Abstract
Previous studies suggested odor stimulation may influence feeding of premature neonates. Therefore, this systematic review and meta-analysis of randomized controlled trials was conducted to assess the effect of human milk odor stimulation on feeding of premature infants. All randomized controlled trials related to human milk odor stimulation on feeding in premature infants published in PubMed, Cochrane, Library, Medline, Embase, Web of science databases and Chinese biomedical literature databases, China National Knowledge Infrastructure, China Science and Technology Journal Database (VIP) and Wanfang Chinese databases were searched, and The Cochrane Handbook 5.1.0 was used to evaluate the quality and authenticity of the literature. Relevant information of the included studies was extracted and summarized, and the evaluation indexes were analyzed using ReviewManager5.3. The retrieval time was from the establishment of the database to July 28, 2022.12 articles were assessed for eligibility, and six randomized controlled studies were eventually included in the meta-analysis (PRISMA). A total of 6 randomized controlled studies with 763 patients were finally included in the study, and the quality evaluation of literatures were all grade B. Human milk odor stimulation reduced the transition time to oral feeding in premature infants [SMD = − 0.48, 95% CI (− 0.69, − 0.27), Z = 4.54, P < 0.00001] and shortened the duration of parenteral nutrition [MD = − 1.01, 95% CI (− 1.70, − 0.32), Z = 2.88, P = 0.004]. However, it did not change the length of hospitalization for premature infants [MD = − 0.03, 95% CI (− 0.41, 0.35), Z = 0.17, P = 0.86]. The implementation of human milk odor stimulation can reduce the transition time to oral feeding and the duration of parenteral nutrition in premature infants, but further studies are needed to determine whether it can reduce the length of hospital stay in premature infants. More high-quality, large-sample studies are needed to investigate the effect of human milk odor stimulation on the feeding process and other outcomes in premature infants.
Similar content being viewed by others
Introduction
Premature infants are babies born alive before 37 weeks of pregnancy are completed. According to the relevant World Health Organization (WHO) report, 15 million premature infants are born every year in the world1. Preterm birth is an important public health issue, as it is associated to a high burden of mortality and morbidities2. Premature infants are at high risk for aspiration due to poor coordination of sucking and swallowing3. Thus, they usually need tube feeding for nutritional needs with a gradual transition to oral feeding. Premature infants need to start oral feeding at the youngest possible age to improve survival and recovery4.
It has been shown5,6,7 that fetal olfactory receptors begin to appear in the 8th week of pregnancy, ciliated olfactory receptors mature in the 24th week, and the nasopharyngeal epithelium can express olfactory marker proteins in the 28th week. Premature infants, just like full-term infants, possess a more advanced olfactory system at birth, enabling them to detect, selectively process, retain, and recall odor information. They are able to distinguish between different odors, including those of human milk, even without history of postpartum exposure to such odors8,9. Olfactory stimulus refers to an environmental stimulus that uses a familiar odor or aromatic odor and is transmitted to the cerebral cortex through olfactory receptors and olfactory nerves to produce an olfactory response. In recent years, an increasing number of studies have used olfactory stimulation as a non-drug intervention to improve the effects of feeding. For example, human milk odor stimulation has a sedative effect on neonates10,11 and relieve the pain12 caused by venipuncture. The milk odor can also prevent apnea13 and improve oxygen saturation14 in premature infants.
Nutritional status parameters, including body weight and oral feeding, are key in determining whether the premature infants can be discharged in time. At present, many reports on the application of human milk odor stimulation to improve the nutritional status of premature infants, but there are differences in the research results and a lack of comprehensive evaluation. Therefore, this systematic review and meta-analysis was conducted to comprehensively evaluate the effect of human milk odor stimulation, and to provide updated evidence for the development of nursing measures in clinical practice.
Methods
This study was conducted in conformity to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)15.
Search strategy
Literature search was conducted from the establishment of the database to July 2022. We searched PubMed/Medline, Cochrane, Library, Embase, Web of science, Chinese biomedical literature databases, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), and Wanfang Data Knowledge Service Platform to retrieve published studies on the human milk odor stimulation as an intervention to improve nutritional status in premature infants. Keyword selection and search included both medical subject headings (MeSH) and life science term indexes (EMBASE TREE; EMTREE). The relevant retrieval strategy was as follows: (“Infant newborn” OR “infant” OR “newborn” OR “neonate”) AND (“Feeding” OR “nutrition” OR “feed” OR “nourishment” OR “pabulum”) AND (“Olfactory stimulation” OR “breast milk stimulation” OR “olfactory” OR “human milk” OR “breast milk” OR “odorant” OR “odor” OR “odour” OR “smell”).
Inclusion criteria
Study characteristics used as criteria for eligibility are as follows: (1) Premature infants born at less than 37 weeks gestation who are receiving tube feeding and/or parenteral nutrition; (2) randomized controlled trials; (3) both groups of premature infants were given tube feeding and/or parenteral nutrition, with interventions involving the application of human milk odor stimulation in the intervention group and routine care in the control group; (4) evaluation metrics included transition time to oral feeding, length of stay, duration of parenteral nutrition, and/or body weight; (5) English or Chinese.
Exclusion criteria
(1) Duplicate articles; (2) Preclinical study, meta-analysis, case reports, reviews, guidelines; (3) Valid ending data unable to be extracted or calculated; (4) Full text of the study is not available; (5) The quality evaluation is grade C.
Data extraction
Two authors (YW and AP) carried out the data extraction process independently. Any disagreement was resolved with a senior researcher (CS) through discussion and consensus. Extracted contents were listed as follows: (1) Basic information of the included articles (title, the first author’s name, year of publication, geographic locations, the quality of the studies). (2) Baseline characteristics of the subjects in the eligible literature. (3) Detail of interventions or exposure factors. (4) The outcome indicators and outcome measures of interest (MD and SMD with the corresponding 95% CI).
Quality assessment
The quality of the selected studies was evaluated by two investigators using a revised tool for assessing risk of bias in Review Manager software. According to the Cochrane intervention research system evaluation manual 5.1.0, the document authenticity evaluation standard is carried out16. It mainly includes five aspects of bias (selection bias, performance bias, detection bias, attrition bias, reporting bias), six evaluation items: the generation method of random sequence, the concealment of random scheme allocation, the blind method of subjects and interventions, the blind method of outcome evaluators, the integrity of outcome data (loss of follow-up), and the possibility of selective reporting of research results. The single evaluation item is divided into three grades: (1) “low risk of bias” when a low risk of bias was determined for all domains, (2) “high risk of bias” when high risk of bias was reached for at least one domain or the study judgment included some concerns in multiple domains, and (3) unclear risk of bias17. The final quality evaluation grades of the literature are Grade A, grade B and grade C.
Statistical analysis
The main statistical software used in this study was ReviewManager5.3; Cochrane library) software. Measures such as length of hospital stay, duration of transitional oral feeding, and duration of parenteral nutrition use were statistically analyzed using the mean ± standard deviation and 95% CI. Standardized conversions could be performed with different measurement instruments to calculate MD/SMD values and 95% CI. The heterogeneity of included studies was examined by the I2 index. If the test showed a high level of heterogeneity (I2 > 50%), a random effect model was used, otherwise a fixed-effect model (I2 < 50%) was used18. Sensitivity analysis was also performed to investigate the potential interference to the pooled effect size19. Statistical significance was set at P < 0 0.05.
Ethics approval and consent to participate
This is a systematic review, no ethics review.
Results
Literature search results
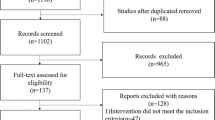
Initially, 322 literatures related to olfactory stimulation applied to premature infant were searched until July 28, 2022, of which 145 literatures related to human milk odor feeding of premature infants were screened. After excluding duplicate publications and those without full texts, 49 studies remained for full text screening. Reading through the full text, 26 articles were finally retained after excluding the inconsistent literature from the three aspects of study topic, overall design, and evaluation index. Then 12 articles were assessed for eligibility, and six randomized controlled studies were eventually included in the meta-analysis20,21,22,23,24,25 (Fig. 1). General information and characteristics of the included literature are detailed (Table 1).
Quality assessment of the selected studies
The qualities of the six included literatures were evaluated as Grade B (partially meeting all criteria). Most of the studies failed to demonstrate the concealment of random scheme allocation and the blind method of outcome evaluators (Fig. 2, Table 2).
Effects of human milk odor stimulation on the transition time of oral feeding in premature infants
Five studies20,21,22,23,24 evaluated the transition time of oral feeding for premature infants. Due to the small heterogeneity among studies (P = 0.39, I2 = 3%), fixed-effect model analysis was conducted. The result showed that the transition time of oral feeding for premature infants in the intervention group was statistically significantly shorter than that in the routine care group,the statistical unit of the outcomes were days [SMD = − 0.48, 95% CI (− 0.69, − 0.27), Z = 4.54, P < 0.00001] (Fig. 3).
Effects of human milk odor stimulation on duration of parenteral nutrition in premature infants
Two studies24,25evaluated the duration of parenteral nutrition of premature infants. Due to the small heterogeneity among studies (P = 0.43, I2 = 0%), the fixed effect model was used to analyze the duration of parenteral nutrition. The result indicated a statistically significantly shorter duration of parenteral nutrition support in the intervention group than that in the routine care group, the statistical unit of the outcomes were days [MD = − 1.01, 95% CI (− 1.70, − 0.32), Z = 2.88, P = 0.004] (Fig. 4).
Effects of human milk odor stimulation on the length of hospital stay for premature infants
Four20,21,24,25 studies explored the impact of human milk odor stimulation intervention on the length of hospital stay. Random-effect model was applied given the high heterogeneity (P = 0.04, I2 = 65%), which found no statistically significant difference between the intervention group and routine nursing care group [MD = − 0.28, 95% CI (− 1.19, 0.63), Z = 0.06, P = 0.55] (Fig. 5A). To explore the source of heterogeneity, sensitivity analysis was conducted by omitting one study at a time, which found that the study conducted by Yildiz could be have a significant impact the heterogeneity20. After removing this study20, heterogeneity was dramatically reduced (P = 0.50, I2 = 0%), and fixed effect model was used to assess the effect of human milk odor stimulation intervention on the length of hospital stay. The result still showed no statistically significant difference between the two groups, the statistical unit of the outcomes were days [MD = − 0.03, 95% CI (− 0.41, 0.35), Z = 0.17, P = 0.86] (Fig. 5B).
Discussion
The systematic review and meta-analysis of six randomized controlled studies found improved outcomes of premature infants associated with human milk odor stimulation. Premature infants are difficult to be fed through bottles by mouth due to underdeveloped oral motor function and uncoordinated sucking, swallowing and respiratory movements, which usually require formula or human milk delivered through a gastric tube26. In addition, oral exercise by oral intake contributes to weight gain and neurological development and accelerate their recovery process, while non-oral feeding deprives premature infants of oral exercise27,28,29. Moreover, prolonged tube feeding affects the oral motor skills of the child, leading to reduced respiratory coordination, late sensory problems, and malnutrition30. Malnutrition leads to lack of stable weight gain, prolonged hospitalization, and even neurological deficits and readmission31. In contrast, adequate nutrition, maintenance of weight gain, and physiological stability play crucial roles in the successful recovery of premature infants from hospitalization22. Therefore, the transition from parenteral or tube feeding to complete oral feeding will contribute significantly to sufficient nutrition and prompt recovery of premature infants. The results of the pooled analyses in the present study showed that human milk odor stimulation was able to reduce the time required for transition to normal oral feeding in premature infants. It is well known that normal oral feeding (sucking, swallowing and respiratory coordination) is an early sign of neuromotor integrity in premature infants and an important indication for hospital discharge28.
Premature infants admitted to the newborn intensive care unit (NICU) for further treatment and care after birth often require controlled number, frequency, and volume of feedings. Therefore, these newborns may lack adequate stimulation and sensory experiences related to feeding, such as hunger, fullness, taste, and smell32. Olfactory and gustatory stimulation alone or in combination can reduce gastrointestinal-related adverse reactions and effectively improve the nutritional status of premature infants by activating complex pathways and triggering cephalic responses, which increase intestinal motility, digestive enzyme secretion, and hormone release33,34,35. Therefore, the application of human milk odor stimulation plays a vital role in promoting the recovery process of premature infants.
Although human milk odor stimulation was associated with reduced transition time to oral feeding and short duration of parental feeding, it did not change the length of hospitalization. Hospitalization in premature infants is affected by a variety of confounding factors, such as body weight, gestational age, the occurrence of complications, family economic status, and medical environment factors. Relevant reports found that very low birth weight infants had significantly longer hospital stays. Moreover, it also revealed that the smaller the gestational age was, the more likely it would be to have complications such as infection, cerebral hemorrhage, and pulmonary hemorrhage, thus prolonging the length of hospital stay36. Due to differences in medical and economic levels in different countries, there will also be inconsistencies in the length of hospitalization for premature infants37.
It is noteworthy that the study by Küçük23, Beker25, Khodagholi21, and Yildiz20 reported the weight of premature infants at discharge, and the mean weight at discharge of the control group of these infants in the four studies was 1933.10 ± 90.50 g, 2913 ± 577 g, 1588.1 ± 84.4 g, and 1922.25 ± 230.82 g, respectively. In contrast, the mean weight of the intervention group at discharge were 1908.00 ± 87.86 g, 2986 ± 672 g, 1565.6 ± 93.6 g, and 1893.50 ± 189.04, respectively. However, the difference of the discharge weight between the intervention group and the control group in each study was not statistically significant. Because the initial weight of the premature infants at admission was different between the control group and the intervention group, a simple comparison of body weight at discharge did not yield enough information. Therefore, the effects of the weight of premature infants were not included and observed.
Several inherent limitations need to be noticed when interpreting the results of this meta-analysis. First, the number of studies included was small with overall small sample size. Second, all included studies had a quality rating of B, which may have had an impact on the evaluation results. Third, other parameters, such as weight gain in premature infants, were not available and their effects were not assessed.
Conclusion
This systematic review and meta-analysis found that human milk odor could reduce the transition time to oral feeding and duration of parenteral nutrition for premature infants, suggesting a cheap, effective, and easily accessible method to improve the overall outcomes of premature infants. However, the findings are limited by the number and quality of included studies, therefore, more well-designed studies are still needed to verify our findings.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
World Health Organization. Preterm birth [EB/OL] [2018-12-27].
Sadeghzadeh, M. et al. Early and late outcome of premature newborns with history of neonatal intensive care units admission at 6 years old in Zanjan, Northwestern Iran. Iran. J. Child Neural. 10(2), 67 (2016).
Lau, C. Development of infant oral feeding skills: What do we know?. Am. J. Clin. Nutr. 103(2), 616S-S621 (2016).
Costa, J., Neves, A., Camargo, J., et al. Characterization of the transition to oral feeding in premature newborns. Caracterização da transição alimentar para via oral em recém-nascidos prematuros. CoDAS 34(5), e20210136 (2022).
Schaal, B., Hummel, T. & Soussingnan, R. Olfaction in the fetal and premature infant: Functional status and clinical implications. Clin. Perinatol. 31(2), 261–285 (2004).
Chuah, M. I. & Zheng, D. R. Olfactory marker protein is present in olfactory receptor cells of human fetuses. Neuroscience 23, 363–370 (1987).
Bartocci, M. et al. Activation of olfactory cortex in newborn infants after odor stimulation: A functional near-infrared spectroscopy study. Pediatr. Res. 48, 18–23 (2000).
Marlier, L., Gaugler, C. & Astruc, D. La sensibilité olfactive du nouveau-né prématuré [The olfactory sensitivity of the premature newborn]. Arch. De Pediatr. 14(1), 45–53 (2007).
Marlier, L. & Schaal, B. Human newborns prefer human milk: Conspecific milk odor is attractive without postnatal exposure. Child Dev. 76(1), 155–168 (2005).
Badiee, Z., Asghari, M. & Mohammadizadeh, M. The calming effect of maternal breast milk odor on premature infants. Pediatr. Neonatol. 54(5), 322–325 (2016).
Tasci, B. & Kuzlu, A. T. The calming effect of maternal breast milk odor on term infant: A randomized controlled trial. Breastfeed Med. 15(11), 724–730 (2020).
Baudessonde Chanville, A., Brevaut-Malaty, V., Garbi, A., et al. Analgesic effect of maternal human milk odor on premature neonates: A randomized controlled trial [published correction appears in J Hum Lact. 2017 Nov;33(4):822]. J. Hum. Lact. 33(2), 300–308 (2017).
Kanbur, B. N. & Balci, S. Impact of the odors of vanilla extract and breast milk on the frequency of apnea in preterm neonates. Jpn J. Nurs. Sci. 17(1), e12271 (2020).
Neshat, H. et al. Effects of breast milk and vanilla odors on premature neonate’s heart rate and blood oxygen saturation during and after venipuncture. Pediatr. Neonatol. 57(3), 225–231 (2016).
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6(7), e1000097 (2009).
Hu, Y., & Hao, Y. F. Evidence-based nursing. Second edition vol. 1, pp. 56–60 (People's Health Publishing House, Beijing, 2018).
Sterne, J. A. C. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Br. Med. J. 366, 1–8 (2019).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558 (2002).
Haidich, A. B. Meta-analysis in medical research. Hippokratia 14(Suppl 1), 29–37 (2010).
Yildiz, A. et al. The effect of the odor of breast milk on the time needed for transition from gavage to total oral feeding in preterm infants. J. Nurs. Scholar. 43(3), 265–273 (2011).
Khodagholi, Z. et al. The effect of non-nutritive sucking and maternal milk odor on the independent oral feeding in preterm infants. Iran. J. Child Neurol. 12(4), 55–64 (2018).
Lee, E. J. The effects of breast milk olfactory stimulation on physiological responses, oral feeding progression and body weight in preterm infants. J. Korean Acad. Nurs. 49(2), 126–136 (2019).
Küçük Alemdar, D. & İnal, S. The effect of individualized developmental care practices in preterm infants. Complement Med. Res. 27(2), 97–104 (2020).
Le, Q. et al. Effects of olfactory and taste stimulation on tube-fed premature infants. J. Nurs. 36(19), 32–34 (2021).
Beker, F. et al. Effects on growth of smell and taste of milk during tube feeding of preterm infants: A randomized clinical trial. JAMA Pediatr. 175(11), 1115–1123 (2021).
Lessen, B. S. Effect of the premature infant oral motor intervention on feeding progression and length of stay in preterm infants. Adv. Neonatal Care. 11(2), 129–139 (2011).
Li, X. L. et al. Early premature infant oral motor intervention improved oral feeding and prognosis by promoting neurodevelopment. Am. J. Perinatol. 37(6), 626–632 (2020).
Thabet, A. M. & Sayed, Z. A. Effectiveness of the premature infant oral motor intervention on feeding performance, duration of hospital stay, and weight of preterm neonates in neonatal intensive care unit: Results from a randomized controlled trial. Dimens Crit. Care Nurs. 40(4), 257–265 (2021).
Jadcherla, S. Dysphagia in the high-risk infant: Potential factors and mechanisms. Am. J. Clin. Nutr. 103(2), 622S-628S (2016).
Toly, V. B. et al. Neonates and infants discharged home dependent on medical technology: Characteristics and outcomes. Adu Neonatal Care. 16(5), 379–389 (2016).
Evereklian, M. & Posmontier, B. The impact of Kangaroo care on premature infant weight gain. J. Pediatr. Nurs. 34, e10–e16 (2017).
Beker, F. et al. The effect of smell and taste of milk during tube feeding of preterm infants (the Taste trial): A protocol for a randomised controlled trial. BMJ Open. 9(7), e027805 (2019).
Kitamura, A. et al. Role played by afferent signals from olfactory, gustatory and gastrointestinal sensors in regulation of autonomic nerve activity. Biol. Pharm. Bull. 33(11), 1778–1782 (2010).
Power, M. L. & Schulkin, J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite. 50(2–3), 194–206 (2008).
Teff, K. Nutritional implications of the cephalic-phase reflexes: Endocrine responses. Appetite. 34(2), 206–213 (2000).
Gupta, S. et al. Short term outcome and predictors of mortality among very low birth weight infants—a descriptive study. Indian J. Pediatr. 88(4), 351–357 (2021).
Ahmadzadeh, N. et al. Estimation of economic burden of preterm and premature births in Iran. Med. J. Islamic Republic Iran 1, 31–78 (2017).
Funding
1. Henan Traditional Chinese Medicine Culture and management Research Projece (NO.TCM2023001); 2. Special Scientific Research Project of Traditional Chinese Medicine in Henan Province (NO.2022JDZX075) 3. Soft Science Projece of Medical Science and Technology Research Programme of Henan Province (NO.RKX202302026); 4. Special Research Project of National TCM Inheritance and Innovation Centre of Henan Provincial Health Commission (NO2023ZXZX1102); 5. Special Research Project of National TCM Inheritance and Innovation Centre of Henan Provincial Health Commission NO.2023ZXZX1114.
Author information
Authors and Affiliations
Contributions
Yangyang Qin and Shu Liu were responsible for the topic selection, literature search, draft of the paper and data analysis. Han Han participated in literature screening, literature quality evaluation, and made the final revision of the paper. All authors conducted the search of literature, reviewed the articles, helped with data synthesis and interpretation, and played a major role in writing the manuscript. All authors agree to publish.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, Y., Liu, S., Yang, Y. et al. Effects of human milk odor stimulation on feeding in premature infants: a systematic review and meta-analysis. Sci Rep 14, 8964 (2024). https://doi.org/10.1038/s41598-024-59175-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59175-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.