Abstract
We analyzed chemoport insertion procedures to evaluate infectious morbidity and factors causing infection. This single-center retrospective study included 1690 cases of chemoport implantation between January 2017 and December 2020. Overall, chemoports were inserted in 1582 patients. The average duration of chemoport use was 481 days (range 1–1794, median 309). Infections occurred in 80 cases (4.7%), with 0.098 per 1000 catheter-days. Among the 80 cases in which chemoports were removed because of suspected infection, bacteria were identified in 48 (60%). Significantly more cases of left internal jugular vein punctures were noted in the infected group (15 [18.8%] vs. 147 [9.1%]; p = 0.004). Pulmonary embolism was significantly different between the infection groups (3 [3.8%] vs. 19 (1.2%), p = 0.048). The hazard ratio was 2.259 (95% confidence interval [CI] 1.288–3.962) for the left internal jugular vein, 3.393 (95% CI 1.069–10.765) for pulmonary embolism, and 0.488 (95% CI 0.244–0.977) for chronic obstructive pulmonary disease. Using the right internal jugular vein rather than the left internal jugular vein when performing chemoport insertion might reduce subsequent infections.
Similar content being viewed by others
Introduction
Since the introduction of the port system by Niederhuber et al.1, the use of chemoports in patients undergoing chemotherapy has increased considerably. The use of chemoports has enabled the safe administration of high-vesicant chemotherapeutic drugs. In addition, it can be used for parenteral nutrition, transfusion of blood products, antibiotics, intravenous fluid administration, and intravenous sampling, and has the advantage of avoiding frequent cannulation2,3. Another advantage of the chemoport is that it requires minimal care and maintenance to remain functional when not in use.
However, because chemoports act as foreign substances in the body, serious complications, such as infection, sepsis, venous thrombosis, mechanical dysfunction, catheter disconnection, and embolism may occur4. Infection is one of the most common complications associated with chemoport use. Infection is a serious complication that may require early chemoport removal, and treatment may delay chemotherapy. Approximately 4.8% chemoport-associated infections have been reported in the literature5. Infections that occur during treatment can result in prolonged hospitalization and higher healthcare costs.
Patients with cancer have a weakened immune system during the course of the disease; therefore, preventing infection is essential for caring for patients with cancer. We analyzed chemoport insertion procedures performed in a single institute for 4 years to evaluate infectious morbidity and the factors causing infection.
Patients and methods
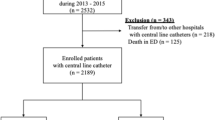
This study was retrospectively conducted on 1690 cases of chemoport implantation in patients who underwent chemoport insertion for chemotherapy at a single institute in Busan, Republic of Korea, between January 2017 and December 2020. Based on the patients’ medical records, we investigated their sex, age, cancer type, use of antithrombotic drugs, and basic medical history. Information related to chemoport insertion, duration of use, vein used for insertion, location of the catheter tip, angle of catheter entry, and other complications such as infection and blood clots were investigated.
All procedures involving human participants performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Pusan National University Hospital Institutional Review Board (IRB No. 2307-007-128). Before all procedures, the potential risks and benefits were explained in detail to the patients, and written informed consent was obtained.
Two cardiovascular surgeons inserted the chemoports. Contraindications included a platelet count < 50,000, an absolute neutrophil count < 500/mm3 and a prothrombin time international normalized ratio > 1.5. For prophylaxis, first-generation cephalosporin was intravenously administered once immediately before surgery, and no additional antibiotics were used thereafter. All patients underwent chemoport insertion in the operating room under local anesthesia. In all patients, the internal jugular vein was punctured using the percutaneous Seldinger technique under ultrasound, a catheter was inserted, and the right internal jugular vein was preferentially used in all patients except for patients with right breast cancer. The left internal jugular vein was used when it was impossible to puncture the right internal jugular vein or in patients with right breast cancer. After insertion, the position of the distal tip of the catheter was confirmed using the C-arm. A pocket for port insertion was created using an electrocautery to create a 2 cm × 2 cm space under the skin through an incision of approximately 1.5–2 cm. After port insertion, the pocket was sutured subcutaneously and continuously using Vicryl 4-0 (Ethicon). Dermabond Advanced (Ethicon, Cincinnati, Ohio, USA) was applied to the skin. As Dermabond Advanced was used, no additional dressing was applied after the operation, and the wound was usually checked on an outpatient basis on postoperative day 7.
The chemoport, including a dressing, was primarily managed by a trained nurse. While using the chemoport, a skilled nurse changed the dressing once every 7 days but immediately if sweaty or dirty. If the port had not been used long, it was flushed every 4 weeks. The port was flushed with 10 mL of 0.9% saline and closed with 4–8 mL of heparinized saline (100 IU/mL) every 4 weeks after insertion and after access to prevent blockage.
Local infection is a case in which symptoms, such as feeling warm to the touch, redness, swelling, discharge or pus, long-lasting pain, and tenderness, occur at the site where the catheter passes through the subcutaneous layer, such as the chemoport insertion site, needling site, and chemoport to the internal jugular vein. Systemic infection was defined as fever, chills, leukocytosis, C-reactive protein elevation, and bacterial identification in blood culture, even in the absence of signs of local infection. In all cases, the chemoport was removed immediately after infection was strongly suspected, and empirical antibiotics and antibiotics appropriate for the bacteria found in the blood culture or wound culture were administered in consultation with the infectious medicine specialist. Empirical antibiotics used were vancomycin and vancomycin plus cefepime. Antibiotic treatment was administered for at least 2 weeks, and when bacteria were not identified at least twice in the blood and wound cultures, antibiotic treatment was discontinued in consultation with an infectious medicine specialist. If pus was present at the pocket or incision site where the chemoport was inserted, betadine soaking was performed without wound closure. If bacteria were not identified more than twice at the wound site, simple suturing was performed using nylon 3-0 on the wound.
Thrombosis was catheter-related, and deep vein thrombosis unrelated to the catheter was excluded. The catheter tip location was measured as the distance from the carina to the catheter tip, and the catheter angle was defined as the angle between the apex inserted into the port and the internal jugular vein and the catheter tip. We investigated whether there were differences according to the type or location of catheter insertion. A change in the position of the catheter was defined as a change in the position of the catheter tip during use, causing malfunction and requiring removal.
Statistical analyses were performed using the SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). Data are presented as frequencies, proportions, and means ± standard deviations. All variables were used to compare the datasets of the infected and non-infected groups. Independent t-tests or Mann–Whitney U tests were used for continuous variables. The chi-square test was used for continuous variables. Pearson’s correlation coefficient and Fisher’s exact test were used to evaluate the degree of correlation between variables. We used propensity score matching (PSM) and a t-test to eliminate bias between groups. Cox hazard regression was used to estimate the risk ratio for sex, age, solid cancer, antiplatelet therapy, left internal jugular vein, catheter tip location, catheter angle, occlusion, catheter-related thrombosis, hypertension, diabetes, coronary artery occlusive disease, peripheral artery occlusive disease, cerebrovascular accident, chronic obstructive pulmonary disease (COPD), and renal insufficiency based on the infection status. Additionally, a Cox proportional hazards regression model was used to perform multivariate prognostic analyses for left internal jugular vein, catheter tip location, occlusion, malfunction, catheter-related thrombosis, catheter angle, and pulmonary embolism. Statistical significance was set at p < 0.05.
Results
A total of 1690 chemoports were inserted in 1582 patients. In majority of cases (1582 cases), the procedure was performed without immunosuppression because the chemoport was performed prior to initiating anticancer treatment. Among the remaining 108 cases, there were 3 instances of reinsertion in 4 patients. In cases where reinsertion followed an infection, the port was first removed for infection treatment and then reinserted at least 1 month after no bacteria were identified or no signs of infection occurred. In cases where the port was reinserted due to recurrence, chemotherapy was not performed for at least 3 months. The average duration of chemoport use was 481 days (range 1–1794 days, median 309 days). Infections occurred in 80 cases (4.7%), with 0.098 per 1000 catheter days. No cases infected more than twice were observed. Among the 80 cases in which chemoports were removed because of suspected infection, bacteria were identified in 48 (60%). Among the 80 cases of infection, 34 cases had signs of systemic infection, and 48 cases had local signs of infection. In 2 cases, both local and systemic infection signs were observed. Notably, 48 cases of infection occurred during outpatient chemotherapy, with 3 cases showing systemic infection signs and 47 cases showing local infection signs. Additionally, 32 cases experienced infection during long-term hospitalization, with 31 exhibiting systemic infection signs and 1 showing local infection signs. The infection group comprised 16, 10, 7, 3, and 2 patients with breast, lung, stomach, colon, and thymic carcinoma, respectively. It also comprised two cases of bladder and pancreatic cancer, respectively. In addition, one patient each had cholangiocarcinoma, esophageal cancer, neurofibromatosis, ovarian cancer, peritoneal cancer, supraglottic cancer, thymic epithelial tumor, and thyroid cancer. In the infection group, the hematological malignancies were lymphoma in 11 patients, leukemia in 2, and multiple myeloma in 3. The characteristics of the patients are described in Table 1. No significant differences were observed between the infected and non-infected groups.
The identified bacterial species are listed in Table 2. Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterococcus faecium were also identified in one patient with Candida albicans. Enterobacter cloacae and Enterococcus faecium were also identified in one patient with Candida tropicalis. In the infection group, there were four in-hospital deaths due to sepsis, and all bacteria were identified only by blood culture. The causative organisms include Staphylococcus epidermidis (methicillin-resistant Staphylococcus epidermidis), Candida parapsilosis, Candida glabrata, and Enterococcus faecium.
The results are described in Table 3. The duration of use was significantly shorter in the infection group than in the non-infection group (230.81 ± 221.23 days vs. 465.39 ± 387.62 days; p < 0.001). There were significantly more cases of left internal jugular vein punctures in the infected group (15 [18.8%] vs. 147 [9.1%]; p = 0.004). No difference was observed in catheter-related thrombosis between the infection and non-infection groups (1 [1.3%] vs. 23 [1.4%]; p = 0.895); however, pulmonary embolism was significantly different in the infection group (3 (3.8%) vs. 19 (1.2%); p = 0.048). Table 4 shows the results of the transformations using PSM. The infected group had a significantly shorter duration of catheter use compared to the non-infected group (244.34 ± 228.00 days vs. 447.68 ± 344.70 days; p < 0.001). Moreover, the infected group had significantly more left internal jugular vein punctures (11[16.2%] vs 3[4.4%]; p = 0.024).
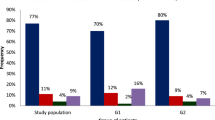
Figure 1 shows the hazard ratios for disease incidence based on infection status and catheter use duration. The hazard ratio was 2.259 (95% confidence interval [CI] 1.288–3.962) for the left internal jugular vein, 3.393 (95% CI 1.069–10.765) for pulmonary embolism, and 0.488 (95% CI 0.244–0.977) for COPD, indicating that patients who developed an infection had a 2.259-, 3.393-, and 0.488-times higher risk, respectively. The hazard ratios for the transformations using PSM are shown in Table 5. The multivariate prognostic analysis for the left internal jugular vein complications yielded a hazard ratio of 3.120 (95% confidence interval [CI] 1.498–6.498).
Discussion
Infection is the most common and feared complication in patients undergoing chemotherapy via a chemoport. However, completely implantable venous access ports have a lower risk of infection and last longer than other intravenous devices6. Approximately 3–10% of patients experience infection associated with the chemoport, which is the most frequent cause of chemoport removal6,7,8,9,10,11. In our study, the incidence of infection was 4.7%, which is not significantly different from that reported in previous studies. However, the infection rate in this study (0.098 per 1000 catheter days) was lower than that of previous studies (range 0.11–0.37 per 1000 catheter-days)12. Once a catheter-associated infection is diagnosed, the chemoport must be removed, broad-spectrum antibiotic therapy is administered, and chemotherapy is deferred. Prevention of catheter-associated infections is crucial, with strict adherence to universal precautions for asepsis, such as hand washing and aseptic techniques5. Nurses accessing the chemoport must be trained to wear face masks, caps, and sterile gloves12. The needle should be disinfected with alcohol-based chlorhexidine or povidone-iodine each time it is inserted13. The Huber needle should be replaced every week if vascular access is continuously maintained6. Patients should be educated about the potential risk of catheter-related infections and informed that only staff trained in aseptic techniques should have access to the device.
In our study, port infection occurred on average 230.81 ± 221.23 days after implantation. These results are more likely caused by infection due to the long-term use of chemoports or reduced immunity of patients associated with long-term chemotherapy, rather than immediate postoperative infection. Therefore, most bacteria identified in this study were skin flora, non-glucose-fermentative gram-negative bacilli, and Candida species. Staphylococcus, the predominant species found on human skin, is the most common cause of catheter-associated infections14,15. To prevent and reduce infection by skin flora and non-glucose fermentative gram-negative bacilli, centralized management and maintenance standards for port insertion sites, especially needling sites, must be established and thoroughly managed15. Candida infections usually occur in immunocompromised hosts16. Staphylococcus and Candida species bind well to the host proteins and attach better to silicone catheters. Total parenteral nutrition can easily cause fungal infections, and ports may not be suitable for delivering it to cancer patients6.
The use rate of the left internal jugular vein was higher in the infected group (15 [18.8%] vs. 147 [9.1%]; p = 0.004). In addition, the hazard ratio for the left internal jugular vein was 2.259 (95% CI 1.288–3.962). We could not find a reference that could prove the difference in the risk of infection between the right and left sides of the central venous catheter. Further investigation through additional literature revealed a study that reported no difference in the occurrence of complications between patients who had a port inserted on the left and those who had a port inserted on the right after the right port was removed17. In addition, another study found more frequent infections with right-sided insertion within the first 2 weeks, but there was no difference in the incidence of infection in the later stages18. Nevertheless, this study does not clearly reveal the mechanism by which infection occurs. Insertion of the chemoport through the left internal jugular vein requires a longer silicone catheter than insertion through the right internal jugular vein because it must pass through the left innominate vein. We speculated that a longer catheter length might increase the risk of infection. No difference in catheter-related thrombosis was observed between the two groups; however, pulmonary embolism occurred more frequently in the infected group (3 [3.8%] vs. 19 [1.2%]; p = 0.048), and the risk ratio was 3.393 (95% CI 1.069–10.765). Therefore, the evaluation of pulmonary embolism can be considered in patients with chemoport-related infections.
Our study results were similar to those of other studies. Infection rates can be reduced through appropriate preventive strategies and chemoport management by well-trained nurses. In this study, we recommend the use of the right internal jugular vein rather than the left internal jugular vein when performing a chemoport procedure to reduce subsequent infection. In addition, the evaluation of the risk of pulmonary embolism in infected patients should be considered.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Niederhuber, J. E. et al. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 92, 706–712 (1982).
Munck, A. et al. Follow-up of 452 totally implantable vascular devices in cystic fibrosis patients. Eur. Respir. J. 23, 430–434 (2004).
Davies, M. G., Feeley, T. M., Moore, D. J. & Shanik, G. D. Home parenteral nutrition using a totally implanted subcutaneous venous access device. Br. J. Clin. Pract. 44, 750 (1990).
Jahangiri, F., Salek, M., Nassiri, S. J., Samadi, F. & Koohian Mohammadabadi, M. Results of port-a-cath implantation: A cross-sectional study about a single tertiary cancer center experience. Med. J. Islam Repub. Iran. 36, 64 (2022).
Zaghal, A. et al. Update on totally implantable venous access devices. Surg. Oncol. 21, 207–215 (2012).
Chang, L., Tsai, J. S., Huang, S. J. & Shih, C. C. Evaluation of infectious complications of the implantable venous access system in a general oncologic population. Am. J. Infect. Control. 31, 34–39 (2003).
Barbetakis, N., Asteriou, C., Kleontas, A. & Tsilikas, C. Totally implantable central venous access ports. Analysis of 700 cases. J. Surg. Oncol. 104, 654–656 (2011).
Costerton, J. W., Stewart, P. S. & Greenberg, E. P. Bacterial biofilms: A common cause of persistent infections. Science. 284, 1318–1322 (1999).
Fischer, L. et al. Reasons for explantation of totally implantable access ports: A multivariate analysis of 385 consecutive patients. Ann. Surg. Oncol. 15, 1124–1129 (2008).
Groeger, J. S. et al. Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann. Intern. Med. 119, 1168–1174 (1993).
Hsieh, C. C. et al. Analysis of risk factors for central venous port failure in cancer patients. World J. Gastroenterol. 15, 4709–4714 (2009).
Lebeaux, D. et al. Management of infections related to totally implantable venous-access ports: Challenges and perspectives. Lancet Infect. Dis. 14, 146–159 (2014).
O’Grady, N. P. et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 52, e162–e193 (2011).
Raad, I. & Hanna, H. Nosocomial infections related to use of intravascular devices inserted for long-term vascular access in Hospital epidemiology and infection control, 2nd ed. (ed. Mayhall, C. G.) 165–172 (Lippincott Williams & Wilkins, 1999).
Maki, D. G. Infections causes by intravascular devices used for infusion therapy: pathogenesis, prevention and management. in Infections associated with indwelling medical devices, 2nd ed. (eds. Bisno, A. & Waldvogel, F. A.) 155–212 (ASM Press, 1994).
Wheat, L. J. Fungal infections in the immunocompromised host in Clinical approach to infection in the compromised host, 3rd ed. (eds. Rubin, R. H. & Young, L. S.) 211–232 (Plenum Medical Book Company, 1994).
Sun, D., Kobayashi, K., Samuel, M., Stewart, G. & Skummer, P. Right- versus left-sided chest ports in oncologic patients with a history of right-sided port removal: Are there any differences in the complication rates?. J. Vasc. Interv. Radiol. 30, 726–733 (2019).
Jones, M. et al. Catheter-associated bloodstream infection in patients with cancer: Comparison of left- and right-sided insertions. J. Hosp. Infect. 118, 70–76 (2021).
Acknowledgements
This work was supported by a clinical research grant from Pusan National University Hospital (2023).
Author information
Authors and Affiliations
Contributions
Conceptualization, C.W. L. and U.H.; methodology, G.K. and M.B.; software, G.K. and D.Y.K.; validation, U.H., J.W.K., and S.H.K.; formal analysis, U.H.; investigation, C.W.L. and D.Y.K.; resources, S.S. and U.H.; data curation, G.K., S.H.K., and S.S.; writing—original draft preparation, U.H.; writing—review and editing, G.K. and U.H.; visualization, G.K.; supervision, U.H.; project administration, U.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, GM., Song, S., Kim, D.Y. et al. Impact of insertion into the left internal jugular vein in chemoport-associated infections: a retrospective single-center study of 1690 cases. Sci Rep 14, 8925 (2024). https://doi.org/10.1038/s41598-024-59749-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59749-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.