Abstract
Potentially fatal fungal sphenoid sinusitis (FSS) causes visual damage. However, few studies have reported on its visual impairment and prognosis. Five hundred and eleven FSS patients with ocular complications treated at Beijing Tongren Hospital were recruited and clinical features and visual outcomes were determined. Thirty-two of the 511 patients (6%) had visual impairment, with 13 and 19 patients having invasive and noninvasive FSS, respectively. Eighteen patients (56.25%) had diabetes and 2 patient (6.25%) had long-term systemic use of antibiotics (n = 1) and corticosteroids (n = 1). All patients had visual impairment, which was more severe in invasive FSS than in noninvasive FSS. Bony wall defects and sclerosis were observed in 19 patients (59.38%), and 11 patients (34.38%) had microcalcification in their sphenoid sinusitis on computed tomography (CT). After a 5-year follow-up, three patients (9.38%) died. Patients with noninvasive FSS had a higher improvement rate in visual acuity than their counterparts. In the multivariate analysis, sphenoid sinus wall sclerosis on CT was associated with better visual prognosis. FSS can cause vision loss with persistent headaches, particularly in those with diabetes. CT showed the sphenoid sinus wall sclerosis, indicating a better visual prognosis in FSS with visual impairment.
Similar content being viewed by others
Introduction
Fungi occur widely in nature as fungal spores, which can be inhaled into the nasal cavity, leading to colonization of the upper respiratory tract mucosa1. They can be engulfed and destroyed by tissue macrophages and neutrophils. However, with impaired host immunity, spores can germinate into hyphae, invade host mucosa, and cause invasive disease2. Fungal sphenoid sinusitis (FSS) is an uncommon and potentially fatal disease. The causative pathogen or disease-related inflammation affects the adjacent important structures, including the cavernous sinus, optic nerve, internal carotid artery, and cranial nerves III, IV, V, and VI, causing severe orbital or intracranial complications3. The early symptoms of FSS are atypical, which can manifest as head and face pain and swelling, often resembling bacterial infections and idiopathic inflammatory conditions of the sinus or orbit. Nasal symptoms are frequently absent4,5,6. In addition to the symptoms of sinusitis, FSS often combined with ocular complications, manifest as monocular or binocular visual impairment7. This infection’s rarity and vague symptoms, make its identification challenging, sometimes resulting in misdiagnosis and inappropriate corticosteroid treatment, which may worsen the condition. Furthermore, there are limited reports of FSS patients with visual damage. They mainly focus on the clinical manifestations of the disease and lack an analysis of the treatment results and factors associated with the long-term visual prognosis. This study aimed to present the clinical presentations, imaging features, treatment, and outcomes in FSS with visual impairment and analyze the factors associated with visual prognosis.
Materials and methods
This retrospective study included patients with ocular complications caused by FSS treated at Beijing Tongren Hospital, Capital Medical University, between January 2006 and December 2020. All study participants provided informed consent. For patients who had died, informed consent was obtained from their legal guardians. Patients with fungal aggregation in the sphenoid sinus, confirmed by histopathology, were divided into invasive and non-invasive groups, according to the presence of fungal components in the mucosa, submucosa, vascular tissue, or bone8. The noninvasive subtypes included allergic fungal sinusitis (AFS) and fungal ball. AFS refers to the presence of allergic mucin and sparse fungal hyphae in one sinus cavity. Fungal balls are described as the presence of a noninvasive conglomeration of dense fungal hyphae within the affected sinuses. The invasive subtypes included acute invasive fungal sinusitis (≤ 4 weeks), chronic invasive fungal sinusitis (> 4 weeks), and granulomatous invasive fungal sinusitis9. Fungal etiology was determined through tissue histopathology or culture. Data collected included those on medical history, demographics, time interval between ocular symptom occurrence and surgery, clinical presentation, imaging features, treatment, and outcomes. Histopathological findings, culture results, and neutrophil counts were also reviewed.
According to Schulze-Bonsel et al.10, visual acuity on the Snellen chart was converted to the log of the minimum angle of resolution (log MAR) scale for quantitative assessment. Count fingers (CF), light perception (LP), and no LP (NLP) were substituted with 1.85, 2.70, and 3.00 log MAR11, respectively. Improvement in visual acuity was evaluated qualitatively based on the best-corrected Snellen visual acuity (BCVA) changes measured during a follow-up period of 6 months to 5 years. When the preoperative BCVA was 20/200 or better, treatment success was defined as an improvement over two lines of the visual acuity chart. Preoperative BCVA worse than 20/200 was grouped into five grades: NLP, LP, CF, 20/1000, and 20/400. The improvement in postoperative BCVA by one or more grades compared with preoperative BCVA was considered significant.
All statistical analyses were completed using IBM SPSS V.26.0 (Armonk, NY, USA). Differences in clinical manifestations between patients with invasive and noninvasive FSS were tested using Pearson’s chi-square test, Fisher’s exact test, and the Mann–Whitney U test. Regression analysis of visual prognosis was performed using generalized estimating equation (GEE) models accounting for inter-eye dependencies. Baseline variables with a P-value < 0.05 in univariate analysis were included in the multivariate analysis. Statistical significance was defined as P < 0.05.
Ethics declarations
This study was approved by the Ethics committee of Beijing Tongren Hospital, Capital Medical University (Approval No. TRECKY2020-0425) and followed the principle of the declaration of Helsinki.
Results
Of 511 FSS patients, we identified visual impairment in 32 patients (6%), including 21 women and 11 men. The mean age was 62.81 years (29–83 years) (Table 1).
There were 18 patients with diabetes and one with breast cancer. One patient was taking antibiotics for chronic bronchiectasis and one was taking long-term corticosteroids for rheumatoid arthritis. Complete blood counts were within normal limits for all patients. By definition, 13 patients showed tissue invasion and one patient showed eosinophils and scanty fungal elements in the biopsy specimen. The fungal etiology was mucormycosis and Aspergillus in four patients (12.5%) and 28 patients (87.5%), respectively. The mean time from symptom onset to diagnosis in noninvasive FSS was longer than that in invasive FSS (P = 0.049, Table 2).
Clinical manifestation
All patients had decreased visual acuity, and it was bilateral in six patients (18.75%). The visual impairment in patients with invasive FSS was more serious than that in patients with noninvasive FSS (P < 0.001, Table 2). Nine patients (nine eyes [64.3%]) with invasive FSS ultimately declined to NLP and three patients (four eyes [16.7%]) with noninvasive FSS had NLP (P = 0.005, Fig. 1). Other ocular symptoms included diplopia (5, 15.63%), motility deficits (8, 25%), blepharoptosis (10, 31.25%), and proptosis (4, 12.5%). The prevalence of motility deficits and blepharoptosis in the invasive FSS group was significantly higher than that in the noninvasive FSS group (Table 2). Of the 18 patients with diabetes, 14 had diabetic retinopathy (DR): two cases showed mild non-proliferative DR without macular edema, and 12 showed moderate non-proliferative DR with non-center-involved macular edema. The pallor of the optic disc was noted in eight eyes, and mild optic disc swelling was observed in two eyes, and there was no abnormality in the fundus of other patients. Headache was a presenting symptom in 26 patients (81.25%), and only 10 (23%) had nasal complaints (Table 1).
Imaging features
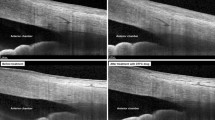
Computed tomography (CT) of the paranasal sinus or orbit showed partial or complete opacification and mucosal thickening of the affected sinus in these patients. We observed microcalcification in 11 cases (34.38%); its prevalence in the noninvasive FSS group was three times that in the invasive FSS group (n = 9, 47.4% vs. n = 2, 15.4%, Table 2). However, the difference was not significant. Bone changes were noted in 28 patients: 19 (59.38%) showed bony erosion, 19 (59.38%) showed bony thickening and sclerosis. There was no significant difference between the invasive and noninvasive FSS groups (Table 2, Fig. 2).
Computed tomography (CT) findings of fungal sphenoid sinusitis (FSS). (a, b) show complete opacity of the left sphenoid sinus with calcification (white arrow), part opacity of the right sphenoid sinus in noninvasive FSS (white arrow). (c, d) show complete opacity of the right sphenoid sinus with surrounding bone destruction (white arrow) in invasive FSS.
Magnetic resonance imaging (MRI) of the paranasal sinus or orbit was performed in 17 cases of noninvasive FSS and 12 cases of invasive FSS. On T1-weighted images (T1WI), eight (8/17, 47.06%) of the noninvasive fungal masses were hyperintense (compared with brain parenchyma) and seven (7/17, 41.18%) were hypointense, two (11.76%) of which were isointense. All cases were marked hypointense on T2-weighted images (T2WI), of which, two were uneven and accompanied by an isointense signal. On T1WI, six cases (6/12, 50%) of invasive FSS showed homogeneous hypointensity and six (6/12, 50%) were isointense, of which, one had an accompanied small amount of hypointensity. The lesions showed variable signal intensity on T2WI: three cases (3/12, 25%) were mainly hypointense with different numbers and shapes of hyperintensity (Fig. 3), the duration of symptoms were > 6 months, surgery confirmed that the lesions had prominent fibrous tissue hyperplasia; nine cases (9/12, 75%) showed hyperintensity, the duration of symptoms were < 5 months, histopathology showed tissue necrosis with varying numbers of inflammatory cells, of which five cases had granulomas (Fig. 4). The mucosa around the lesion was significantly thickened with isointensity on T1WI and hyperintensity on T2WI. There were 17 cases with infiltration of the orbital apex, 14 of which involved the cavernous sinus. The optic nerve showed hyperintensity on T2WI in four cases (4/29, 13.79%). Thickening and enhancement of the optic nerve sheath were observed in four cases (4/29, 13.79%).
MRI findings of FSS. (a, b) show low signal of right sphenoid sinus on T2WI (white arrow), peripheral mucosal thickening and linear enhancement (white arrow). Irregular low T2 signal shadows can be seen in the left sphenoid sinus (white arrow). (c, d) show the hypointensity in bilateral sphenoid sinus on T1WI , without enhancement (white arrow). The lesion invades the skull base, and enhancement can be seen in the adjacent skull base membrane (white box).
Treatment and outcome
Excision of the necrotic tissues and sinuses was performed in all patients, and four patients (12.5%) had received glucocorticoid treatment preoperatively because they were misdiagnosed as having optic neuritis. Postoperatively, 13 patients were treated with antifungal drugs. Two patients were treated with intravenous liposomal amphotericin B, and 11 with intravenous voriconazole combined with irrigation of the involved paranasal sinuses with amphotericin B. Three patients died of progressive disease owing to intracranial spread 1, 3, and 5 months postoperatively, respectively. All of them were found to have a mucormycosis infection. Of the remaining cases, patients with ptosis or limited eye movement improved partially or even completely. The visual acuity of 22 eyes from 19 patients (57.89%) improved significantly, whereas there was no improvement in 16 eyes of 13 patients (42.11%). Patients with noninvasive FSS showed significantly greater improvement in visual acuity (17 eyes, 70.8%) than those with invasive FSS (5 eyes, 35.7%) (P = 0.026). Univariate and multivariate GEE model results revealed that sphenoid sinus wall sclerosis on CT was an independent factor for better visual prognosis (odds ratio = 4.434, 95% confidence interval (CI) 1.032–19.059, P = 0.045) (Table 3).
Discussion
Fungal sinusitis is a relatively uncommon disease and is broadly classified into two categories: invasive and noninvasive6. In the present study, we found that FSS with ocular complications was more common among the older women with a mean age of > 62 years, similar to that described in previous reports12. This suggests that advanced age may be a risk factor for FSS.
Abnormal immune function is an important inducer of fungal invasion of the paranasal sinuses13,14. The typical patients with noninvasive FSS are immunocompetent, while affected patients with invasive FSS tend to be critically ill, and demonstrate some degree of compromised immune function, such patients typically include those with decreased host cell-mediated immunity, specifically with impaired neutrophil function, hematologic malignancies, aplastic anemia, hemochromatosis, poorly controlled diabetes, acquired immunodeficiency syndrome, or organ transplantation; or are undergoing immunosuppressive treatments, such as systemic steroids or chemotherapeutic agents. Infrequently, invasive FSS has been reported in patients with normal immune function9. In this study, 18 patients (56.25%) had a diagnosis of diabetes mellitus, 4 of whom had uncontrolled diabetes. In addition, one patient was on antibiotic therapy for chronic bronchiectasis and one was taking long-term corticosteroids for rheumatoid arthritis. We have assessed the neutrophil count of all patients to categorize the degrees of impaired immune function. However, the neutrophil count of all patients is within the normal range. Patients with uncontrolled diabetes are known to have poor humoral immunity and neutrophilic dysfunction, which provide a favorable environment for the fungus to thrive15. The long-term systemic use of antibiotics or corticosteroids is also an important risk factor for altered immune function. Although the neutrophil counts of these patients were within normal limits, we suspected that impaired neutrophil function could be the main reason for the poor immunity, which reminded us to pay attention to neutrophil function in our future work.
In our study, 13 eyes had NLP, of which nine exhibited fungal invasion of the mucosa or submucosal tissue of the sphenoid sinus on histopathology. The other four fungal ball or allergic fungal rhinosinusitis were accompanied by infiltration of acute and chronic inflammatory cells. These findings suggest that the mechanism of visual impairment in FSS sinusitis may be related to the direct invasion of fungi or the local immune response caused by the fungal infection.
It is generally not difficult to locate the lesion at the orbital apex or cavernous sinus16 when vision loss coincides with binocular diplopia, motility deficits, ptosis, or exophthalmos, the diagnostic process becomes more straightforward, typically involving the exclusion of tumors. Sinonasal malignancy is always with a short history and rapid progression, commonly arise from the maxillary sinus. CT scan shows a soft tissue mass filling the sinus cavity and extensive osteolytic destruction of the sinus wall. The masses have uneven density and irregular morphology17. However, if there is only headache or ocular pain followed by monocular or binocular vision loss, it is easy to misdiagnose as noninfectious optic neuritis, which is a relatively common disease, and administer high-dose glucocorticoids as treatment. In our study, four patients were treated with glucocorticoids preoperatively. Visual acuity of three patients did not show any improvement, while the other one's visual acuity further decreased after glucocorticoids treatment. For these patients, there is a potential risk of infection spread and, even fatal consequences. Fortunately, there were not any other complications due to the glucocorticoid therapy in these four patients, and they were correctly diagnosed in time during the subsequent treatment. Therefore, in order to avoid the serious consequences caused by misdiagnosis, for older women with decreased vision and persistent headache, especially those with diabetes, orbital or cranial neuroimaging examinations should be performed to exclude the FSS.
CT typically is effective in delineating the fine bone structures and intra-sinus calcification18,19. Noninvasive FSS is mainly characterized by calcification, bone thickening, and sclerosis of the sinus wall. Conversely, invasive FSS usually causes bone destruction of the sinus, but calcification is rare. MRI is useful for diagnosing and identifying FSS, as it provides better details of lesions of the orbital apex, cavernous sinus, brain parenchyma, and pterygomaxillary fossa. In this study, noninvasive FSS showed marked hypointensity on T2WI, which may be related to heavy metals, such as calcium salt, iron, and magnesium in fungal secretions20. The manifestations of invasive FSS are different from those of noninvasive FSS on T2WI. The invasive FSS showed variable signals on T2WI, which was related to the course of the disease. The early stage is dominated by hyperintensity and the late stage by hypointensity, but it is usually uneven. Histopathology showed tissue necrosis with varying numbers of inflammatory cells in the early stage, some cases formed granulomas, and there was prominent fibrosis in the late stage. Most of them were accompanied by peripheral mucosal inflammation, manifested as mucosal thickening, and appeared isointense on T1WI and hyperintense on T2WI, the edge was evidently enhanced, which is valuable to distinguish from noninvasive FSS.
Surgical exenteration and reconstruction of a sufficient sinus cavity are effective treatments21. All patients in this study underwent surgical debridement, and 13 were treated with antifungal drugs after exenteration. All patients survived, except three patients with Mucor infection died of subsequently intracranial dilation at 1, 3, and 5 months postoperatively, respectively. Cavernous carotid artery occlusion was identified in two patients and Mucor meningitis was identified in the other one. These three patients had a diagnosis of diabetes mellitus. Aspergillus and Mucor are saprophytic fungi, which thrive in an environment of low oxygen tension and hyperglycemia21. Both have an affinity for invading blood vessels, however, Mucor has been suggested to be more aggressive with a strong affinity for arteries15. It can grow along the vascular wall or into the lumen, leading to fungal vasculitis and thrombosis22.
Visual acuity was improved after treatment. The overall improvement rate of visual acuity was higher in noninvasive FSS than in invasive FSS. However, there was no correlation between fungal invasion and visual prognosis in multivariate analysis. We noted that the P value was exactly 0.05 in multivariate analysis, which may have resulted from the sample size limitation, and future studies with larger sample sizes may have led to different conclusions. In addition, sphenoid sinus wall sclerosis on CT was an important independent factor affecting visual prognosis. Remodeling of the sphenoid sinus wall is due to stimulation of fungal reproduction or secondary chronic inflammation, indicating that the pathogen has low virulence and poor invasiveness and causes less damage to the optic nerve. Therefore, the vision of some patients will be improved to a certain extent.
This study had some limitations. First, this was a single–center retrospective study. Second, our study lacked other visual function evaluation methods, such as the visual field. Therefore, these assessment methods should be included in future prospective studies.
Conclusion
Fungal sinusitis, although relatively rare, can cause varying degrees of visual impairment. When symptoms are nonspecific, it can be misdiagnosed as noninfectious optic neuritis and inappropriate treatment with high-dose glucocorticoids may lead to infection spread, even fatal consequences. Immediate imaging of the orbit and sinuses is imperative to avoiding misdiagnosis and prompt initiation of appropriate treatment as soon as possible is essential for the preservation of visual function. In severe cases, especially in invasive FSS, early endoscopic sphenoidotomy and antifungal therapy are effective treatments.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- FSS:
-
Fungal sphenoid sinusitis
- AFS:
-
Allergic fungal sinusitis
- DR:
-
Diabetic retinopathy
- BCVA:
-
Best-corrected visual acuity
- log MAR:
-
Log of the minimum angle of resolution
- CF:
-
Count fingers
- LP:
-
Light perception
- NLP:
-
No light perception
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- T1WI:
-
T1-weighted images
- T2WI:
-
T2-weighted images
- GEE:
-
Generalised estimating equation
- CI:
-
Confidence interval
- OR:
-
Odds ratio
References
Lee, J. T., Frank, D. N. & Ramakrishnan, V. Microbiome of the paranasal sinuses: Update and literature review. Am. J. Rhinol. Allergy. 30, 3–16 (2016).
Craig, J. R. Updates in management of acute invasive fungal rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 27, 29–36 (2019).
Grosjean, P. & Weber, R. Fungus balls of the paranasal sinuses: A review. Eur. Arch. Otorhinolaryngol. 264, 461–470 (2007).
Thery, A., Espitalier, F., Cassagnau, E., Durand, N. & Malard, O. Clinical features and outcome of sphenoid sinus aspergillosis: A retrospective series of 15 cases. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 129, 179–184 (2012).
Jung, J. H., Cho, G. S., Chung, Y. S. & Lee, B. J. Clinical characteristics and outcome in patients with isolated sphenoid sinus aspergilloma. Auris Nasus Larynx. 40, 189–193 (2013).
Chen, L., Jiang, L., Yang, B. & Subramanian, P. S. Clinical features of visual disturbances secondary to isolated sphenoid sinus inflammatory diseases. BMC Ophthalmol. 17, 237 (2017).
Kim, J. S., Kim, B. K., Hong, S. D., Kim, H. J. & Kim, H. Y. Clinical characteristics of sphenoid sinus fungal ball patients with visual disturbance. Clin. Exp. Otorhinolaryngol. 9, 326–331 (2016).
Chakrabarti, A., et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope. 119, 1809–1818 (2009).
Raz, E. et al. Fungal sinusitis. Neuroimaging Clin. N. Am. 25, 569–576 (2015).
Schulze-Bonsel, K., Feltgen, N., Burau, H., Hansen, L. & Bach, M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest. Ophthalmol. Vis. Sci. 47, 1236–1240 (2006).
Sparrow, J. M. et al. The Cataract National Dataset electronic multi-centre audit of 55,567 operations: Risk indicators for monocular visual acuity outcomes. Eye (London). 26, 821–826 (2012).
Promsopa, C., Polwiang, P., Chinpairoj, S. & Kirtsreesakul, V. Complications of isolated fungal sphenoiditis: patient clinical characteristics. ORL J. Otorhinolaryngol. Relat. Spec. 82, 15–24 (2020).
Nicolai, P. et al. Fungus ball of the paranasal sinuses: Experience in 160 patients treated with endoscopic surgery. Laryngoscope. 119, 2275–2279 (2009).
Toussain, G. et al. Sinus fungal balls: Characteristics and management in patients with host factors for invasive infection. Rhinology. 50, 269–276 (2012).
Trief, D. et al. Invasive fungal disease of the sinus and orbit: A comparison between mucormycosis and Aspergillus. Br. J. Ophthalmol. 100, 184–188 (2016).
Mukherjee, B., Raichura, N. D. & Alam, M. S. Fungal infections of the orbit. Indian J. Ophthalmol. 64, 337–345 (2016).
Kuan, E. C. et al. International consensus statement on allergy and rhinology: Sinonasal tumors. Int. Forum Allergy Rhinol. 14, 149–608 (2024).
Chen, J. C. & Ho, C. Y. The significance of computed tomographic findings in the diagnosis of fungus ball in the paranasal sinuses. Am. J. Rhinol. Allergy. 26, 117–119 (2012).
Groppo, E. R., El-Sayed, I. H., Aiken, A. H. & Glastonbury, C. M. Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol. Head Neck Surg. 137, 1005–1010 (2011).
Phuttharak, W., Hesselink, J. R. & Wixom, C. MR features of cerebral aspergillosis in an immunocompetent patient: Correlation with histology and elemental analysis. AJNR Am. J. Neuroradiol. 26, 835–838 (2005).
Wali, U., Balkhair, A. & Al-Mujaini, A. Cerebro-rhino orbital mucormycosis: An update. J. Infect Public Health. 5, 116–126 (2012).
Zinreich, S. J. et al. Fungal sinusitis: Diagnosis with CT and MR imaging. Radiology. 169, 439–444 (1988).
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
The study was funded by Capital Health Development Research Project (No: 2020-2-1082).
Author information
Authors and Affiliations
Contributions
FC and YS: writing – original draft; formal analysis; writing – review and editing; QH: Resources. YC: Investigation and Resources BY: Validation and writing – original draft. LJ: funding acquisition; writing – review and editing. Written informed consent for publication of this report and the accompanying images was obtained from the patient.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, F., Shao, Y., Huang, Q. et al. Visual function loss in fungal sphenoid sinusitis: clinical characteristics and outcomes. Sci Rep 14, 8649 (2024). https://doi.org/10.1038/s41598-024-59107-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59107-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.