Abstract
For rewilding the depleted crocodylian populations in India, a targeted ‘one-species one area’ based conservation approach was adopted in the early-1970s. Suitable habitats were identified and designated as protected areas, specifically targeted to recover a particular crocodylian species. A ~ 610 km stretch of Chambal River in the Ganga River Basin was declared as National Chambal Sanctuary to restore the ‘Critically Endangered’ gharial (Gavialis gangeticus), where active management of mugger (Crocodylus palustris) was discouraged. In the present study, we examined the population trends, occupancy, and genetic status of mugger by conducting population monitoring and genetic assessment to understand the status of potentially competitive mugger in the Sanctuary. Our finding suggests that the mugger population has notably increased and colonised the Sanctuary. We observed a moderate level of genetic diversity in the mugger, which was relatively higher compared to the gharial in the Sanctuary. The rapid colonization of ecological generalist mugger raises concerns about potential competition with ecological specialist gharial threatening its long-term sustainability. Considering the coexistence dynamics between the species, it is essential to extend adaptive management strategies for mugger to ensure successful recovery of gharial population in the Sanctuary.
Similar content being viewed by others
Introduction
Over the last few centuries, ecosystem degradation led by anthropogenic activities has triggered massive biodiversity loss resulting in demographic declines, range contractions, population isolations, and extinction of numerous species1,2,3. In response to this declining trend, micro-scale conservation measures such as species translocation have become imperative and integral for species restoration and rewilding to buffer the impact of human-driven biodiversity loss4,5,6. In the past, translocation efforts were primarily motivated by the desire to establish or enhance populations of target species for various purposes like food resources, pest control and trophy hunting7,8,9. Improved scientific understanding led to recognition of ecological implications of species translocations, promoting its global adoption as a conservation strategy and substantial increase in species translocation programs globally10,11. Species translocation has successfully prevented extinction of several wildlife species, such as the Arabian oryx (Oryx leucoryx)12, Californian condor (Gymnogyps californianus)13, and Lord Howe Island woodhen (Hypotaenidia sylvestris)14.
By the late-1960s, the crocodylian populations in India had also witnessed a significant population and range decline primarily attributed to habitat loss caused by sand mining and agriculture, poaching, and mortality in passive and detrimental fishing15,16. To address this situation, the government of India listed all three crocodylians i.e. gharial (Gavialis gangeticus Gmelin, 1789), mugger (Crocodylus palustris Lesson, 1831), and saltwater crocodile (Crocodylus porosus Schneider, 1801), in the Schedule I of the then enacted Wild Life (Protection) Act, 1972, prohibiting their hunting, and trade in any form17. Subsequently, a dedicated crocodile conservation and management program, commonly termed ‘Project Crocodile,’ was launched in 1975 and aimed at protecting the remaining natural populations of crocodylians by creating new protected areas, enhancing population recruitment and recovery through a 'head-start’ assisted conservation translocation, and establishing captive-rearing and breeding facilities within species range18. In a decade of its inception, more than 12 Wildlife Sanctuaries were created under the Wild Life (Protection) Act, 197218,19. Simultaneously, rearing and captive breeding facilities were established, and crocodylians of wild origins were captively reared in these facilities and later released into the protected areas16,20,21.
The mugger is widespread in India and occurs sympatrically with gharial especially in the Northern and Eastern Indian rivers22. Due to the sympatric occurrence and likelihood of interspecific competition, a targeted 'one-species one area’ based conservation approach was suggested19,20. Following which, there has been a scientific consensus to restrict the release of mugger in the riverine sanctuaries dedicated for gharial conservation17,20. Consequently, gharial specific conservation management interventions were implemented in the riverine protected areas viz. National Chambal Sanctuary (NCS) in the Chambal River, Katerniaghat Wildlife Sanctuary in the Girwa River, Son Gharial Sanctuary in the Son River, and Ken Gharial Sanctuary in the Ken River of the Northern region and Satkosia Gorge Wildlife Sanctuary (SWGS) in the Mahanadi River of the Eastern region18,23. Whereas, conservation and management efforts for the mugger were primarily concentrated in selected protected areas viz. Krishnagiri Wildlife Sanctuary, Coringa Wildlife Sanctuary, Papikonda Wildlife Sanctuary and Manjira Wildife Sanctuary in the southern region of India19,22.
Conservation management has facilitated the rapid recovery of the severely depleted gharial populations in the riverine protected area16. The gharial population in the Chambal River has increased from 107 individuals in 1979 to approximately 1675 individuals in 201815,24. In subsequent years, despite the initial precautionary measures taken, the lack of effective communication and coordination has resulted in inadvertent release of mugger individuals into the NCS and SGWS21,23. The increase in mugger population in the SGWS has resulted in interspecific aggression and conspicuous dominance of mugger25. The dominance of mugger in the SGWS also been attributed to the non-survival of the gharials in the past23. The increase in mugger population in prime gharial habitat, combined with depleting resources may intensify the competition and disrupt the coexistence dynamics26,27, undermining decades long gharial conservation efforts. Hence, monitoring of sympatrically occurring mugger is essential for guiding the gharial recovery program and develop a comprehensive framework for maintaining the coexistence between theses sympatric species in a single riverscape28.
In the present study, we intend to address the following research questions to understand the status of mugger population in the National Chambal Sanctuary in the Chambal River, (a) What is the present population trend of the mugger? (b) What is the extent of occupancy of the mugger, and what factors (habitat, ecological and anthropogenic) influence its occupancy? (c) What is the genetic status of the mugger population in the Chambal River?
Results
Population trends
We observed a consistent increase in the numbers of mugger crocodiles over time, as indicated by the relative density index (RDI) (Supplementary Fig. 1). The adult and sub-adult size classes constituted a significant portion, ranging between 81 and 84% of the population. Conversely, the percentage composition of the juvenile and below-size classes varied between 0 and 8% (Supplementary Table 1). This observation highlights the presence of different age cohorts within the mugger population. The exponential rate of increase (r) during the period was 0.101 (F—901.07; P < 0.001; R2—0.98) (Supplementary Fig. 2). The annual finite population growth rate (λ) was 1.106 (95% CI = 1.099–1.113). The percentage change estimated from λ was 10.6% (95% CI = 9.9–11.3%) per year (Table 1).
Single-season site occupancy
The estimated naïve occupancy for the mugger in the 134 composite blocks was 0.88 (± 0.04) (95% CI = 0.80–0.96). The univariate model containing channel width yields the lowest Akaike Information Criterion (AIC) for the probability of detection. Further, the additive models predicted five best models under ΔAIC ≤ 2, and the AICwt ranged between 0.12 to 0.28 (Table 2). The covariates associated with the best-predicted model (ΔAIC = 0) were river width for detection (p), and channel depth and sand for occupancy (Ψ). The goodness of fit test of the most parameterized multivariate occupancy model showed no evidence of overdispersion or underdispersion. The best model (ΔAIC) contained three covariates: river width, depth, and sand. The covariate river width has a negative influence on detection (p), and both site-covariates, viz., river depth and sand, positively impact occupancy (Ψ) (Table 2 and Supplementary Table 2). The estimated 95% confidence interval of the beta coefficient [β ± 1.96 × SE (Standard Error)] was used to determine the impact of covariates on detection and occupancy. Two covariates, river width for detection and river depth for occupancy, yielded strong support with non-zero overlapping. The river width had non-zero overlapping estimates in all candidate models (ΔAIC ≤ 2). In contrast, river depth had non-zero overlap in four out of five candidate models for influence on occupancy (Supplementary Table 2). In contrast, other covariates did not obtain much support for impact on mugger detection and occupancy.
The best variables for mugger detection and occupancy predictors were estimated using summed Akaike weights (ΣAICwt). The ΣAICwt for detection (p) was highest for river width (ΣAICwt—0.99) followed by air temperature (ΣAICwt—0.12), and for occupancy (Ψ), it was highest for river depth (ΣAICwt—0.99) followed by sand substrate type (ΣAICwt—0.28) and frequency of mining (ΣAICwt—0.22). The estimated mean model-averaged prediction for best candidate models was 0.57 ± 0.005 (mean ± SE) for detection and 0.88 ± 0.01 for occupancy (Supplementary Fig. 3).
Genetic assessment
We successfully extracted DNA from 106 of the 122 samples (86%) and genotyped 81 of the 106 samples (76%) (Supplementary Table 2). The average amplification success across 10 polymorphic loci rates was 88.61 ± 1.44. The quality index was 0.93 ± 0.008 across the 10 polymorphic loci, and the average error rate per locus was 0.07 ± 0.008 (Supplementary Table 4). We found no evidence of a large allele dropout in our data, and the null allele frequency for each locus was below 5%. No single locus showed significant deviation from Hardy–Weinberg Equilibrium across all nesting sites. Therefore, all loci genotyped were used for further analyses.
The cumulative probability of identity PID or probability of identity, PID-unbiased was 9.93 × 10–8, and PID-sibs was 1.17 × 10–3 for a panel of 10 polymorphic loci. We identified 60 unique individuals from 122 biological samples collected across five mugger nesting sites using multilocus genotype data (Fig. 1). The Dangbasai (DG) nesting site had only one individual genotype; therefore, we excluded it from further analysis. The number of polymorphic loci varied across the nesting sites, with the Baroli (BR) nesting site showing the most (n = 10), followed by the Nadigaon (NG) (n = 9), Pali (PA) (n = 8), and Tigri (TG) (n = 8). The number of alleles observed at each locus ranged from 2 to 14. The overall mean number of alleles per locus was 5.2 ± 1.4 (Table 3). The BR showed the highest number of alleles per locus BR (3.9 ± 0.7), followed by NG (3.8 ± 0.8), TG (2.0 ± 0.6), and PA (2.1 ± 0.3). The allelic richness based on a minimum sample size of six individuals was 2.7 ± 0.7 (Supplementary Table 5). The overall observed (Ho) and expected (He) heterozygosities were 0.57 ± 0.08 and 0.55 ± 0.08, respectively.
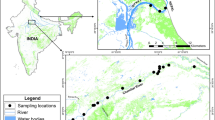
Map showing study area, survey stretches, and sampling locations for genetic analysis in the Nation Chambal Sanctuary along the Chambal River, India. The survey was conducted between Pali (start) and Panchnada (end) ~ 400 km stretch. The labeled sodalite blue represents sampling locations (1 = Pali, 2 = Baroli, 3 = Nadigaon, 4 = Dangbasai, and 5 = Tigri). The map was prepared using ArcGIS v.10.3.1 software developed by ESRI (https://www.esri.com).
The Bayesian clustering analysis implemented in Structure v2.3.4 suggests the presence of three (K = 3) clusters inferred by the delta K estimates (Supplementary Fig. 4). All individuals sampled from PA were assigned to Cluster-I with an overall proportion of membership (q) equal to 0.975, and 92% individuals sampled from TG were assigned to cluster-III with q equal to 0.901 and 8% to Cluster-I. The individuals sampled from BR and NG were assigned to multiple clusters. (Fig. 2). The Discriminant Analysis of Principal Components (DAPC) identified five clusters (K = 5) using the Bayesian Information Criterion (BIC) (Supplementary Fig. 5), segregating them into three units (Fig. 3). We observed significant (P < 0.05) genetic differentiation measures between all sites except BR and NG. The estimated FST value ranged between 0.087 (PA-BR) and 0.286 (PA-TG), and Jost’s D value between 0.039 (PA-BR) and 0.188 (PA-TG) (Table 4). Both analyses showed presence of three genetic clusters within the sampled stretch (I-PA, II-BR/NG, and III-TG).
The Structure barplot of 59 mugger individuals from four nesting sites (Pali, Baroli, Nadigaon, and Tigri) in the National Chambal Sanctuary, India. Each bar indicates an individual, and the extent of color in the bar indicates the probability of assigning the individual to a particular cluster. The plot was prepared using Distruct v1.1 (https://rosenberglab.stanford.edu/distruct.html).
Discriminant Analysis of Principal Component (DAPC) scatterplot of 59 mugger individuals in the National Chambal Sanctuary, India. The scatter points represent individual observations, and each color represents a genetic cluster. The plot was prepared in RStudio 2023.03.1 (https://posit.co/download/rstudio-desktop/) using package adgenet (https://adegenet.r-forge.r-project.org/).
The estimated M ratio across 10 polymorphic loci analysed was 0.33 ± 0.08, and the Heterozygosity Excess Test (HET) performed under both mutational models yielded significant (P < 0.05) values for heterozygosity excess (Stepwise Mutation Model P = 0.04; and Two-Phase Mutation Model P = 0.04). Both the approaches i. e., M ratio and HET, detected a signature of genetic bottleneck. We also observed a shift in allele frequency distribution mode. However, the pooling of samples from different clusters may have affected the result in performing bottleneck detection.
Discussion
Conservation translocations have significantly averted the risk of pushing several threatened species to the brink of extinction5,29. Despite widespread acceptance and common use in achieving targeted conservation benefits, there are instances where such efforts fall short of achieving desired goals of establishing and/or restoring populations10. Guidelines incorporating adaptive conservation measures and information on key biological and ecological aspects of target species and monitoring have proven crucial for identifying conservation shortfalls and planning conservation strategies for effective solutions to increase the overall success of such programs5. The guidelines to monitor target species alone may not be adequate in situations, when the target species occur sympatrically and the presence of sympatric species could induce competition impeding the intended recovery of the target species26,30. Therefore, information on the status of sympatric species could provide valuable insights to guide program outcomes. In the present study, we assessed the population trend, occupancy and genetic diversity to understand the status of mugger in Chambal River.
Our findings revealed an increase in the population trend and high area of occupancy with moderate level of genetic diversity in the mugger population. This confirms colonization of NCS by mugger, though active management was discouraged. The successful mugger colonization is likely to induce competition and interspecific aggression, which may negatively impact the gharial survival imposing a threat to the long-term conservation effort in the Chambal River. Similar reports of increase in mugger in SGWS confirms that mugger now rapidly colonizing prime gharial habitats. Their increase might have been a key contributing factor to the escalated interspecific aggression between gharials and mugger, ultimately leading to the competitive dominance of the mugger23,28. This also rationalise the initial decision of debarring translocation of mugger in gharial-centric riverine protected areas31. As a result, this validates the need for extensive monitoring of the mugger along with gharial to develop a robust road map for the recovery of gharial22.
Population trend
Abundance indices provide a valuable tool to assess population trends e.g., increasing, declining, or stable over extended time periods, enabling insights into population dynamics. It is crucial for understanding the status and trajectory of a species, as well as for making informed conservation and management decisions. The population dynamics of recovered populations are often estimated using time-series data over a short period, which lacks the statistical ability to predict the linear population trend accurately and hence may be erroneous32. In this study, we used 17 year-long continuous time-series data to obtain reliable estimates of population trends in the mugger population in the NCS. Similar to other crocodilian species, we have also observed an age-structured size class composition within the mugger population33, where adult size class constituted the highest percentage (Supplementary Table 1). The population growth analysis showed an increasing trend in the mugger population. Although a ‘one-species one area’ based targeted conservation approach was adopted to restrict interspecific competition, 28 muggers were released in the Sanctuary in 198421. The primary focus of the translocation program was to re-establish a self-sustaining gharial population. However, the mugger population witnessed a significant increase, surging from 19 individuals in 1984 to 674 individuals in 2019 (Supplementary Table 1). The increase in the mugger population can be attributed to the protected nature of the Chambal River and its ecological generalist traits, i.e. broader dietary choice, habitat versatility, and resilience towards environmental fluctuations. In addition to the NCS in the Chambal River, mugger population in other riverine protected areas, such as Katerniaghat Wildlife Sanctuary in the Girwa River, Satkosia Gorge Wildlife Sanctuary in the Mahanadi River and the Corbett National Park in the Ramganga River has shown a considerable increase in numbers and breeding activity in the past31,34,35. Population dynamic studies on various crocodylians have revealed differential trends, with some species exhibiting increasing trends while others have shown a decline in population over the study period36,37. These trends are often linked to the conservation efforts invested to safeguard the species and the level of threats. Additionally, the timeframe and spatial scale of the study can also impact the observed trends. Therefore, comprehensive and long-term monitoring efforts coupled with effective conservation strategies are crucial for ensuring crocodilian populations' long-term viability and sustainability.
Single-season site occupancy
The estimated mugger occupancy across the study area indicates that the species is faring well in the Chambal River. In the last couple of decades, they have grown in numbers, as shown by the population trend, and so has their spread, as evidenced by the naïve occupancy (0.88 ± 0.04). The National Chambal Sanctuary within the Chambal River is the longest riverine protected area in India, providing considerable space and suitable protective measures for mugger populations to grow in numbers and expand. The generalist species with high dispersal capacities and broader dietary choices as observed in mugger are less constrained by habitat are expected to colonize sub-optimal habitats38.
We observed a strong influence of river width on detection probability (p) wherein the detectability of the mugger decreased with an increase in river width. This may be attributed to the boat surveys being conducted along the mid-river channel, and in the broader channel, the distance between the observer and the river bank increases, hindering the observer's ability to detect mugger individuals basking on the river bank. Similarly, river depth strongly influenced mugger occupancy, which increased till the mean river depth (4.7 m) and saturated after that. The preferred depth of the sympatric species, gharial, in the same riverscape was ≥ 4 m17. The river depth preference is possibly related to food availability, movement, and cover from predators. Previous studies have indicated that mugger prefer basking on open river banks with a rocky substrate39. Also, their presence in human-dominated riverscapes and water bodies in urban landscapes suggests their remarkable adaptability40. Considering its preference and adaptability, we anticipated a positive association with rocky substrate and minimal influence of disturbances on mugger occupancy. However, none of the candidate models (ΔAIC ≤ 2) included rocky substrate as the best predictor variable. Instead, the best-predicted model indicated that sandy substrate was among the associated covariates. This highlights that sandy substrate may be more influential in determining mugger occupancy than previously anticipated. The positive association with sandy substrate type might be because the crocodile prefers an open surface for basking. Mugger may use rocky substrate in the absence of other substrate types, such as sand and clay. This also indicates that species preference for substrate might as well rely on relative availability and suitability when alternative substrate options are limited or absent. The absence of any significant influence of anthropogenic factors on mugger occupancy aligns with the species' adaptability in riverscape with high anthropogenic disturbances. This could be attributed to the generalist nature of the species, where they do not exhibit strict habitat preferences. Unsurprisingly, disturbances resulting from human activities have minimal impact on their occupancy. This further supports the notion that the mugger is highly adaptable and can persist in various environments, including those influenced by high human presence. Unlike this, the saltwater crocodile and American alligator (Alligator mississippiensis) were shown to occupy areas with low human interference36,41.
Genetic assessment
The overall genetic diversity for the species in the Chambal River showed moderate values (Table 3). The level of genetic diversity in the mugger population is higher than the gharial population when estimated using a similar set of microsatellite loci16. This could be attributed to species life-history traits and widely distributed species retaining high genetic diversity42,43,44. In the absence of studies on the movement of the muggers in Chambal and its tributaries, the possibility of the dispersion of muggers from the tributaries of Chambal contributing to the increasing population and gene-flow to maintain the genetic diversity cannot be ruled out.
The population structure suggests the presence of three genetic units among the four nesting sites (Figs. 2 and 3). The identified genetic units followed a pattern where individuals from nesting sites situated at a short distance (~ 10 km; BR-NG) from each other were assigned to a single cluster and did not show a significant level of genetic differentiation, whereas individuals from sites situated at longer distances (> 100 km) were assigned to separate clusters and showed significant genetic differentiation.
The limited long-distance movement probably explains the observed differentiation pattern observed in released muggers21 and might be due to the species life-history traits, such as philopatry, observed in crocodiles45,46. As an ecological generalist, the mugger can efficiently utilize resources in a given habitat without dispersing across longer distances in search of optimal basking, nesting, and foraging habitats. Hence, the presence of multiple genetic clusters within Chambal River sites may have been due to the limited movement of the individuals across sites. Since muggers are adapted to move on land with agility, the possibility of obstructing movement due to natural and artificial barriers is very low. The current mugger population in the Chambal River was recovered from an initial population of 33 individuals of all size classes in 1984–198518,21. The M ratio and HET in the mugger population confirmed the genetic bottleneck. Since, the crocodile population across India suffered a severe decline in its range during the late-1960s our result corroborates with the past demographic decline in the mugger population.
The utilization of cross-species microsatellite markers is likely to introduce ascertainment bias. Hence, the results should be interpreted with discretion. Further, it is required to increase the number of samples and utilize High Throughput Sequencing techniques, such as Single Nucleotide Polymorphisms (SNPs), to substantiate the findings of the study and gain insights into the fine-scale population genetic structure, timing of occurrence, and magnitude of the bottleneck experienced by the mugger population.
Conclusions
There has been a pressing need for a scientifically supported evaluation of the conservation efforts for gharials. In the past, no attempts were made to gather comprehensive information on all facets recommended under monitoring guidelines that are essential for the evaluation of the ongoing conservation translocation program. The combination of increasing population trends, widespread distribution, and a moderate level of genetic diversity hint toward the successful colonisation of mugger in the Chambal River and pose serious threat to the recovery of gharial. The rapid colonization of mugger also indicates that mugger is benefitting from gharial specific conservation measures such as habitat protection, nest protection implemented in the National Chambal Sanctuary. In the future, continuous monitoring and follow-up assessments of key facets, including population dynamics and genetic status of the gharial as well as the sympatric mugger, is essential for developing adaptive management strategies to minimize potential conflict from interspecific competition and secure the gharial population in Chambal and other rivers. For safeguarding the gharial population in Chambal River, the strategies should aim at developing mechanisms to control mugger population growth in prime gharial habitats. For instance, by translocating breeding individuals and/or nest to a suitable habitat conducive for mugger. Further, studies to fill knowledge gaps are important to prepare adaptive management plans in the absence of empirical evidence on ecological aspects of the mugger, such as size-class-specific survivability, nest count, and interspecific interactions between gharial and mugger. Further, the increasing mugger population in Chambal and other North Indian rivers needs to be studied to understand the dynamics of interspecies competition and develop differential management strategies where muggeroccur sympatrically with the gharial.
Methodology
Study area, permits, and ethical considerations
The Chambal River originates in the Vindhyan Range in Mhow, Indore, Madhya Pradesh, and traverses a course of around 965 km up to its confluence with the Yamuna River in Uttar Pradesh, India47. Since 1979, ~ 600 km stretches of the Chambal River between Kota barrage in Rajasthan and Chambal-Yamuna confluence in Uttar Pradesh, and ~ 60 km stretches of the Parbati River in Madhya Pradesh (South bank) between Badodiya Bindi and Pali have been protected as National Chambal Sanctuary for the conservation and management of aquatic fauna (Fig. 1).
The Forest Department of Madhya Pradesh (Letter No. 8200 and 2545), Rajasthan (Letter No. 1399), and Uttar Pradesh (Letter No. 3093) provided the necessary permissions for the surveys and collection of the biological samples. No humans were involved during the experiments, and animal ethical clearance was not required as all biological samples were collected from dead hatchlings or hatched eggshells and no animal was captured or handled for sample collection.
Data collection
Population monitoring
The river stretch from Pali (confluence of Chambal-Parvati) to Panchnada (the endpoint of National Chambal Sanctuary) was surveyed annually during the winter months (January–February) of 2017–2019 (Supplementary Fig. 6). We used the total count method to estimate the RDI of mugger i.e. individuals sighted per km of river stretch48. The survey was conducted during daylight between 1000 and 1600 h. A motorboat fitted with a 25HP outboard engine was used to survey the river, and the speed of the boat was maintained between 8 and 10 km/hr. Two observers equipped with 8 × 40 mm binoculars were positioned to spot the basking muggers15. The mugger was identified based on their morphology using established description keys following the identification guide49. When spotted, the number of mugger individuals, visually estimated size of the mugger and associated habitat variables were recorded for each sighting. The observed mugger crocodiles were categorized into size classes following Khadka et al.50: Hatchlings (< 30 cm), Yearlings (30 < 50 cm), Juveniles (50 < 125 cm), Sub-adults (125 < 180 cm), and Adults (> 180 cm).
Single-season site occupancy
We used spatial replicates in a linear system to determine the drivers of mugger distribution in the Chambal River using single-season site occupancy modeling51. We surveyed ~ 400 km in a total of 134 composite blocks, each composite block consisting of three one km spatially replicated blocks. Only survey data for the year 2019 was used for site occupancy analysis. The presence-absence of the mugger was recorded in each block along with associated detection and site covariates (Supplementary Table 6). The covariates were selected based on a priori understanding of crocodylian ecology and literature39,52, and assumed to be essential in determining their occupancy and detection probability. A detection history matrix was built using the sightings as presence locations to model the occupancy of the mugger. The detection covariates are thought to potentially influence the probability of detecting a species given the presence in a site (p), and site covariates influence the probability of occupancy (Ψ).
Genetic assessment
Biological samples for genetic assessment were collected from ~ 400 km stretch of the Chambal River during 2017 and 2018 (Fig. 1). The biological samples include tissue obtained from dead remains of hatchlings and chorioallantoic membrane from hatched eggshells. We collected 122 biological samples, including tissue (n = 22) and eggshells containing chorioallantoic membrane (n = 100), from five nesting sites (Pali, Baroli, Nadigaon, Dangbasai, and Tigri) (Supplementary Table 3). The samples were collected opportunistically during post-nesting surveys. The eggshells were kept in a sterile container and air-dried at room temperature, and tissue samples were stored in absolute ethanol at room temperature and later at -20 °C in the laboratory for long-term storage. A significant portion of the samples collected for genetic assessment were dry eggshells and tissue from dead hatchlings; hence clutch information on these samples is not available. Total genomic DNA was extracted from tissue (80–100 mg) and chorioallantoic membrane (cotton swab) following the phenol–chloroform method53. DNeasy blood and tissue kit (Qiagen Inc. USA) were used for samples that failed to recover DNA using phenol–chloroform method.
We used a panel of 10 microsatellite loci previously developed in other crocodile species54,55,56,57,58. The loci were selected based on a screening of 36 microsatellite loci developed across 10 species (Supplementary Table 7). Polymerase chain reactions (PCR) were performed following the universal primer-multiplex method59,60. The amplified PCR products were subjected to fragment analysis in Applied Biosystems Genetic Analyser, ABI 3500xl as per the manufacturer's protocol with the GeneScan 500 LIZ as a size standard. The alleles were scored using the automated allele scoring feature of GeneMarker v2.7.4 (SoftGenetics, LLC, State College, PA, United States), and finally validated through visual inspection. To produce reliable multilocus genotypes from noninvasive samples, a multiple-tube method was used with three replicates of each sample61.
Data analyses
Population trend
The population trend was estimated using contiguous RDI data from 2003 to 201631 and from 2017 to 2019. Altogether we have used time-series data over 17 years to obtain population trend estimates in the mugger population in the Chambal River (Supplementary Fig. 1). The population trends were calculated using the exponential rate of increase (r), the annual finite population growth rate (λ), and the percentage change in population over time48,62. We estimated the exponential rate of increase (r) as the slope of the linear regression of RDI transformed to natural logarithms (loge) and years62,63. The annual finite population growth rate (λ) was estimated by back transformation (λ = er, where e is the base of natural logarithms, ~ 2.71828, and r is the exponential rate of increase). The percentage change was calculated here from mean λ; if λ = 1.15, the population increases at 15% per year.
Single-season site occupancy
We used the site occupancy modeling to derive the occupancy of mugger and factors influencing their occupancy in the Chambal River. The analysis was performed using unmarked package64 implemented in R v4.1.265. Each pair of covariates were tested for spatial correlation using Spearman’s correlation, continuous variables were standardised and categorical variables were provided as a factor for the analysis.
We opted for a two-step approach to model the probability of detection (p) and occupancy (Ψ) as a function of detection and site covariates, respectively66. Initially, we built univariate detection (p) model, holding the site covariates constant. The detection models were then ranked based on AIC, and the model corresponding to the lowest AIC was selected. Finally, we used an additive combination of site covariates with selected detection covariates from the previous step to produce the final set of models. Again, these models were ranked according to AIC and models below Δ2 AIC were considered important for predicting the mugger occupancy. We performed the MacKenzie and Bailey goodness of fit test for single-season occupancy models based on the best models to assess the goodness of fit and over dispersion67,68. The relative contribution of each variable was assessed using summed Akaike weights69. The covariates were considered significant when the estimated beta coefficient at 95% confidence interval did not overlap with zero. We predicted the detection and occupancy probabilities were estimated for each site via model averaging of the final model set using MuMin package70 in R v4.1.265.
Genetic assessment
The genotype of each individual was obtained through the consensus of three replicates, and the quality index was estimated for each locus genotyped following Miquel et al.71. The average amplification success was calculated as a percentage of positive PCR amplification against the total number of PCR. The genotyping errors rate per locus was estimated manually following Pompanon et al.61, null alleles frequency was estimated using FreeNa72 and large allele dropout using Micro-Checker v2.2.373.
PID (unbiased) and PID (sibs) was calculated using Gimlet v1.3.374 and deviations from Hardy–Weinberg equilibrium (HWE) for each locus using GenAlEx v6.5175. The genetic diversity estimates (the number of alleles per locus, observed heterozygosity, and expected heterozygosity) were estimated using Arlequin v3.076, and allelic richness was estimated using a rarefaction approach implemented in HP-Rare v1.1 to account for the uneven sample size across nesting sites77.
The genetic differentiation indices FST78 and Jost’s D79 were estimated in R studio 2023.03.165 using the strataG package80 with 103 bootstrap iterations. The population genetic structure was inferred using two approaches: (a) Systematic model-based Bayesian clustering approach that uses allele frequencies at each locus to infer the population structure implemented in Structure v2.3.481. The analysis was performed for 1 to 10 clusters (K). For each K, 10 iterations were run under the admixture with correlated allele model. The simulations were run for 105 burn-in and 106 Markov chain Monte Carlo iterations (MCMC). The optimum number of K was inferred using delta K82, which was estimated using the web version of Structure Harvester, v0.6.9483. The assignment plot was prepared using the program Distruct v1.184. (b) Discriminate Analysis of Principal Component (DAPC), a multivariate non-model-based approach to identify and describe genetic clusters85,86. The analysis was performed using the adgenet in R studio 2023.03.165. The optimal number of clusters in DAPC was estimated based on the lowest associated BIC.
We examined the evidence of a genetic bottleneck using two different approaches. First, the heterozygosity excess test approach was performed in Bottleneck v1.2.0287. A one-tailed Wilcoxon test was used to determine the presence of a significant number of loci with excess heterozygosity to confirm the presence of a bottleneck. The estimates were calculated under two mutation models: Single-step Mutation Model (SMM) and Two-phase Mutation Model (TPM). TPM tends to be the most appropriate mutation model for microsatellite loci88. TPM was carried out at 95% SMM (variance at 12), and the simulations were run for 104 iterations87. Second, we used the Garza-Williamson index (or M ratio) implemented in Arlequin v3.176. The M ratio estimates the ratio of the observed number of alleles to the size of the allele range based on the assumption that the ratio is expected to decrease due to the random loss of alleles in a recently reduced population. The calculated M ratio was then compared with the critical value (MC = 0.68). An M ratio below MC is considered a genetic bottleneck signature89. Due to fewer samples than recommended to obtain a reliable result for evidence of genetic bottleneck in two of three identified genetic clusters, the tests were performed by pooling samples together.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Ceballos, G., Ehrlich, P. R. & Raven, P. H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. USA 117(24), 13596–13602. https://doi.org/10.1073/pnas.1922686117 (2020).
Díaz, S., Fargione, J., Stuart Chapin, F. & Tilman, D. Biodiversity loss threatens human well-being. PLoS Biol. https://doi.org/10.1371/journal.pbio.0040277 (2006).
Spielman, D., Brook, B. W. & Frankham, R. Most species are not driven to extinction before genetic factors impact them. Proc. Natl. Acad. Sci. 101, 15261–15264 (2004).
Dodd, K. C. & Seigel, R. A. Relocation, repatriation, and translocation of amphibians and reptiles: Are they conservation strategies that work?. Herpetologica 47(3), 336–350. https://doi.org/10.1016/0006-3207(92)91063-x (1991).
IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations IUCN. Version 1.0. Gland, viiii + 57, (IUCN Species Survival Commission, 2013).
Wolf, M. C., Griffith, B., Reed, C. & Temple, S. A. Avian and mammalian translocations: Update and reanalysis of 1987 survey data. Conserv. Biol. 10(4), 1142–1154. https://doi.org/10.1046/j.1523-1739.1996.10041142.x (1996).
Griffith, B., Scott, J. M., Carpenter, J. W. & Reed, C. Translocation as a species conservation tool: Status and strategy. Science 245, 477–480. https://doi.org/10.1126/science.245.4917.477 (1989).
Seddon, P. J., Armstrong, D. P. & Maloney, R. F. Developing the science of reintroduction biology. Conserv. Biol. 21(2), 303–312 (2007).
Alves, J. M. et al. A single introduction of wild rabbits triggered the biological invasion of Australia. Proc. Natl. Acad. Sci. 119(35), e2122734119 (2022).
Berger-Tal, O., Blumstein, T. D. & Swaisgood, R. R. Conservation translocations: A review of common difficulties and promising directions. Anim. Conserv. 23(2), 121–131. https://doi.org/10.1111/acv.12534 (2020).
Seddon, P. J., Griffiths, C. J., Soorae, P. S. & Armstrong, D. J. Reversing defaunation: Restoring species in a changing world. Science 345(6195), 406–412 (2014).
Price, M. R. S. Animal Reintroductions: The Arabian Oryx in Oman (Cambridge University Press, 1989).
Walters, J. R. et al. Status of the california Condor (Gymnogyps californianus) and efforts to achieve its recovery. Auk 127(4), 969–1001. https://doi.org/10.1525/auk.2010.127.4.969 (2010).
Frith, C. The Woodhen: A Flightless Island Bird Defying Extinction (CSIRO Publishing, 2013).
Hussain, S. A. Reproductive success, hatchling survival and rate of increase of gharial Gavialis gangeticus in National Chambal Sanctuary, India. Biol. Conserv. 87(2), 261–268 (1999).
Sharma, S. P. et al. Microsatellite analysis reveals low genetic diversity in managed populations of the critically endangered gharial (Gavialis gangeticus) in India. Sci. Rep. 11(1), 1–10. https://doi.org/10.1038/s41598-021-85201-w (2021).
Hussain, S. A. Basking site and water depth selection by gharial Gavialis gangeticus Gmelin 1789 (Crocodylia, Reptilia) in National Chambal Sanctuary, India and its implication for river conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 19, 127–133 (2009).
Choudhury, B. C. & Chowdhury, S. Lessons from crocodile reintroduction projects in India. Indian For. 112(10), 881–890 (1986).
de Vos, A. Crocodile conservation in India. Biol. Conserv. 29(2), 183–189 (1984).
Sharma, S. P. et al. Mitochondrial DNA analysis reveals extremely low genetic diversity in a managed population of the Critically Endangered Gharial (Gavialis gangeticus, Gmelin 1789). Herpetol. Journal 30, 202–206 (2020).
Singh, L. A. K. Gharial Population Trend in National Chambal Sanctuary With Notes on Radio Tracking. (1985).
Rao, R. J. & Choudhury, B. C. Sympatric distribution of gharial Gavialis gangeticus and mugger Crocodylus palustris in India. J. Bombay Nat. Hist. Soc. 89, 312–315 (1992).
Singh, L. A. K. Status of gharial and mugger in Orissa. ENVIS (Wildl. Protect. Areas) 2(1), 17–23 (1999).
Lang, J. W., Jailabdeen, A. & Kumar, P. Gharial ecology project—update 2018–2019. IUCN-SSC Crocodile Specialist Group Newsl. 37(4), 15–17 (2018).
Maharana, S. & Mohapatra, R. K. Interspecific conflict between gharial (Gavialis gangeticus) and mugger (Crocodylus palustris) in the Mahanadi River, Odisha, India. Crocodile Specialist Group Newsl. 40(2), 7–8 (2021).
Büchi, L. & Vuilleumier, S. Coexistence of specialist and generalist species is shaped by dispersal and environmental factors. Am. Nat. 183(5), 612–624 (2014).
Nie, Y., Zhou, W., Gao, K., Swaisgood, R. R. & Wei, F. Seasonal competition between sympatric species for a key resource: Implications for conservation management. Biol. Conserv. 234(March), 1–6. https://doi.org/10.1016/j.biocon.2019.03.013 (2019).
Da Silva, A. & Lenin, J. Mugger crocodile Crocodylus palustris. Crocodiles. Status survey and conservation action plan, vol. 3 (2010).
Weeks, A. R. et al. Assessing the benefits and risks of translocations in changing environments: A genetic perspective. Evol. Appl. 4(6), 709–725 (2011).
Clavel, J., Julliard, R. & Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization?. Front. Ecol. Environ. 9(4), 222–228 (2011).
Sharma, R. K. & Singh, L. A. K. Status of Mugger crocodile (Crocodylus palustris) in National Chambal Sanctuary after thirty years and its implications on conservation of Gharial (Gavialis gangeticus). Zoo’s Print 30(5), 9–16 (2015).
White, E. R. Minimum time required to detect population trends: The need for long-term monitoring programs. BioScience 69(1), 26–39. https://doi.org/10.1093/biosci/biy144 (2019).
Briggs-Gonzalez, V. et al. Life histories and conservation of long-lived reptiles, an illustration with the American crocodile (Crocodylus acutus). J. Anim. Ecol. 86(5), 1102–1113 (2017).
Choudhary, S., Choudhury, B. C. & Gopi, G. V. Spatio-temporal partitioning between two sympatric crocodilians (Gavialis gangeticus & Crocodylus palustris) in Katarniaghat Wildlife Sanctuary, India. Aquat. Conserv. Mar. Freshw. Ecosyst. 28(5), 1067–1076. https://doi.org/10.1002/aqc.2911 (2018).
Jacobson, C. Reintroduction of the Mugger crocodile, Crocodylus palustris, in India. Restor. Reclam. Rev. 4(3), 1–7 (1999).
Than, K. Z., Strine, C. T., Sritongchuay, T., Zaw, Z. & Hughes, A. C. Estimating population status and site occupancy of saltwater crocodiles Crocodylus porosus in the Ayeyarwady delta, Myanmar: Inferences from spatial modeling techniques. Glob. Ecol. Conserv. 24, e01206 (2020).
Wallace, K. M., Leslie, A. J., Coulson, T. & Wallace, A. S. Population size and structure of the Nile crocodile Crocodylus niloticus in the lower Zambezi valley. Oryx 47(3), 457–465 (2013).
Van Looy, K. et al. Are generalist and specialist species influenced differently by anthropogenic stressors and physical environment of riparian corridors?. Riparian Ecol. Conserv. 1(2013), 25–35 (2013).
Venugopal, D. P. & Prasad, D. K. V. Basking behavior and survey of marsh crocodiles Crocodylus palustris in Ranganthittu Bird Sanctuary, Karnataka, India. Hamadryad 27(2), 241–247 (2003).
Vyas, R. Results of the 2015 mugger crocodile (Crocodylus palustris) count at Vadodara, Gujarat, India. Reptiles Amphib. 25(1), 20–25 (2018).
Gardner, B., Garner, L. A., Cobb, D. T. & Moorman, C. E. Factors affecting occupancy and abundance of American alligators at the northern extent of their range. J. Herpetol. 50(4), 541–547 (2016).
Brüniche-olsen, A., Kellner, K. F., Belant, J. L., Dewoody, J. A. & Brüniche-olsen, A. Life-history traits and habitat availability shape genomic diversity in birds: Implications for conservation. Proc. R. Soc. B. 288, 20211441. https://doi.org/10.1098/rspb.2021.1441 (2021).
Ellegren, H. & Galtier, N. Determinants of genetic diversity. Nat. Rev. Genet. 17(7), 422–433. https://doi.org/10.1038/nrg.2016.58 (2016).
Jimmy, G. & Lafontaine, P. Life history traits and dispersal shape neutral genetic diversity in metapopulations. J. Math. Biol. 84(6), 45 (2022).
van Asch, B. et al. Phylogeography, genetic diversity, and population structure of Nile crocodile populations at the fringes of the southern African distribution. PLoS ONE 14(12), 1–20. https://doi.org/10.1371/journal.pone.0226505 (2019).
Cunningham, S. W., Shirley, M. H. & Hekkala, E. R. Fine scale patterns of genetic partitioning in the rediscovered African crocodile, Crocodylus suchus (Saint-Hilaire 1807). Peer J https://doi.org/10.7717/peerj.1901 (2016).
Nath, M. L. The Upper Chambal Basin: A Geographical Study in Rural Settlements (Northern Book Centre, 1989).
Bayliss, P. Survey method and monitoring within crocodile management programmes. In Wildlife Management; Crocodiles and Alligators 1st edn 157–175 (Surrey Beatty & Sons Pty Ltd, 1987).
Brazaitis, P. The identification of living crocodilians. Zoologica 58, 59–101 (1973).
Khadka, B. B., Maharjan, A., Thapalia, B. P. & Lamichhane, B. R. Population status of the mugger in Chitwan National Park, Nepal. Crocodile Specialist Group Newsl. Karama (Northern Territory, Australia) 33(3), 9–12 (2014).
Charbonnel, A. et al. Spatial replicates as an alternative to temporal replicates for occupancy modelling when surveys are based on linear features of the landscape. J. Appl. Ecol. 51(5), 1425–1433 (2014).
Grigg, G. & Kirshner, D. Biology and evolution of crocodylians. CSIRO Publishing https://doi.org/10.1071/9781486300679 (2015).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual 2nd edn. (Cold Spring Harbor Laboratory Press, 1989).
Aggarwal, R. K., Lalremruata, A. & Dubey, B. Development of fourteen novel microsatellite markers of Crocodylus palustris, the Indian mugger, and their cross-species transferability in ten other crocodilians. Conserv. Genet. Resour. 7, 197–200 (2014).
Fitzsimmons, N. N. Single paternity of clutches and sperm storage in the promiscuous green turtle (Chelonia mydas). Mol. Ecol. 7(5), 575–584 (1998).
Hinlo, M. R. P. et al. Population genetics implications for the conservation of the Philippine Crocodile Crocodylus mindorensis Schmidt, 1935 (Crocodylia: Crocodylidae). J. Threat. Taxa 6, 5513–5533 (2014).
Jogayya, K. N., Meganathan, P. R., Dubey, B. & Haque, I. Novel microsatellite DNA markers for Indian Gharial (Gavialis gangeticus). Conserv. Genet. Resour. 5, 787–790. https://doi.org/10.1007/s12686-013-9908-6 (2013).
Miles, L. G., Isberg, S. R., Moran, C., Hagen, C. & Glenn, T. C. 253 Novel polymorphic microsatellites for the saltwater crocodile (Crocodylus porosus). Conserv. Genet. 10(4), 963–980 (2009).
Ge, C., Cui, Y. N., Jing, P. Y. & Hong, X. Y. An alternative suite of universal primers for genotyping in multiplex PCR. PLoS ONE 9(3), 1–8. https://doi.org/10.1371/journal.pone.0092826 (2014).
Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 18(2), 233–234. https://doi.org/10.1038/72708 (2000).
Pompanon, F., Bonin, A., Bellemain, E. & Taberlet, P. Genotyping errors: Causes, consequences and solutions. Nat. Rev. Genet. 6(11), 847–859. https://doi.org/10.1038/nrg1707 (2005).
Caughley, G. Analysis of Vertebrate Populations (Wiley, 1977).
Dinsmore, S. J. & Johnson, D. H. Population analysis in wildlife biology. Techniques for wildlife investigations and management, 154–184 (2005).
Fiske, I. J. & Chandler, R. B. Unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 43(10), 1–23. https://doi.org/10.18637/jss.v043.i10 (2011).
R. C. Team. R: A Language and Environment for Statistical Computing (Foundation for Statistical Computing, 2021).
Kroll, A. J. et al. Factors influencing stream occupancy and detection probability parameters of stream-associated amphibians in commercial forests of Oregon and Washington, USA. For. Ecol. Manag. 255(11), 3726–3735. https://doi.org/10.1016/j.foreco.2008.03.005 (2008).
MacKenzie, D. I. et al. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence (Elsevier Inc, 2006).
Sillett, T. S., Chandler, R. B., Royle, J. A., Kéry, M. & Morrison, S. A. Hierarchical distance-sampling models to estimate population size and habitat-specific abundance of an island endemic. Ecol. Appl. 22(7), 1997–2006 (2012).
Burnham, K. P. & Anderson, D. R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33(2), 261–304 (2004).
Barton, K. & Barton, M. K. Package ‘mumin’. Version 1(18), 439 (2015).
Miquel, C. et al. Quality indexes to assess the reliability of genotypes in studies using noninvasive sampling and multiple-tube approach. Mol. Ecol. Notes 6, 985–988 (2006).
Chapuis, M. P. & Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 24, 621–631 (2007).
Oosterhout, C. V., Hutchinson, W. F., Wills, D. P. M. & Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 4, 535–538 (2004).
Valière, N. GIMLET: A computer program for analysing genetic individual identification data. Mol. Ecol. Notes 2, 377–379 (2002).
Peakall, R. & Smouse, P. E. GenALEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28, 2537–2539 (2012).
Excoffier, L., Laval, G. & Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 1, 117693430500100 (2005).
Kalinowski, S. T. HP-RARE 1.0—A computer program for performing rarefaction on measures of allelic richness. Mol. Ecol. Notes 5, 187–189 (2005).
Weir, B. S. & Cockerham, C. C. Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (1984).
Jost, L. GST and its relatives do not measure differentiation. Mol. Ecol. 17, 4015–4026 (2008).
Archer, F. I., Adams, P. E. & Schneiders, B. B. strataG: An r package for manipulating, summarizing and analysing population genetic data. Mol. Ecol. Resour. 17, 5–11 (2017).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Genetics 155, 945–959 (2000).
Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 14, 2611–2620 (2005).
Earl, D. A. & VonHoldt, B. M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361 (2012).
Rosenberg, N. A. DISTRUCT: A program for the graphical display of population structure. Mol. Ecol. Notes 4, 137–138. https://doi.org/10.1046/j.1471-8286.2003.00566.x (2004).
Jombart, T., Devillard, S. & Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 11, 94 (2010).
Jombart, T., Devillard, S., Dufour, A. B. & Pontier, D. Revealing cryptic spatial patterns in genetic variability by a new multivariate method. Heredity 101, 92–103 (2008).
Piry, S., Luikart, G. & Cornuet, J. M. BOTTLENECK: A computer program for detecting recent reductions in the effective population size using allele frequency data. J. Hered. 90, 502–503 (1999).
Rienzo, A. D. et al. Mutational processes of simple-sequence repeat loci in human populations. Proc. Natl. Acad. Sci. USA 91, 3166–3170 (1994).
Garza, J. C. & Williamson, E. G. Detection of reduction in population size using data from microsatellite loci. Mol. Ecol. 10, 305–318 (2001).
Acknowledgements
This study was carried out with funding support from grant-in-aid fund of the Wildlife Institute of India (WII), Dehra Dun and through the projects "Biodiversity conservation and Ganga rejuvenation" and "Planning & Management for Aquatic Species Conservation and Maintenance of Ecosystem Services in the Ganga River Basin" funded by the National Mission for Clean Ganga (NMCG), Ministry of Jal Shakti, Government of India. We acknowledge the help and support provided by the Director and Dean, WII and Mr. G. Asok Kumar, Director General (DG), NMCG, Mr. Rajiv Ranjan Mishra, Former DG, and their team in executing this study. We would like to thank the Chief Wildlife Wardens of Government of Madhya Pradesh, Rajasthan and Uttar Pradesh for providing and facilitating timely research permits. We especially thank Dr. A. A. Ansari, Divisional Forest Officer for facilitating this study. We fondly remember the help and support provided to us by and Mr. J. P. Dandotiya at National Chambal Sanctuary and Mr. A. Madhanraj and Mr. Goura Chandra Das at WII for their help during the laboratory work and data analysis.
Funding
This study was funded by the grant-in-aid funds of the Wildlife Institute of India (WII), Dehra Dun, and the National Mission for Clean Ganga (NMCG), Ministry of Jal Shakti, Government of India sponsored project Biodiversity Conservation and Ganga Rejuvenation (No. B-02/2015-16/1259/NMCG-WII-PROPOSAL and B-03/2015-16/1077/NMCG–NEW PROPASAL). S.P.S was awarded the Department of Science and Technology-INSPIRE Research Fellowship (IF160549). The funding agency has no role in the designing study, collection, analysis, interpretation of data and writing the manuscript.
Author information
Authors and Affiliations
Contributions
S.A.H and R.B. conceived the study, raised funds, supervised the study and did review and editing of the manuscript. S.P.S designed the methodology, collected biological samples, conducted population surveys, performed the experiment, data curation, data analysis and wrote the original manuscript. M.G.G contributed data analysis and did review and editing of the manuscript. S.K conducted population surveys, collected biological samples and contributed in writing original manuscript. All authors agreed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharma, S.P., Ghazi, M.G., Katdare, S. et al. Population status and genetic assessment of mugger (Crocodylus palustris) in a tropical regulated river system in North India. Sci Rep 14, 7438 (2024). https://doi.org/10.1038/s41598-024-57983-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57983-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.