia 169, 23–31 (2012).
Bevier, C. R. Utilization of energy substrates during calling activity in tropical frogs. Behav. Ecol. Sociobiol. 41, 343–352 (1997).
Luedtke, J. A. et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 622, 308–314 (2023).
Bucher, T. L., Ryan, M. J. & Bartholomew, G. A. Oxygen consumption during resting, calling, and nest building in the frog Physalaemus pustulosus. Physiol. Zool. 55, 10–22 (1982).
Emerson, S. B., Greene, H. W. & Charnov, E. L. Allometric aspects of predator-prey interactions. In Ecological Morphology: Integrative Organismal Biology (eds. Wainwright, P. C., Reilly, S. M.) 123–139 (University of Chicago Press, 1994).
Paluh, D. J., Stanley, E. L. & Blackburn, D. C. Evolution of hyperossification expands skull diversity in frogs. Proc. Natl. Acad. Sci. U.S.A. 117, 8554–8562 (2020).
Wolda, H. Insect seasonality: Why?. Annu. Rev. Ecol. Evol. Syst. 19, 1–18 (1988).
Tong, Q. et al. Environmental and host factors shaping the gut microbiota diversity of brown frog Rana dybowskii. Sci. Total Environ. 741, 140142 (2020).
Hovda, M. B., Fontanillas, R., McGurk, C., Obach, A. & Rosnes, J. T. Seasonal variations in the intestinal microbiota of farmed Atlantic salmon (Salmo salar L.). Aquac. Res. 43, 154–159 (2012).
Drovetski, S. V., O’Mahoney, M. J., Matterson, K. O., Schmidt, B. K. & Graves, G. R. Distinct microbiotas of anatomical gut regions display idiosyncratic seasonal variation in an avian folivore. Anim. Microbiome 1, 1–11 (2019).
Maurice, C. F. et al. Marked seasonal variation in the wild mouse gut microbiota. ISME J. 9, 2423–2434 (2015).
Sepulveda, J. & Moeller, A. H. The effects of temperature on animal gut microbiomes. Front. Microbiol. 11, 384 (2020).
Couch, C. E. et al. Diet and gut microbiome enterotype are associated at the population level in African buffalo. Nat. Commun 12, 2267 (2021).
Ramakrishna, B. S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 28, 9–17 (2013).
Castaner, O. et al. The gut microbiome profile in obesity: A systematic review. Int. J. Endocrinol. 2018, 1–9 (2018).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Murphy, E. et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 59, 1635–1642 (2010).
Schlaghecke, R. & Blüm, V. Seasonal variations in fat body metabolism of the green frog Rana esculenta (L.). Experientia 34, 1019–1020 (1978).
Guo, P., Zhang, K., Ma, X. & He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 11, 1–10 (2020).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U.S.A. 101, 15718–15723 (2004).
Rollins-Smith, L. A. Amphibian immunity–stress, disease, and climate change. Dev. Comp. Immunol. 66, 111–119 (2017).
Wells, K. D. Anuran vocal communication. In The Ecology and Behavior of Amphibians (ed. Wells, K. D.) 268–337 (University of Chicago Press, 2010).
Park, J.-K. & Do, Y. Seasonal pattern of advertisement calling and physiology in prolonged breeding Anurans, Japanese Tree Frog (Dryophytes japonicus). Animals 13, 1612 (2023).
Wexler, H. M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 20, 593–621 (2007).
Barsotti, A. M. G. et al. ACTH modulation on corticosterone, melatonin, testosterone and innate immune response in the tree frog Hypsiboas faber. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 204, 177–184 (2017).
Assis, V. R., Titon, S. C. M. & Gomes, F. R. Acute stress, steroid plasma levels, and innate immunity in Brazilian toads. Gen. Comp. Endocrinol. 273, 86–97 (2019).
Braude, S., Tang-Martinez, Z. & Taylor, G. T. Stress, testosterone, and the immunoredistribution hypothesis. Behav. Ecol. 10, 345–350 (1999).
Desprat, J. L., Lengagne, T., Dumet, A., Desouhant, E. & Mondy, N. Immunocompetence handicap hypothesis in tree frog: Trade-off between sexual signals and immunity?. Behav. Ecol. 26, 1138–1146 (2015).
Figueiredo, A. C., Titon, S. C., Cyrino, J. C., Nogueira, L. A. & Gomes, F. R. Immune and hormonal modulation in the postprandial period of bullfrogs (Lithobates catesbeianus). J. Exp. Biol. 224, jeb243153 (2021).
Elmberg, J. Factors affecting male yearly mating success in the common frog, Rana temporaria. Behav. Ecol. Sociobiol. 28, 125–131 (1991).
Girish, S. & Saidapur, S. Interrelationship between food availability, fat body, and ovarian cycles in the frog, Rana tigrina, with a discussion on the role of fat body in anuran reproduction. J. Exp. Zool. 286, 487–493 (2000).
Wright, K. M. & Whitaker, B. R. Amphibian Medicine and Captive Husbandry (Krieger Publishing Company, 2001).
AmphibiaWeb. Pelophylax nigromaculatus: Dark-Spotted Frog. University of California, Berkeley. https://amphibiaweb.org/species/5109 (2007).
Madelaire, C. B., de Oliveira Cassettari, B. & Gomes, F. R. Immunomodulation by testosterone and corticosterone in toads: Experimental evidences from transdermal application. Gen. Comp. Endocrinol. 273, 227–235 (2019).
Titon, S. C. M. et al. Hormonal daily variation co-varies with immunity in captive male bullfrogs (Lithobates catesbeianus). Gen. Comp. Endocrinol. 303, 113702 (2021).
Gentz, E. J. Medicine and surgery of amphibians. ILAR J. 48, 255–259 (2007).
Wojick, K. B., Langan, J. N. & Mitchell, M. A. Evaluation of MS-222 (tricaine methanesulfonate) and propofol as anesthetic agents in Sonoran desert toads (Bufo alvarius). J. Herpetol. Med. Surg. 20, 79–83 (2010).
Assis, V. R., Titon, S. C. M., Barsotti, A. M. G., Spira, B. & Gomes, F. R. Antimicrobial capacity of plasma from anurans of the Atlantic Forest. S. Am. J. Herpetol. 8, 155–160 (2013).
llumina. Preparing 16S ribosomal RNA gene amplicons for the Illumina MiSeq system. Illumina technical note (2011).
Asshauer, K. P., Wemheuer, B., Daniel, R. & Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31, 2882–2884 (2015).
Barreira, T. V. & Tseh, W. The effects of acute water ingestion on body composition analyses via dual-energy X-ray absorptiometry. Clin. Nutr. 39, 3836–3838 (2020).
Chua, K. W., Liew, J. H., Shin, K. H. & Yeo, D. C. Effects of ethanol preservation and formalin fixation on amino acid stable isotope analysis (δ13C and δ15N) and its ecological applications. Limnol. Oceanogr. Methods 18, 77–88 (2020).
Tieszen, L. L., Boutton, T. W., Tesdahl, K. G. & Slade, N. A. Fractionation and turnover of stable carbon isotopes in animal tissues: Implications for δ13C analysis of diet. Oecologia 57, 32–37 (1983).
Sweeting, C., Polunin, N. & Jennings, S. Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid. Commun. Mass. Spectrom. 20, 595–601 (2006).
Parnell, A., Jackson, A. & Parnell, M. A. Package ‘siar’ Stable Isotope Analysis in R. R Packag. Version 4.2. (2008).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022).
Liu, C., Cui, Y., Li, X. & Yao, M. microeco: an R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 97, fiaa255 (2021).
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF), grant funded by the Korean government (MSIT) (No. 2022R1A2C1004240); by the Korea Environmental Industry & Technology Institute (KEITI) through the Wetland Ecosystem Value Evaluation and Carbon Ab-sorption Value Promotion Technology Development Project; and also funded by the Korea Ministry of Environment (MOE) (No. 2022003640001).
Author information
Authors and Affiliations
Contributions
Y.D.: Conceptualization, funding acquisition, methodology, project administration, resources, validation, writing—review and editing, supervision. J.-K.P.: Conceptualization, data curation, formal analysis, investigation, methodology, roles/writing—original draft, software, visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, JK., Do, Y. Combined effect of seasons and life history in an anuran strengthens the response and relationship between their physiology and gut microbiota. Sci Rep 14, 10137 (2024). https://doi.org/10.1038/s41598-024-60105-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60105-7
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.