Abstract
The oral cavity harbors complex communities comprising bacteria, archaea, fungi, protozoa, and viruses. The oral microbiota is establish at birth and develops further during childhood, with early life factors such as birth mode, feeding practices, and oral hygiene, reported to influence this development and the susceptibility to caries. We here analyzed the oral bacterial composition in saliva of 260 Swedish children at two, three and five years of age using 16S rRNA gene profiling to examine its relation to environmental factors and caries development at five years of age. We were able to assign the salivary bacterial community in each child at each time point to one of seven distinct clusters. We observed an individual dynamic in the development of the oral microbiota related to early life factors, such as being first born, born by C-section, maternal perinatal antibiotics use, with a distinct transition between three and five years of age. Different bacterial signatures depending on age were related to increased caries risk, while Peptococcus consistently linked to reduced risk of caries development.
Similar content being viewed by others
Introduction
The oral bacterial communities consist of a diverse range of bacteria comprising more than 700 unique species with a composition reflecting the different ecological niches found in the oral cavity1. The acquisition and establishment of the oral bacterial communities have been addressed in several prospective investigations in recent years2,3,4,5. Thus, it is clear that the compositional pattern is heterogenic, but shifts as the child matures, starting from birth with early colonizers (Streptococcus and Veillonella) while certain Gram-negative genera, such as Neisseria, appear after one year of age6,7. In the very early stages, perinatal factors and circumstances such as mode of delivery, breastfeeding, and prescribed antibiotics are determinants for the overall distribution of taxa and relative abundance of specific species2,4,8. Once established, the composition of the oral bacterial communities remains relatively stable over time9. However, ecological perturbations that affect the structure and function can induce dysbiosis in the local environment and lead to outbreak of oral diseases such as dental caries10.
Early childhood caries is a biofilm-mediated, sugar-driven, multifactorial, non-communicable disease detected before six years of age11. Cross-sectional studies have demonstrated an enrichment of species with an acid-producing and acid-tolerating phenotype in biofilms overlying caries lesions12. For causality however, longitudinal trials provide best evidence since these have the advantage of determining temporal changes in the bacterial community prior to the caries diagnosis. Such data are available from prospective birth cohorts in which children have been sampled repeatedly over the preschool years6,7,13,14,15. A common observation was that dental caries development seems associated with diverging microbial composition over time, with distinct differences in the composition of the oral bacterial communities between caries-active and caries-free children. Interestingly, the discriminatory role of S. mutans and several acid tolerating Actinomyces/Bifidobacterium species was a main feature although these species account for only a tiny fraction of the bacterial communities6,12. In the dental biofilm, newly described species such as Scardovia wiggsiae, Slackia exigua, and Granulicatella elegans were detected in caries-active children, while commensals like Streptococcus cristatus, Streptococcus gordonii, Streptococcus sanguinis, Corynebacterium matruchotii and Neisseria flavescens were overabundant at non-carious tooth surfaces13. Another study found Rothia mucilaginosa, Streptococcus sp., and Veillonella parvula to be important biomarkers of risk for caries onset in preschool children14.
In longitudinal trials, the salivary bacterial community is of particular interest; firstly, saliva samples can be considered as a representation of various ecological habitats in the oral cavity and, secondly collection of saliva is a non-invasive procedure and thereby convenient for young children. For example, one study showed that the salivary levels of the genera Atopobium, Megasphaera, and Veillonella increased significantly in preschool children before the development of dental caries15. Another study found that the saliva bacterial community was diverse already two days after birth and underwent transformations up to five years of age and beyond, with fluctuations possibly reflecting age-related environmental influences5. We have previously reported on the influence of perinatal and metabolic risk factors, as well as family and nursing determinants on early childhood caries development in a prospective cohort study16,17. In connection with the oral examinations at two, three and five years of age, saliva samples were collected and analyzed. This gave us the opportunity to deepen the insight into the longitudinal maturation of the oral bacterial community during the preschool years and its possible role in caries etiology. The primary aim of the present study was therefore to display ecological changes, including taxa profiles and community structures of the oral salivary bacterial community from two to five years of age. A second aim was to evaluate the microbial diversity in relation to selected environmental exposures such as mode of delivery and infant feeding, and the development of early childhood caries.
Results
Oral microbial community structure during early childhood
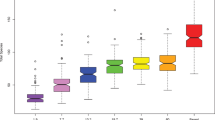
The oral bacterial composition in saliva was examined at two, three and five years of age in a longitudinally followed cohort consisting of 260 Swedish children18 using 16S ribosomal RNA (rRNA) gene amplicon sequencing of the V3-V4 region. In the 780 samples, we identified a total of 6978 amplicon sequence variants (ASVs), corresponding to 115 unique taxa (Fig. 1A, where the inner, middle, and outer rings depict the mean relative abundance of each of the 115 genera at age two, three and five years, respectively, Supplementary Table 1). Several genera were present and dominated the community structure across the years, with the most abundant being Streptococcus, Haemophilus (annotated as Haemophilus 1 and 2 in the SILVA database with Haemophilus 2 being more abundant at 5y compared to 2y and 3y), Neisseria, Gemella and Porphyromonas. The diversity, characterized as the Shannon index and ASV richness, dropped significantly at five years of age, as compared to the two earlier time points (Fig. 1B). At the genus level, we observed a slight, but significant shift in beta-diversity (Bray–Curtis dissimilarity) from two to three years of age (Fig. 1C, PERMANOVA, 2y vs 3y r2 = 0.008, P = 0.001) with communities characterized by 19 genera. By 5y, compared to 2y and 3y, we observed a less diverse community characterized by four genera Haemophilus 2, Bergeyella, Gemella, and Streptococcus, which dominated the oral bacterial community at five years of age (Fig. 1C, PERMANOVA, 2y vs 5y, r2 = 0.065, P = 0.001, 3y vs 5y, r2 = 0.055, P = 0.001).
Oral salivary microbial community structure during early childhood. (A) The inner, middle, and outer ring represent the mean relative abundance of taxa in the saliva microbiota from children at two, three and five years of age, respectively. (B) Shannon index and ASV richness at the three time points are represented by boxplots. The Wilcoxon rank-sum test was used to compare groups with ***, P < 0.001. C) A NMDS of Bray–Curtis dissimilarities at all time points. Circles represent the covariance centered on the mean of each time point. PERMANOVA was used to test differences in beta diversity between age groups with ***, P < 0.001. Top 20 most abundant taxa across samples are correlated to the first and second dimension and represented by arrows. The length of arrows corresponds to r2-values. The bacterial abundance is aggregated to genus level. If genus-level information was missing, the taxa is named after the lowest level available and annotated with “f” or “o" for family- or order-level, respectively. N = 260 for each time point.
Temporal shifts of the oral salivary bacterial community
To investigate temporal shifts of the salivary bacterial community, we made a Dirichlet-Multinomial modelling (DMM) using all 3 × 260 samples as input (Supplementary Fig. 2). This enabled us to assign the salivary bacterial community in each child and time point to one of seven identified clusters. To identify which bacterial taxa that best defined each of these clusters, we identified the top 10 most abundant bacteria in the children of each cluster, which resulted in 17 unique bacterial genera (Fig. 2A). Each of the 17 cluster-defining bacteria is part of the top 20 most abundant bacterial genera found across children at all time points (Fig. 2B), and accounted for an average of 96.6% of the relative abundance in all saliva samples.
Temporal shifts of the oral salivary microbiota community. DMM was used to cluster the saliva microbiota from children (N = 260) into seven clusters. (A) Heatmap showing Z-scored mean relative abundances of the top 10 taxa in each cluster – corresponding to 17 taxa accounting for 96.6% of the mean abundance across children. Mean richness and Shannon index of each cluster are shown to the left of the heatmap. Taxa are ordered by complete hierarchical clustering. (B) Heatmap of top 20 most abundant genera in the saliva microbiota of children split according to clustering (C) Transition model showing the movement of children between clusters over time. The size of clusters is indicative of the number of children in a cluster, while the width and color of connecting lines indicate the percentage of children following the same trajectory between time points. (A, D) NMDS of Bray-Curtis dissimilarities at all time points (each N = 260). Circles represent the covariance centered on the mean of each cluster. Top 20 most abundant taxa across samples are correlated to the first and second dimension and represented by arrows. The length of arrows corresponds to r2-values.
After assigning bacteria to each cluster, it became apparent that cluster 1 was best defined by Alloprevotella, Granulicatella, Porphyromonas. Cluster 2 was defined by many genera that were equally distributed in abundance and was the cluster displaying the highest richness and Shannon index amongst the seven clusters. Cluster 3 was the third most dominant cluster at age two and three years, and the one holding the lowest microbial diversity at this age. Streptococcus and Gemella dominated cluster 3. Cluster 4 and 5 were each dominated by six bacterial genera, with Prevotella and Fusobacterium overlapping between the two. Haemophilus 2 was the sole bacterial taxon to define cluster 6, while cluster 7 was defined by Bergeyella and Porphyromonas, and four other bacterial genera, including Streptococcus.
By illustrating how children transition between clusters over time, we demonstrate that the salivary bacterial community structure within children is subject to marked fluctuations over time (Fig. 2C). Most children exhibited a dominance of bacteria within clusters 1 to 3 at two and three years of age and progressed towards especially cluster 6 (from 2 to 15%) and 7 (from 11 to 41%) at five years of age. Streptococcus dominated the salivary bacterial community of all clusters, and the same applied to Haemophilus 1, although the latter was most abundant in cluster 4, while Haemophilus 2 was prevalent in cluster 6. By the use of non-metric multidimensional scaling (NMDS) of the Bray–Curtis dissimilarities, we illustrated how similar the children of each cluster were to each other, with cluster 1, 3, 6 and 7 clearly separating from other clusters, while cluster 2, 4 and 5 appeared more similar (Fig. 2D).
Pre- and early post-natal environmental factors affect the salivary microbial community structure
We next aimed to define pre and early postnatal environmental factors associated with each of the seven salivary microbial community clusters. The different environmental factors and their distribution are presented in Table 1. We found that sex influenced the overall bacterial community diversity (PERMANOVA: r2 = 0.003, P = 0.03), where the oral microbiota in females compared to males exhibited lower diversity and lower abundance of the cluster 5-defining bacterial genus, Neisseria. Among prenatal factors, being the first-born child, water breakage prior to delivery, delivery by caesarean section (C-section), and administration of antibiotics to the mother during delivery differentially associated with salivary bacterial abundances, diversity, and richness later in life (Fig. 3). Being first born was associated with increased relative abundance of two cluster 5 bacterial genera, Corynebacterium and Lautropia, in addition, water breakage prior to delivery associated with increased salivary bacterial community diversity. C-section was linked to lower relative abundance of the cluster 5 dominating bacterial genera, Actinomyces and Lautropia, and to lower richness of the salivary bacterial community. We found a reduced relative abundance of Actinomyces and Lautropia, and lower richness in children of mothers who received antibiotics according to the Swedish guidelines (see methods) during delivery, resembling that of C-section children.
Early environmental factors affect oral salivary microbial community structure. Heatmap representing relations between DMM cluster-defining taxa and sex as well as pre- or post-natal environmental factors in addition to Shannon index and richness (each N = 260). Z-values represent test statistics of coefficients for generalized linear mixed models modelled over a negative binomial distribution or linear mixed models for Shannon index and richness. Z-values show the number of standard deviations the value is from the mean of the distribution. Negative scores indicate that the value lies below the mean and positive values are above the mean. Significant adjusted P-values are labeled as following: *: P < 0.05, **: P < 0.01, ***: P < 0.001. P-values significant before adjustment are labeled with a dot.
We identified less strong associations between the salivary bacterial community structure and postnatal factors, but breastfeeding habits, bottle-feeding, and tooth brushing frequency were all associated with presence or absence of distinct cluster-defining salivary bacteria. Breastfeeding during the first week of life was associated with higher relative abundance of the cluster 5-defining bacterial genus Lautropia, while breastfeeding for less than four months resulted in reduced relative abundance of Actinomyces. Bottle-feeding beyond one year of age was associated with increased relative abundance of the cluster 1-defining genus Alloprevotella. We also found that tooth brushing twice a day enhanced the relative abundance of the cluster 6-defining bacterial taxon Haemophilus 2 but reduced the relative abundance of the cluster 1-defining bacterial genus Alloprevotella.
Environmental factors and oral bacteria of importance for caries onset until five years of age
We next aimed to examine if any of the environmental early-life factors or the salivary microbial community were associated with an increased or reduced risk of developing caries by the age of five. Caries was expressed as the proportion of children with any tooth with a cavitated or non-cavitated (initial) carious lesion. Among the 260 children, two had developed caries by two years of age, 11 at three years of age, and 52 by the age of five. We generated Cox proportional hazard models for each factor and used caries at five years as the outcome. Tooth brushing twice a day lowered the risk of developing caries until five years of age (HR: 0.3 (0.18–0.49), Fig. 4A). Children born by C-section (N = 87) had an increased risk of developing caries until five years of age (HR: 2.1 (1.2–3.4)), thus, 26 children (30%) born by C-section had developed caries by the age of five. None of the other pre- or postnatal factors were significantly associated to caries development at five years of age.
Environmental factors and oral bacteria of importance for caries onset until five years of age. Cox proportional hazard models showing risks associated with environmental factors or specific bacteria and later onset caries at five years of age. (A) Hazard ratios and 95% confidence intervals for pre- and post-natal environmental factors in relation to later onset caries at five years of age. All hazard ratios were adjusted for sex. (B) Hazard ratios and 95% confidence intervals for cluster-defining bacteria in relation to later onset caries at five years of age. (C) Hazard ratios and 95% confidence intervals for oral bacteria in relation to later onset caries at five years of age. Only bacteria significant after adjustment for sex are shown. B + C) Green and purple hazard ratios were adjusted for tooth brushing habits or C-section, respectively, in addition to sex. Significant adjusted P-values are labeled by an asterisk. For all analyses, N: 2y = 258, 3y = 249, 5y = 260; total = 767.
When focusing on the association between the cluster-defining bacterial taxa and risk of caries development until five years of age, we found that cluster 5-associated Leptotrichia associated with an increased the risk of developing caries until five years of age (Fig. 4B). This remained significant after adjusting for tooth brushing frequency and C-section. After adjusting for C-section, two other cluster 5-defining genera, Corynebacterium and Actinomyces, were also found to associate with an increased risk of caries at five years of age.
As few cluster-defining bacterial genera associated to a risk of caries development until five years of age, we also examined if any other oral bacterial taxa were associated with caries development, independently of the age at which they appeared in the saliva. We identified members of the Prevotellaceae family, members of the Porphyromonadaceae family, Megasphera and Centipeda to be associated with an increased the risk of developing caries until five years of age, while Peptococcus associated with a reduced risk (Fig. 4C). Adjusting for tooth brushing frequency, we found that the increased caries risk remained significant for members of the Prevotellaceae family, while adjustment for C-section did not affect the association between caries and individual bacterial taxon.
Specific oral bacteria in early life predict caries development at five years of age
We next investigated if the presence of certain oral bacterial taxa enabled prediction of the risk of caries development in children. To identify the most pro-cariogenic bacterial taxa at each sampling time point, we constructed a cross-validated sparse partial least square discriminant analysis (sPLS-DA) model for each of the three age groups (2y, 3y and 5y) and used caries at five years as the outcome. The sPLS-DA-selected oral bacterial taxa at 2 years of age were found to be slightly better in predicting caries development at five years (AUC = 0.84) than the oral bacterial communities at 3 and 5 years of age (AUC = 0.81 and = 0.78, respectively, Supplementary Fig. 3, Supplementary Table 2). In general, the taxa that were most predictive for caries development changed over time (Fig. 5A), but Peptococcus was consistently identified to reduce the risk of caries development. We then calculated a bacterial caries score for each child at each time point based on the models. The individual bacterial caries scores were used to predict the risk of developing caries at five years of age using a Cox proportional hazard model. This showed that a high bacterial caries score at two and three years of age was associated with later onset caries at five years (HR at 2 years: 1.35 (1.26–1.45), HR at three years: 1.38 (1.20–1.59), Fig. 5B). Additionally, the bacterial score at five years of age was also capable of separating children with caries from non-caries at five years of age (HR at five years: 1.32 (1.20–1.46)). Overall, this analysis demonstrated that the structure of a child’s salivary bacterial community in early life predicts the later risk of caries development at five years of age.
A subset of oral bacteria in early life predicts caries development at five years of age. A sPLS-DA was used to distinguish the salivary bacterial community at two, three and five years between children who did and did not develop caries at five years. Each child was assigned a bacterial caries score. For balanced model prediction, the number of samples from children without caries was randomly downsampled to the number of children who developed caries after that year (2y: N = 50, 3y: N = 41) or had caries at 5y (N = 52). Downsampling was repeated 1,000 times, and results averaged. (A) Top 20 most important bacteria at 2, 3 and 5 years of age for discriminating between children who did not and children who developed caries at 5 years of age. The dot size indicates how many times a given bacterium was selected during the 1,000 times repeated downsampling of samples from children without caries. (B) Hazard ratios and 95% confidence intervals for bacterial caries scores at two (N = 258), three (N = 249) and five years (N = 260) in relation to later onset caries at five years of age. All hazard ratios are adjusted for sex.
Tooth brushing as a mediator in lowering the bacterial caries score and thereby the risk of caries development
We used a mediation analysis to examine the causal inference of tooth brushing twice daily on the bacterial caries score at two years of age and caries development at five years (Fig. 6A). The analysis revealed that 49% (P = 0.006) of the effect of tooth brushing at two years of age on the risk of caries at five years was mediated via lowering of the bacterial caries score (Fig. 6B). This implies that tooth brushing makes the oral bacterial community less cariogenic, and thereby lowers the risk of caries development. At 3 years of age, only 12.6% of the effect of tooth brushing on caries at five years of age was mediated via lowering of the bacterial caries score, and this was not statistically significant.
Tooth brushing as a mediator in lowering the bacterial caries score and thereby the risk of caries development. (A) Schematic representation of the mediation analysis model structure with tooth brushing frequencies as the predictor, the bacterial caries scores as the mediator, and caries at five years as the outcome. (B) Mediation analysis showing that the effect of tooth brushing habits at two years on later caries development at five years is mediated via the effect of tooth brushing on the bacterial caries score (N = 258). All tests were adjusted for sex.
Discussion
Here we characterized the bacterial composition in saliva from 260 Swedish children at two, three and five years of age and its relation to peri- and postnatal factors and early childhood caries. According to a recent systematic review, there are only two previous prospective birth cohorts available that address this topic19. In this study, participants were enrolled before birth and perinatal and medical data were extracted from hospital records. In addition, self-reported information on family characteristics, behavioral factors, as well as nursing and dietary habits, were captured continuously during the 5-year course to minimize recall bias. We sampled and analyzed unstimulated whole (mixed) saliva as a proxy for the oral bacterial community as a practical non-invasive procedure considering the young age of the children and their ability to cooperate. As saliva does not harbor endogenous bacteria, the salivary bacterial community constitutes a compilation of bacteria shed from all oral surfaces, including the tongue and the throat4,20. It is however important to point out that the low-volume saliva samples do not fully mirror biofilm samples collected directly from the teeth, and thereby most relevant for caries development. For example, one recent study found enrichment of cariogenic species in samples from teeth, while fungal species were more abundant in oral swab samples21. The spatial structure of the bacterial communities (biogeography) with localized rotund corona-like arrangements may also have direct implications for the initiation of dental caries22. Of note, all samples were collected with the same technique throughout the study and could thereby be considered as internally comparable. In addition, it should also be noted the use of a swab placed under the tongue of the infants might constitute a potential limitation, as this protocol may not provide a full representation of the bacterial composition of saliva collected using other protocols.
Our present findings concerning the community structure and temporal shifts were partly in support and partly in conflict with previous findings. The observation that the diversity dropped between 3 and 5 years is in contrast to previous reports5,6, while others found no significant changes with age14. On the other hand, it has been shown that the oral microbiota of children displays a higher diversity than that of adults23. In this context, it is important to underline that the structure of the bacterial community differed markedly within and between children in this study. One may speculate whether the sharp increase in caries prevalence between three and five years may have influenced the observed reduction in the diversity of the saliva microbiota, since dysbiotic biofilms, preceding the disease, are characterized by a reduced community diversity with enrichment of acid tolerant bacteria12. To expand the information on this matter, future studies must be designed with more frequent samplings between 3 and 5 years of age, as this age interval represents a knowledge gap today.
Consistent with past research5, we showed age-related temporal shifts in the salivary community and transitions between clusters over time, with a domination of Streptococcus in all the defined clusters. We also noted that birth-related events, such as mode of delivery, water breakage, and exposure to antibiotics affected bacterial abundance, α-diversity, and richness up to five years of age4. This clearly suggests that very early-life events play a role in shaping the oral bacterial community later in life. Among the postnatal factors, breastfeeding habits and oral hygiene habits seemed to influence the bacterial clusters, which is in concert with previous findings4,23. Breast milk contains both gut-derived lactic acid bacteria and oligosaccharides that some bacteria, like Actinomyces, are capable of utilizing as a carbon source24,25. Consequently, we found a reduced relative abundance of Actinomyces in children being breastfed for less than four months. Infrequent tooth brushing (less than twice a day) is known to be associated with an increased chance of incidence or increment of caries in the primary dentition26. The fact that we found that tooth brushing frequency influenced the composition of the salivary bacterial community is most likely due to the mechanical disruption of the dental biofilm. In this context, it is of note that tooth brushing was associated by a decrease in the relative abundance of the genus Alloprevotella and more surprisingly that bottle-feeding beyond one year of age was associated with an increased relative abundance of Alloprevotella, given the observation that Alloprevotella in a prospective cohort study of Japanese students was shown to be enriched in individuals who developed caries compared to individuals who did not develop caries27.
Almost all children brushed their teeth with fluoride toothpaste, and this may have contributed to inhibit biofilm matrix production and hamper the metabolic activity of certain members of the oral biofilm28.
Our microbial findings concerning the onset of dental caries lend support to the emerging understanding that classifies dental caries as a non-communicable disease29. During the past decades, caries has been regarded as an infectious, transmissible disease30, but here we demonstrate that a blend of functional clusters rather than specific pathogens is associated with caries development.
The structure of the oral bacterial community early in life was clearly associated with caries risk and members of the Prevotellacease, Porphymonadacaeae and Veillonellaceae families have previously been linked to caries14,31. The potential impact of Lepthotricia was interesting in the light of past findings in which oral hygiene discontinuation was associated with a significant increase in relative abundance of this bacterial genus32. The negative relation between Peptococcus and caries risk is consistent with a previous study reporting that Peptococcus, along with Rothia and Treponema, were enriched over a 2-year period in preschool children that remained free from caries15. These Gram-positive bacteria are part of the normal bacterial community of the mouth and may be important members of health-associated functional clusters in oral biofilms. Further investigations seem therefore justified in order to elucidate their potential protective role in the caries process.
In predicting clinical outcomes in prospective cohort studies, it is important to acknowledge the limitations of not knowing how many children will develop caries, which can result in low number of cases. Still, in the present study 52 out of 260 Swedish children had developed carries by the age of five, meaning that carries developed in 20% of the children, enabling rather robust statistically analyses by downsizing the number of children without carries at each time point to balance the number of children with caries at 5 years of age. Ideally, future studies would benefit from enrollment of more children or by changing the sampling strategy to focus on the period between 3 and 5y, which, based on our results, seems to be a critical window for caries development.
In summary, our longitudinal study of the development of the oral bacterial community of saliva during the preschool age shows that different bacterial community signatures at two and three years of age are related to increased risk of caries at five years of age. Our results point towards a potential for early analysis of the oral bacterial community in predicting future caries during early childhood. However, further studies are clearly warranted to substantiate this notion.
Materials and methods
Ethical approval
The longitudinal study design was approved by the Regional Ethical Review Board in Lund, Sweden (44/2008, 2010/362, 2012/483) and a written informed consent was obtained from the legal guardians of all the study participants. All research was performed in accordance with relevant guidelines and regulations.
Study group
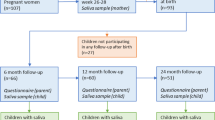
The present study group was derived from children participating in a sub-study (N = 551) of the H2GS birth cohort in southwest Sweden18. Children were invited for dental examinations and saliva samplings. The parents of 346 children (179 boys and 167 girls) accepted and 336 children attended the first visit at two years of age. Follow-up examinations were carried out at three (N = 302) and five years of age (N = 292), respectively. The main reasons for the attrition were relocation or lack of interest or time. Two children rejected the sampling, three children delivered insufficient saliva for analysis and the samples from nine children were discarded due to technical errors. Thus, the findings reported here were based on 260 children (134 girls, 126 boys) with a complete set of medical and dental data together with successfully analyzed saliva samples at all three time points.
Data collection
Maternal and birth-related factors were collected through a validated questionnaire at the maternity ward as previously described33. In addition, medical data, such as prescription of antibiotics, gestational week, and neonatal hospital care, were extracted from the hospital records. Antibiotics administration during labor and C-section in this cohort was performed according to Swedish guidelines, which include mothers with suspected infection or increased risk of infection in vaginal deliveries (i.e., fever during labor or Group B streptococcus colonization during late pregnancy). Mothers delivering with C-section received intraoperative antibiotics only if the C-section was classified as acute or if additional risk factors for postoperative infections were present (i.e., severe obesity). We collected key data covering family characteristics, nursing and feeding habits through comprehensive questionnaires at regular intervals during the project17. The oral hygiene routines were captured by structured personal interviews and two calibrated and experienced dentists performed a visual-tactile dental examination according to the WHO criteria34. The prevalence of dental caries was expressed as the proportion of children with any tooth with a cavitated or non-cavitated (initial) carious lesion35.
Saliva sampling
Resting mixed whole saliva samples (N = 780) were collected at 2, 3 and 5 years of age in connection with the dental examination. The child was seated in an upright position and asked not to swallow. A sterile cotton bud was then inserted under the tongue until soaked and immediately transferred to a sterile Sarstedt tube. The samples were rapidly frozen to -20 °C and transported along with negative controls to the laboratory in Copenhagen for storage at -80 °C until further processing. The amount of saliva collected with this technique was approximately 0.3–0.4 mL.
DNA extraction and 16S rRNA gene library preparation
DNA was extracted from the saliva samples using the NucleoSpin 96 Soil kit, (MACHEREY–NAGEL, Germany), following the manufacturer´s instructions with a few modifications. Briefly, samples were transferred to NucleoSpin Bead Tubes containing 700 µL Buffer SL1 and 150 µL Enhancer SX and lysed on a horizontal vortexer (Vortex-genie 2, Scientific industries) for 15 min at full speed. The filtering, binding, and washing of the NucleoSpin Soil Binding Plate were performed as described in the centrifuge processing protocol, with centrifugation at 4,700xg, and elution in 50 µL Buffer SE. Negative controls were added to check the cotton swabs, bead tubes, and Sarstedt tubes. The obtained DNA was then refrozen at − 80 °C and transported to BGI Europe, Copenhagen for 16S ribosomal RNA (rRNA) gene amplicon sequencing of the V3-V4 region. The library preparation was performed as follows: DNA samples were analyzed by fluorometry and agarose gel electrophoresis, if possible 30 ng DNA were used as input. Since this sample type has low DNA yield, this was not always possible, but library preparation was attempted for all samples. DNA was amplified using primers 341F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT). All PCR reactions were carried out in 30 µL reactions with 15 µL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs); 0.2 µM of forward and reverse primers. Thermal cycling consisted of initial denaturation at 98℃ for 1 min, followed by 30 cycles of denaturation at 98℃ for 10 s, annealing at 50℃ for 30 s, and elongation at 72℃ for 30 s and finally 72℃ for 5 min.
After PCR, the libraries were purified using AmpureXP beads (AGENCOURT) to remove unspecific products, and the purified libraries were evaluated by using the Agilent 2100 Bioanalyzer system (Agilent DNA 1000 Reagents) and quantified by Quantitative PCR (EvaGreenTM) before pooling. Except for four samples, library preparation was successful. The qualified libraries were sequenced pair end on the MiSeq System, with the sequencing strategy PE300 (PE301 + 8 + 8 + 301) (MiSeq Reagent Kit) and the read depth was > 30 k reads per sample. For information on total reads per sample see supplementary table 3.
16S rRNA Gene Data processing
Reads were analyzed and denoised using DADA236. Resulting amplicon sequence variants (ASVs), were mapped to the 99% identity clustered SILVA database v13237 using a naïve Bayes classifier trained on the amplified region, as implemented in DADA2. (Supplementary table 3).
Several control samples were collected as part of the study: the cotton swabs and the sample collection tubes were washed with nuclease-free water, which together with empty tubes from the DNA extraction kit underwent the same procedure and sequencing as the saliva samples. The control samples were used to identify contaminating reads in the samples by the use of the R package decontam (v. 1.12)38 using default settings. Overall, the control samples did not contain the same bacteria as the biological samples and had fewer sequencing counts (Supplementary Fig. 1A + B).
After correction for contaminating reads, we filtered out low-abundant ASVs with less than 20 read counts across all samples and ASVs found in less than five samples with an abundance under 1%. In total we obtained 6978 ASVs distributed across 115 bacterial taxa. The bacterial abundance was aggregated to genus level. If genus-level information was missing the taxa was named after the lowest level available and annotated with either “f” or “o" for family- or order-level, respectively.
Statistics and data analysis
All statistical analyses were performed using R (v4.0.0)39. Shannon index, which was used as a measure for alpha-diversity and richness as a measure of number of unique taxa was calculated using the vegan package (v. 2.6–2)40. Differences in Shannon index and richness between time points were compared using Wilcoxon rank sum test. Visualization of the overall bacterial communities at the three time points or the DMM clusters were done using Bray–Curtis dissimilarities (beta-diversity) made from Hellinger-transformed relative abundances using the decostand function (vegan) and presented by NMDS in seven dimensions identified by iteratively adding dimensions to find a solution with lowest level of stress, which is a measure of remaining variance not captured by the model. The top 20 taxa were added to the NMDS using the function envfit (vegan). The differences in beta-diversity between time points (2y, 3, and 5y) were tested using a permutational multivariate analysis of variance (PERMANOVA with 999 permutations, using Adonis (vegan). The DMM-based temporal transition modelling of the saliva microbiota was performed using the Dirichlet Multinomial package41. The optimal number of Dirichlet components (clusters) were chosen by estimating the goodness of fit using Laplace (Supplementary Fig. 2). Samples from all time points were clustered together, which enabled us to identify how individual children move between clusters over time. The graphical representation was done using an R implemented script from a previous study42. Environmental factors were fitted to bacterial counts using negative binomial generalized linear mixed models with logged sequencing depths as offset term to compensate for differences in sequencing depth between samples and children IDs as random effect to include information of repeated sampling in the model using the glmmADMB package (v. 0.8.3.3). Shannon index and richness were fitted to environmental factors using linear mixed models with children IDs as random effect. Associations between environmental factors, individual bacteria or bacterial caries scores and caries at five years were quantified in terms of hazard ratios by Cox proportional hazard models adjusted for sex and clustered by children IDs to include information of repeated sampling, using the survival package. The individual bacteria significantly associated with caries at five years were also adjusted for tooth brushing frequencies or C-section in addition to sex. Since the aim was to identify at each age (2y, 3y and 5y) which oral bacteria contributed the most in separating children who developed caries at 5y from those who did not, we utilized sPLS-DA (a supervised clustering method) from the mixOmics package (v. 6.20)43. Input data were log10-transformed relative abundances, using random values sampled from a distribution ranging from 1/10 to 1/2 of the lowest non-zero value as pseudocounts44. A separate model was trained for each time point with caries at five years as outcome. Children diagnosed with caries at 2y (N = 2) and 3y (N = 11) were excluded from the respective models. Since the number of prospective caries and non-caries cases led to an unbalanced design (caries cases at 5 years = 52, non-caries = 208), we performed a random downsampling of the caries-free children at 2y, 3y and 5y to match the number of children with caries at 5y, subtracting the number of caries cases at the given year (2y: N = 52–2 = 50, 3y: N = 52–11 = 41, 5y: N = 52). To increase robustness and to avoid potential biases of the random downsampling, the process was repeated 1,000 times and the results were averaged across samples. Within each age group, the samples were subjected to a tenfold cross-validation repeated 100 times to avoid overfitting and to select the most discriminating bacteria. The component values from each of the three age-dependent bacterial risk scores were scaled and combined into a bacterial caries score for each age, the latter explaining how much the bacterial community of each sample resembled the bacterial features associated with caries development at 5y. The final models were evaluated using AUC statistics.. Mediation analysis was performed using the mediation package (v. 4.5.0)45, using a model structure with tooth brushing frequencies as the predictor, the bacterial caries scores as the mediator, and caries at five years as the outcome. P values were deemed significant using 0.05 as significance level; when appropriate, P values were adjusted for multiple testing using FDR with an adjusted significance level of 0.05.
Data availability
Sequencing data are publicly available in European Nucleotide Archive under project: PRJEB60183. Analysis software including quality control, taxonomy, and tools for analysis of ecological communities are publicly available and referenced.
References
Dewhirst, F. E. et al. The human oral microbiome. J. Bacteriol. 192, 5002–5017. https://doi.org/10.1128/JB.00542-10 (2010).
Kennedy, B. et al. Oral microbiota development in early childhood. Sci. Rep. 9, 19025. https://doi.org/10.1038/s41598-019-54702-0 (2019).
Kahharova, D. et al. Maturation of the oral microbiome in caries-free toddlers: A longitudinal study. J. Dent. Re.s 99, 159–167. https://doi.org/10.1177/0022034519889015 (2020).
Kaan, A. M. M., Kahharova, D. & Zaura, E. Acquisition and establishment of the oral microbiota. Periodontol 2000(86), 123–141. https://doi.org/10.1111/prd.12366 (2021).
Lif Holgerson, P., Esberg, A., Sjödin, A., West, C. E. & Johansson, I. A longitudinal study of the development of the saliva microbiome in infants 2 days to 5 years compared to the microbiome in adolescents. Sci. Rep. 10, 9629. https://doi.org/10.1038/s41598-020-66658-7 (2020).
Dashper, S. G. et al. Temporal development of the oral microbiome and prediction of early childhood caries. Sci. Rep. 9, 19732. https://doi.org/10.1038/s41598-019-56233-0 (2019).
Dzidic, M. et al. Oral microbiome development during childhood: an ecological succession influenced by postnatal factors and associated with tooth decay. ISME J 12, 2292–2306. https://doi.org/10.1038/s41396-018-0204-z (2018).
Craig, S. J. C. et al. Child weight gain trajectories linked to oral microbiota composition. Sci. Rep. 8, 14030. https://doi.org/10.1038/s41598-018-31866-9 (2018).
Richards, V. P. et al. Microbiomes of site-specific dental plaques from children with different caries status. Infect Immun. 85, e00106-e117. https://doi.org/10.1128/IAI.00106-17 (2017).
Marsh, P. D. In sickness and in health-what does the oral microbiome mean to us? An Ecological Perspective. Adv. Dent. Res. 29, 60–65. https://doi.org/10.1177/0022034517735295 (2018).
Pitts, N. B. et al. Dental caries. Nat. Rev. Dis. Primers 3, 17030. https://doi.org/10.1038/nrdp.2017.30 (2017).
Tanner, A. C., Kressirer, C. A. & Faller, L. L. Understanding caries from the oral microbiome perspective. J. Calif. Dent. Assoc. 44, 437–446 (2016).
Fakhruddin, K. S., Ngo, H. C. & Samaranayake, L. P. Cariogenic microbiome and microbiota of the early primary dentition: A contemporary overview. Oral Dis 25, 982–995. https://doi.org/10.1111/odi.12932 (2019).
Grier, A. et al. Oral microbiota composition predicts early childhood caries onset. J. Dent. Res. 100, 599–607. https://doi.org/10.1177/0022034520979926 (2021).
Xu, L. et al. Dynamic alterations in salivary microbiota related to dental caries and age in preschool children with deciduous dentition: A 2-year follow-up study. Front. Physiol. 9, 342. https://doi.org/10.3389/fphys.2018.00342 (2018).
Boustedt, K., Roswall, J., Twetman, S. & Dahlgren, J. Influence of mode of delivery, family and nursing determinants on early childhood caries development: A prospective cohort study. Acta Odontol. Scand. 76, 595–599. https://doi.org/10.1080/00016357.2018.1490965 (2018).
Boustedt, K., Roswall, J., Kjellberg, E., Twetman, S. & Dahlgren, J. A prospective study of perinatal and metabolic risk factors for early childhood caries. Acta Paediatr https://doi.org/10.1111/apa.15231 (2020).
Roswall, J. et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe. https://doi.org/10.1016/j.chom.2021.02.021 (2021).
Peres, K. G. et al. Scoping review of oral health-related birth cohort studies: Toward a global consortium. J. Dent. Res. 101, 632–646. https://doi.org/10.1177/00220345211062475 (2022).
Kilian, M. et al. The oral microbiome: An update for oral healthcare professionals. Br. Dent. J. 221, 657–666. https://doi.org/10.1038/sj.bdj.2016.865 (2016).
de Jesus, V. C. et al. Characterization of supragingival plaque and oral swab microbiomes in children with severe early childhood caries. Front. Microbiol. 12, 683685. https://doi.org/10.3389/fmicb.2021.683685 (2021).
Kim, D. et al. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. U S A 117, 12375–12386. https://doi.org/10.1073/pnas.1919099117 (2020).
Burcham, Z. M. et al. Patterns of oral microbiota diversity in adults and children: A crowdsourced population study. Sci. Rep. 10, 2133. https://doi.org/10.1038/s41598-020-59016-0 (2020).
Borewicz, K. et al. The association between breastmilk oligosaccharides and faecal microbiota in healthy breastfed infants at two, six, and twelve weeks of age. Sci. Rep. 10, 4270. https://doi.org/10.1038/s41598-020-61024-z (2020).
Holgerson, P. L. et al. Oral microbial profile discriminates breast-fed from formula-fed infants. J. Pediatr. Gastroenterol. Nutr. 56, 127–136. https://doi.org/10.1097/MPG.0b013e31826f2bc6 (2013).
Kumar, S., Tadakamadla, J. & Johnson, N. W. Effect of toothbrushing frequency on incidence and increment of dental caries: A systematic review and meta-analysis. J. Dent. Res. 95, 1230–1236. https://doi.org/10.1177/0022034516655315 (2016).
Uchida-Fukuhara, Y. et al. Caries increment and salivary microbiome during university life: A prospective cohort study. Int. J. Environ. Res. Public Health 17, 3713. https://doi.org/10.3390/ijerph17103713 (2020).
Ten Cate, J. M. & Buzalaf, M. A. R. Fluoride mode of action: Once there was an observant dentist. J. Dent. Res. 98, 725–730. https://doi.org/10.1177/0022034519831604 (2019).
Pitts, N. B., Twetman, S., Fisher, J. & Marsh, P. D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 231, 749–753. https://doi.org/10.1038/s41415-021-3775-4 (2021).
Simón-Soro, A. & Mira, A. Solving the etiology of dental caries. Trends Microbiol. 23, 76–82. https://doi.org/10.1016/j.tim.2014.10.010 (2015).
Zhu, C. et al. The predictive potentiality of salivary microbiome for the recurrence of early childhood caries. Front. Cell Infect. Microbiol. 8, 423. https://doi.org/10.3389/fcimb.2018.00423 (2018).
Belstrøm, D. et al. Influence of periodontal treatment on subgingival and salivary microbiotas. J. Periodontol. 89, 531–539. https://doi.org/10.1002/JPER.17-0377 (2018).
Roswall, J. et al. Overweight at four years of age in a Swedish birth cohort: influence of neighbourhood-level purchasing power. BMC Public Health 16, 546. https://doi.org/10.1186/s12889-016-3252-1 (2016).
Organization, W. H. Oral Health Surveys: Basic Methods (World Health Organization, 2013).
Boustedt, K., Dahlgren, J., Twetman, S. & Roswall, J. Tooth brushing habits and prevalence of early childhood caries: A prospective cohort study. Eur. Arch. Paediatr. Dent. 21, 155–159. https://doi.org/10.1007/s40368-019-00463-3 (2020).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. https://doi.org/10.1093/nar/gks1219 (2013).
Davis, N. M., Proctor, D. M., Holmes, S. P., Relman, D. A. & Callahan, B. J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6, 226. https://doi.org/10.1186/s40168-018-0605-2 (2018).
Team, R. C. R: A language and environment for statistical computing. (2020). DOI:
Oksanen, J. et al. vegan: Community Ecology Package 2022).
Holmes, I., Harris, K. & Quince, C. Dirichlet multinomial mixtures: generative models for microbial metagenomics. PLoS One 7, e30126. https://doi.org/10.1371/journal.pone.0030126 (2012).
Stewart, C. J. et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562, 583–588. https://doi.org/10.1038/s41586-018-0617-x (2018).
Rohart, F., Gautier, B., Singh, A. & Lê Cao, K. A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 13, e1005752. https://doi.org/10.1371/journal.pcbi.1005752 (2017).
Lubbe, S., Filzmoser, P. & Templ, M. Comparison of zero replacement strategies for compositional data with large numbers of zeros. Chemom. Intell. Lab. Syst. 210, 104248. https://doi.org/10.1016/j.chemolab.2021.104248 (2021).
Vinod, H. D. (ed.) Causal Mediation Analysis Using R (Springer, New York, 2010).
Acknowledgements
We thank children and their parents participating in this study. We are grateful for the excellent work done by research nurses Eivor Kjellberg and Monika Nygren, Department of Paediatrics, Halland Hospital Halmstad, during the recruitment and clinical follow up visits in this study. We are also very thankful for the help of the dental staff at the Maxillofacial Unit, Halland Hospital for support during oral examinations of the participating children. This study was supported by the Halland Regional Research board (Halland-665071, Halland-623561, Halland-566481). The funder played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Funding
Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.B., J.D., S.T., and J.R.; Methodology: C.E., K.B., J.D., S.B.S., K.K., S.T., S.B., and J.R.; formal analysis, C.E., and S.B.; Resources: K.B., S.B.S., J.D., S.T., K.K. and J.R.; Data curation: C.E., K.B., and J.R.; Writing original draft: C.E., K.B., S.T., S.B. and J.R.; Writing—review and editing: all authors; Funding: K.B., J.D., K.K., and J.R.; Supervision: K.K., S.B., J.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eriksen, C., Boustedt, K., Sonne, S.B. et al. Early life factors and oral microbial signatures define the risk of caries in a Swedish cohort of preschool children. Sci Rep 14, 8463 (2024). https://doi.org/10.1038/s41598-024-59126-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59126-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.