Abstract
Persistence is important for the success in the treatment of women with overactive bladder syndrome (OAB). We aimed to identify the predictors of non-persistence in women with OAB after first-line medical treatment. All consecutive women with OAB (n = 608), who underwent urodynamic studies and received first-line medical treatment (5 mg of solifenacin or 25 mg of mirabegron per day) in a referral medical center, were reviewed. Mirabegron (hazard ratio [HR] = 0.711) was associated with a higher persistence rate, compared to solifenacin. Mirabegron treatment (HR = 0.269) was less likely to switch medication; however, a high Urogenital Distress Inventory score (HR = 1.082) was more likely to switch medication. Furthermore, old age (HR = 1.050, especially for ≥ 75 years) and high voided volume (dL, HR = 1.420, especially for voided volume ≥ 250 ml) were associated with added medication at follow-up. Additionally, women with low parity (HR = 0.653, especially for parity ≤ 3) and a low Incontinence Impact Questionnaire (IIQ-7) score (HR = 0.828, especially for IIQ-7 score ≤ 7) were associated with improvement without medication. In conclusion, mirabegron can be considered as the first frontline treatment to increase the persistence rate and decrease the rate of switched medications, compared to solifenacin. In addition, combination therapy or higher-dose monotherapy could be used as the first front-line treatment for women ≥ 75 years of age or with ≥ 250 ml of voided volume.
Similar content being viewed by others
Introduction
Overactive bladder syndrome (OAB) is characterized by urinary urgency, frequency, and nocturia. Both antimuscarinics and beta-3 agonists are considered first-line medical treatments for OAB. Several antimuscarinics (for example, solifenacin and tolterodine) are currently marketed for OAB treatment. Solifenacin has a moderate selectivity for the M3 receptor over the M2 receptor. Beta-3 agonists relax the detrusor muscle during the bladder storage phase and increase bladder capacity. Mirabegron is the first beta-3 agonist approved for OAB treatment. Mirabegron has been reported to have similar efficacy to antimuscarinics1; however, the beta-3 agonist is associated with less bothersome adverse effects, such as dry mouth2. However, the question of whether solifenacin and mirabegron have different persistence rates remains undetermined3,4,5,6.
Persistence is generally referred to the overall duration of drug therapy. OAB typically requires long-term persistence with medical therapy7. Patients who persist in taking OAB medication have a significant improvement in OAB symptoms compared to non-persistent8. Discontinuation of medication or lost follow-up had been used as the definition of non-persistence9; switched medications and added medication were excluded as non-persistence10.
Lack of efficacy, improvement in symptoms, adverse effects, insurance limits, cost concerns, and inconvenience are considered causes of non-persistence5,10,11. Knowledge of non-persistence is important to improve persistence and personal precision treatment.
In addition, urodynamic studies might be helpful in the treatment of complex storage diseases such as OAB12. A Female Urgency, Trial of Urodynamics as Routine Evaluation (FUTURE) study was conducted to evaluate whether routine urodynamics improves treatment results in women with refractory OAB13.
Due to the positive association between the persistence of OAB medication and therapeutic efficacy8, the issue of improving persistence is important. Therefore, this study aimed to analyze clinical and urodyamic predictors of non-persistence.
Results
Between July 2010 and December 2020, a total of 608 women were reviewed in this study. Except age, OAB-wet and pad weight, there were no differences between solifenacin and mirabegron (Table 1).
There was a statistical difference in the persistent curves between the mirabegron and solifenacin groups (log-rank test, p = 0.008, Fig. 1A). The multivariable Cox regression model also showed that mirabegron (hazard ratio [HR] = 0.711, p = 0.019) was the only predictor of non-persistence (Table 2). OAB-wet was not a predictor of non-persistence (HR = 1.047, p = 0.729, Table 2).
Mirabegron (HR = 0.269, p < 0.001) was associated with a lower incidence of switched medications (Fig. 1B, Table 3). Furthermore, a higher baseline Urogenital Distress Inventory (UDI-6) score14 (HR = 1.082, p = 0.029) was associated with a higher incidence of switched medications (Table 3). The UDI-6 score ≥ 10 was the optimal cutoff value for predicting switched medications, with an area under the receiver operating characteristic curve (AUC) of 0.534 (95% confidence interval [CI] = 0.452 to 0.616; sensitivity = 26.6%, specificity = 83.6%).
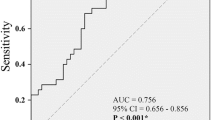
Old age (HR = 1.050, p = 0.009) and larger voided volume (dL, HR = 1.420, p = 0.001) were associated with a higher incidence of added medications (Table 4). Age ≥ 75 years was the optimal cut-off value for predicting added medications, with an AUC of 0.727 (95% CI = 0.611 to 0.842; sensitivity = 33.3%, specificity = 92.2%). Voided volume ≥ 2.50 dL (i.e., 250 mL) was the optimal cut-off value for predicting added medications, with an AUC of 0.657 (95% CI = 0.541 to 0.772; sensitivity = 76.5%, specificity = 50.0%).
Mirabegron was not associated with improvement without treatment (Table 5). However, low parity (HR = 0.653, p = 0.020) and low baseline Incontinence Impact Questionnaire (IIQ-7) score14 (HR = 0.828, p = 0.007) were associated with improvement without medication (Table 5). Parity ≤ 3 was the optimal cutoff value for predicting improvement without medication, with an AUC of 0.692 (95% CI = 0.587 to 0.797; sensitivity = 43.4%, specificity = 89.5%). The baseline IIQ-7 score ≤ 7 was the optimal cut-off value for predicting improvement without medication, with an AUC of 0.705 (95% CI = 0.602 to 0.808; sensitivity = 47.1%, specificity = 83.3%).
The details of the switched medications and the added medications are shown in Table 6.
Discussion
In this study, women who received mirabegron treatment tended to have a higher incidence of persistence (Fig. 1A, Table 2). Similarly, a study from the national cohort database of Korea found that mirabegron has a longer persistence than antimuscarinics4. The PERSPECTIVE (a Prospective Non-interventional Registry Study of Patients Initiating a Course of Drug Therapy for Overactive Bladder) study revealed that the persistence was longer for mirabegron compared with antimuscarinics 5. Chapple et al. also reported that mirabegron had a higher persistence rate compared to antimuscarinics6. Nazir et al. and Yeowell et al. had similar findings on the superiority of mirabegron in persistence15,16. However, Lee et al. reported that there is no difference in persistence between groups4. Sussman et al. reported that persistence rates were similar between solifenacin and mirabegron17.
In our study, mirabegron was associated with a lower incidence of switched medications (Fig. 1B, Table 3). The causes of switched medications could include cost consideration, lack of efficacy,or intolerable adverse effects. In Taiwan, the fee for solifenacin and mirabegron was covered by the National Health Insurance. In addition, the same efficacy between mirabegron and solifenacin has been reported1. Furthermore, higher adverse effects were observed for antimuscarinic drugs2. Thus, the lower adverse effects of mirabegron should contribute to its lower rate of switching medications.
The UDI-6 score (HR = 1.082) is a predictor of switched medications (Table 3). UDI-6 question 2 is the question for urgency incontinence severity. In this study, the UDI-6 score was strongly associated with UDI-6 question 2 (Spearman’s rho = 0.62, p < 0.001); and this meant that higher severity of urgency incontinence was associated with higher rate of switched medications. Thus, a lower side effect of the medication (for example, mirabegron) or a higher dose (for example, 50 mg mirabegron) could be used as an initial first-line treatment for women with severe urgency incontinence to decrease the rate of switched medications.
Age (HR = 1.050, especially for those ≥ 75 years) was a predictor of the added medications (Table 4).Similarly, Soda et al. found that old age was associated with continuous treatment, which included added medication9. In this study, old age was associated with a higher score on UDI-6 Question 2 (that is, the urgency incontinence, Spearman’s rho = 0.27, p < 0.001), and this represents that women of old age had a greater severity of urgency incontinence. Therefore, the medication (5 mg solifenacin or 25 mg mirabegron) might be inadequate in some old women. Higher dose (for example, 50 mg mirabegron) or combination therapy could be used as a first line treatment for old age women.
In our study, high voided volume (HR = 1.420, especially for voided volume ≥ 2.50 dL) was a predictor of added medication (Table 4). High voided volume represents mild severity of OAB18,19. Mild severity of OAB was reported to be associated with a poor response to solifenacin20. That is, high voided volume might be associated with poor therapeutic response; and women with high voided volume might need higher dose medication or combination therapy to improve treatment response.
In this study, low parity (HR = 0.653, especially for parity ≤ 3) was a predictor of improvement without medication (Table 5). Low parity was associated with mild severity of OAB21. Similarly, high parity (HR = 1.81) has been reported to be a predictor of the retreat of OAB symptoms22.
In this study, a low IIQ-7 score (HR = 0.828, especially for IIQ-7 score ≤ 7) was a predictor of improvement without medication (Table 5). A low IIQ-7 score means a mild urgency incontinence severity (i.e., IIQ-7 score versus UDI-6 question 2 score (i.e., urgency incontinence score), Spearman's rho = 0.31, p = 0.001). Thus, our data represent that women with mild OAB severity have a higher incidence of improvement in symptoms without treatment.
Limitations of this study include retrospective nature and non-randomized. Additionally, the sample size was not equal in both groups. Furthermore, this study covered a period of almost 10 years, and 25 mg of mirabegron was only available since 2016 in our hospital, leading to a significant difference in the median follow-up intervals between solifenacin and mirabegron; and the above might also bias our results. In addition, 50 mg mirabegron was not available in our hospital and our data could not be extrapolated to 50 mg mirabegron. In this study, women with improvement without medication were identified from the medical record. However, the true causes responsible for lost to follow-up include improvement of symptoms or no response to treatment. Therefore, in this study, the percentage of improvement without medication in Table 1 should be underestimated.
In conclusion, mirabegron can be considered as the first frontline treatment to increase the persistence rate and decrease the rate of switched medications, compared to solifenacin. In addition, combination therapy or higher-dose monotherapy could be used as the first front-line treatment for women ≥ 75 years of age or with ≥ 250 ml of voided volume.
Methods
Between July 2010 and December 2020, medical records of all consecutive OAB women who underwent pretreatment urodynamic studies and then received first-line medication (5 mg of solifenacin or 25 mg of mirabegron per day) were reviewed. Women with coexistent stress urinary incontinence were also included. However, women who underwent a midurethral sling procedure or vaginal laser therapy for coexisting stress urinary incontinence were excluded. The hospital’s Research Ethics Review Committee approved this study (Far Eastern Memorial Hospital, No.110053E, approval date: April 27, 2021). All methods were performed in accordance with relevant guidelines and regulations. The Research Ethics Review Committee agreed that informed consent was not required due to the retrospective nature of this study.
Urodynamic studies were performed on women in a seated position using a Life-Tech six-channel monitor with computer analysis and the Urolab/Urovision System V (Houston, Texas, USA). Urodynamic studies included uroflowmetry, filling cystometry with 35° C distilled water at a rate of 60 ml/s, a pressure flow study, and a stress urethral pressure profile with a strong desire volume of distilled water in the bladder. In addition, a 1 h pad test was performed23.
All terminology used in this document is consistent with the standards recommended by the joint report of the International Urogynecological Association and the International Continence Society24. All procedures were performed by an experienced technician and the data was interpreted by a single observer to avoid interobserver variability. OAB was defined as the presence of urinary urgency, with or without urgency incontinence, which is generally accompanied by urinary frequency and nocturia24. OAB-wet was diagnosed in women who complained of at least one episode of urgency incontinence in the previous month; otherwise, OAB-dry was diagnosed19.
Non-persistence after first-line medication was defined as the presence of lost follow-up. The switch of medication was defined as the discontinuation of the first-line OAB medication and the switch to other medications. Improvement without medication was referred to spontaneous improvement of OAB symptoms after first-line medical treatment, and the patients did not need additional OAB medication. The persistence interval was calculated as the time interval from the date of the start of the prescribed medication to the date of loss of follow-up, improvement without medication, or the last follow-up for continuous prescription.
Stata version 11.0 (Stata Corp, College Station, TX) was used for statistical analyzes. Survival curves were generated using the Kaplan–Meier method and differences in survival curves were calculated with the log-rank test. A p-value less than 0.05 was considered statistically significant. The multivariable backward stepwise Cox proportional hazards model was used to identify independent predictors using all variables in the univariate analysis with p < 0.10 until all remaining variable with p < 0.10. A receiver operating characteristic (ROC) curve analysis was performed to identify the optimal cut-off value. The optimal cut-off value was determined by the point on the ROC curve that was closest to the upper left corner.
Ethical approval
This study was approved by the Research Ethics Committee (Far Eastern Memorial Hospital Research Ethics Review Committee, No.110053E, approval date: 27 April 2021) at Far Eastern Memorial Hospital.
Informed consent
The Research Ethics Review Committee of Far Eastern Memorial Hospital approved the informed consent waiver.
Data availability
The datasets generated and / or analysed during the current study are available from the corresponding author on reasonable request.
References
Chapple, C. R. et al. Safety and efficacy of mirabegron: Analysis of a large integrated clinical trial database of patients with overactive bladder receiving mirabegron, antimuscarinics, or placebo. Eur. Urol. 77, 119–128 (2020).
Wagg, A. et al. Oral pharmacotherapy for overactive bladder in older patients: Mirabegron as a potential alternative to antimuscarinics. Curr. Med. Res. Opin. 32, 621–638 (2016).
Kinjo, M., Sekiguchi, Y., Yoshimura, Y. & Nutahara, K. Long-term persistence with mirabegron versus solifenacin in women with overactive bladder: Prospective, randomized trial. Low. Urin. Tract. Symptoms 10, 148–152 (2018).
Lee, K. S. et al. Mirabegron has longer treatment persistence than antimuscarinics: Real-world data from a Korean national cohort database. Neurourol. Urodyn. 40, 1972–1980 (2021).
Carlson, K. V. et al. Persistence with mirabegron or antimuscarinic treatment for overactive bladder syndrome: Findings from the PERSPECTIVE registry study. Low. Urin. Tract. Symptoms 13, 425–434 (2021).
Chapple, C. R. et al. Persistence and adherence with mirabegron versus antimuscarinic agents in patients with overactive bladder: A retrospective observational study in UK clinical practice. Eur. Urol. 72, 389–399 (2017).
Schabert, V. F. et al. Challenges for managing overactive bladder and guidance for patient support. Am. J. Manag. Care. 4(Suppl), S118-122 (2009).
Kim, T. H., Choo, M. S., Kim, Y. J., Koh, H. & Lee, K. S. Drug persistence and compliance affect patient-reported outcomes in overactive bladder syndrome. Qual. Life. Res. 25, 2021–2029 (2016).
Soda, T., Tashiro, Y., Koike, S., Ikeuchi, R. & Okada, T. Overactive bladder medication: Persistence, drug switching, and reinitiation. Neurourol. Urodyn. 39, 2527–2534 (2020).
Sexton, C. C. et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: A systematic review of the literature. Int. J. Clin. Pract. 65, 567–585 (2011).
Illiano, E., Finazzi Agrò, E., Natale, F., Balsamo, R. & Costantini, E. Italian real-life clinical setting: The persistence and adherence with mirabegron in women with overactive bladder. Int. Urol. Nephrol. 52, 1035–1042 (2020).
Kim, S. J., Choo, H. J. & Yoon, H. Diagnostic value of the maximum urethral closing pressure in women with overactive bladder symptoms and functional bladder outlet obstruction. Int. Neurourol. J. 26(Suppl 1), S1-7 (2022).
Abdel-Fattah, M. et al. Female Urgency, Trial of Urodynamics as Routine Evaluation (FUTURE study): A superiority randomised clinical trial to evaluate the effectiveness and cost-effectiveness of invasive urodynamic investigations in management of women with refractory overactive bladder symptoms. Trials. 22, 745 (2021).
Uebersax, J. S., Wyman, J. F., Shumaker, S. A., McClish, D. K. & Fantl, J. A Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol. Urodyn. 14, 131–139 (1995).
Nazir, J. et al. A retrospective study of treatment persistence and adherence to mirabegron versus antimuscarinics for the treatment of overactive bladder in Spain. BMC. Urol. 18, 76 (2018).
Yeowell, G. et al. Real-world persistence and adherence to oral antimuscarinics and mirabegron in patients with overactive bladder (OAB): A systematic literature review. BMJ. Open. 8, e021889 (2018).
Sussman, D. et al. Adherence and persistence of mirabegron and anticholinergic therapies in patients with overactive bladder: A real-world claims data analysis. Int. J. Clin. Pract. 71, e12824 (2017).
Hsiao, S. M., Chang, T. C., Chen, C. H., Wu, W. Y. & Lin, H. H. Frequent nocturia episodes, a suboptimal response to treatment, and small bladder capacity predict the need for persistent antimuscarinic therapy or retreatment after discontinuation of antimuscarinics in female overactive bladder. Menopause 24, 100–104 (2017).
Hsiao, S. M., Wu, P. C., Chang, T. C., Chen, C. H. & Lin, H. H. Urodynamic and bladder diary factors predict overactive bladder-wet in women: A comparison with overactive bladder-dry. Int. Neurourol. J. 23, 69–74 (2019).
Hsiao, S. M., Lin, H. H. & Kuo, H. C. Factors associated with a better therapeutic effect of solifenacin in patients with overactive bladder syndrome. Neurourol. Urodyn. 33, 331–334 (2014).
Palma, T. et al. Prospective study of prevalence of overactive bladder symptoms and child-bearing in women of reproductive age. J. Obstet. Gynaecol. Res. 39, 1324–1329 (2013).
Hsiao, S. M., Chang, T. C. & Lin, H. H. The probability of retreatment after discontinuation of a 3-month versus a 6-month course of solifenacin for female overactive bladder: A prospective randomized controlled study. Maturitas 126, 11–17 (2019).
Wu, W. Y., Sheu, B. C. & Lin, H. H. Comparison of 20-minute pad test versus 1-hour pad test in women with stress urinary incontinence. Urology 68, 764–768 (2006).
Haylen, B. T. et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 21, 5–26 (2010).
Funding
This work was supported by Astellas Pharma, Inc. under a general research grant.
Author information
Authors and Affiliations
Contributions
S.-M.H. contributed to study design, data collection, statistical analysis, and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
S-M H has received research support from Astella Pharma Ltd.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsiao, SM. Predictors of non-persistence in women with overactive bladder syndrome. Sci Rep 14, 7499 (2024). https://doi.org/10.1038/s41598-024-58036-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58036-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.