Abstract
Osteoporosis (OP) is a prevalent global disease characterized by bone mass loss and microstructural destruction, resulting in increased bone fragility and fracture susceptibility. Our study aims to investigate the potential of kaempferol in preventing and treating OP through a combination of network pharmacology and molecular experiments. Kaempferol and OP-related targets were retrieved from the public database. A protein–protein interaction (PPI) network of common targets was constructed using the STRING database and visualized with Cytoscape 3.9.1 software. Enrichment analyses for GO and KEGG of potential therapeutic targets were conducted using the Hiplot platform. Molecular docking was performed using Molecular operating environment (MOE) software, and cell experiments were conducted to validate the mechanism of kaempferol in treating OP. Network pharmacology analysis identified 54 overlapping targets between kaempferol and OP, with 10 core targets identified. The primarily enriched pathways included atherosclerosis-related signaling pathways, the AGE/RAGE signaling pathway, and the TNF signaling pathway. Molecular docking results indicated stable binding of kaempferol and two target proteins, AKT1 and MMP9. In vitro cell experiments demonstrated significant upregulation of AKT1 expression in MC3T3-E1 cells (p < 0.001) with kaempferol treatment, along with downregulation of MMP9 expression (p < 0.05) compared to the control group. This study predicted the core targets and pathways of kaempferol in OP treatment using network pharmacology, and validated these findings through in vitro experiments, suggesting a promising avenue for future clinical treatment of OP.
Similar content being viewed by others
Introduction
Osteoporosis (OP) is a systemic bone disease characterized by reduced bone mass and degeneration of bone microstructure, leading to increased bone fragility and the risk of fractures1. OP is a global health problem, especially among the elderly. According to statistics, its prevalence worldwide signifies a pervasive threat to the health of older individuals. This not only results in severe pain and extended recovery periods but also hampers mobility, diminishes quality of life, and elevates the risk of mortality2.According to epidemiological data released by the National Health Commission of China in 2018, the prevalence of OP among individuals aged over 50 in China was 19.2%, comprising 6.0% of males, 32.1% of females, 16.2% in urban areas, and 20.7% in rural areas. As China’s population continues to age, the projected estimate of affected individuals is anticipated to surge to 212 million by 2050, emphasizing OP as a substantial public health concern3. OP arises from an imbalance between bone resorption and formation, relying on the coordination of osteoclasts and osteoblasts4. In OP patients, bone resorption outpaces formation, resulting in decreased bone mass and strength5. Various molecular signaling pathways contribute to OP development, including RANK/RANKL/OPG, Wnt/β- catenin, and estrogen signaling pathway, crucial in regulating osteoclasts and osteoblasts activity. RANKL (osteoprotegerin ligand) activates the formation and activation of osteoclasts by binding to its receptor, thus promoting bone absorption. OPG (osteoprotegerin) is a natural antagonist of RANKL, which can bind to RANKL and prevent it from binding with RANKL, thus inhibiting bone resorption6. Activating the Wnt/β- catenin pathway can promote the proliferation and differentiation of osteoblasts and increase bone formation7,8. Estrogen can inhibit bone resorption by reducing the formation of osteoclasts and prolonging their life span, and can also promote the activity of osteoblasts9. Notably, strategies for OP treatment focus on slowing bone resorption or promoting bone formation, employing options like anti-bone resorption medications (bisphosphonates, SERMs, and PTH analogs) and bone formation promoters (abaloparatide, denosumab, and romosozumab)10,11,12. However, these treatments face challenges due to potential side effects and the limitation of targeting a single molecular target4,13,14,15, necessitating comprehensive approaches to address OP’s intricate pathophysiology. Moreover, a combination of suitable exercise, adequate calcium, and vitamin D remains a crucial strategy for OP prevention and treatment16. Nonetheless, for some elderly patients, these interventions alone may be insufficient17, and the drugs currently used in clinical practice lack specificity. Therefore, there is an urgent need to explore novel approaches to address this pressing issue.
OP is categorized in Traditional Chinese Medicine (TCM) as “bone impotence”, “bone paralysis”, and “kidney deficiency”, with its key pathogenesis attributed kidney deficiency. Currently, the use of kidney-tonifying Chinese medicine in OP treatment has become a research focus. Moreover, the active components of Chinese herbal medicine, including Kaempferol, a flavonoid found in several Chinese herbs, hold significant research value and application prospects in OP treatment. In vitro and in vivo experiments conducted by numerous researchers have demonstrated the anti-OP effects of kaempferol and kaempferol-containing plants. These studies reveal that kaempferol plays a pivotal role in treating OP by inhibiting adipogenesis, inflammation, oxidative stress, osteoclast autophagy, and osteoblast apoptosis, while simultaneously activating osteoblast autophagy18,19,20,21,22,23. Consequently, kaempferol holds excellent clinical application prospects and can serve as a complementary or alternative treatment for OP.
Although there have been reports on the use of kaempferol in the treatment of OP, the underlying mechanism of action has not been investigated using network pharmacology. In view of this, this study utilized network pharmacology analysis and molecular docking technology to systematically analyze the potential mechanism of kaempferol against OP, with further validation through in vitro cell experiments. This objective of this study is to provide insights into the mechanism of kaempferol in the treatment of OP.
Materials and methods
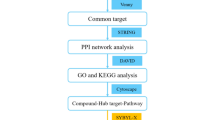
The basic framework of the study is depicted in Fig. 1.
Extraction of target genes
Kaempferol-related targets were predicted using the TCMSP (https://old.tcmsp-e.com/index.php)24 and SwissTargetPrediction (http://www.swisstargetprediction.ch/) databases25. Following screening, the targets were standardized by inputting them into the UniProt database (https: //www.uniprot.org)26,27 to obtain the official names of the targets.
Screening of OP-related targets
Using “osteoporosis” as the keyword and “Homo sapiens” as the species, OP-related targets were collected from the GeneCards (https://www.genecards.org/)28 and DisGeNET (https://www.disgenet.org/) databases29. The intersection of the two databases was then considered to determine the OP-related targets.
Acquisition of kaempferol-OP common targets and construction of protein–protein interaction (PPI) network
The intersection of kaempferol-related targets and OP-related targets was obtained using the R software (v.4.2.2) package “VennDiagram” (v.1.7.3) through Hiplot Pro (https://hiplot.com.cn/)30. The common targets obtained were then imported into the STRING database (https://stringdb.org/)31, and the PPI network was constructed with “Homo sapiens” as the species. Subsequently, the data were imported into Cytoscape 3.7.2 software for visual analysis, and cytoHubba plug-in was utilized to identify the core targets based on the top 10 rankings in maximal neighborhood component (MNC), node connect degree (Degree) and node connect closeness (Closeness).
Gene ontology (GO) functional annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
The GO and KEGG enrichment analysis of the common targets was conducted using the R software (v.4.2.2) package clusterProfiler (v.4.5.0) through Hiplot Pro (https://hiplot.com.cn/)30, a comprehensive web service for biomedical data analysis and visualization. The results were presented in the form of bubble diagrams, bar diagrams, and clustering dendrograms, with significance denoted by p < 0.05.
Molecular docking verification
Molecular docking, a crucial technique in network pharmacology analysis, involves combining known protein targets with small compounds to validate compound-target interactions. In this study, molecular docking analysis was performed using Molecular operating environment (MOE) software version 2019. The protein’s three-dimensional structure was retrieved from the Protein Data Bank (PDB) (http://www.rcsb.org/)32 and preprocessed by removing water molecules, preparing the protein structure, and minimizing the energy. The protein structure was then imported into MOE to construct a docking pocket. Small compound structures were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/)33 and subjected to molecular docking analysis with the docking pocket.
Experimental verification
Preparation of kaempferol solution
Kaempferol (purity = 99.86%) was purchased from MedChemExpress (MCE). The kaempferol dry powder was dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM and stored at -20℃ for subsequent use.
Cell culture
Pre-osteoblastic MC3T3-E1 cells were purchased from Procell Life Science&Technology Co., Ltd (Wuhan, China). The cells were cultured in α-modified Eagle’s medium (α-DMEM; Thermo, MA) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. Simultaneously, the cells were seeded into a cell culture plate and incubated at 37 °C in a humidified atmosphere with 5% CO2.
The effect of kaempferol on cell viability
Cell viability was assessed using cell counting kit-8 (CCK-8) assays following the manufacturer’s instructions. Initially, MC3T3-E1 cells were seeded in 6-well plates at a density of 5 × 103 cells/well and cultured for 24 h at 37 °C with 5% CO2. Subsequently, various concentrations (2.5, 5, 7.5, 10, 12.5, and 15 μM) of kaempferol were added to the cells. After 24 and 48 h, the cells were treated with 10 μL CCK-8 solution for 4 h at 37℃, and the optical density (OD) at 450 nm was measured by a microplate reader.
Total RNA extraction and real-time quantitative polymerase chain reaction (RT-qPCR)
The MC3T3-E1 cells were inoculated into 6-well cell culture plates when they reached a healthy growth condition. Upon reaching 90% cell density, various concentrations of kaempferol (5 μM and 7.5 μM) were added, determined based on the cell viability assay. After 24 h, total RNA was extracted using TRIzol reagent (Sigma, USA), and the RNA concentration was measured with NanoDrop 2000 (Thermo, USA). Reverse transcription was performed using the TaKaRa reverse transcription kit (TaKaRa, Japan). GAPDH served as an internal reference, and the mRNA expression levels of AKT1 and MMP9 in each group were assessed using an ABI 7500 PCR instrument (Thermo, USA). Additionally, the relative mRNA expression levels were calculated using the 2-△△Ct method. Each sample was assayed at least three times. The primer sequences for PCR were as follows:
AKT1: 5′-GGACTACTTGCACTCCGAGAAG-3′, 3′-CATAGTGGCACCGTCCTTGATC-5′;
MMP9: 5′-GCTGACTACGATAAGGACGGCA-3′, 3′-TAGTGGTGCAGGCAGAGTAGGA-5′;
GAPDH: 5′-GAGTCAACGGATTTGGTCGT-3′, 3′-GACAAGCTTCCCGTTCTCAG-5′.
Western blotting
The cells were inoculated and treated as described above. After 24 h of kaempferol treatment, total proteins were extracted and quantified using the BCA quantitative kit (Beyotime, China). Subsequently, they underwent polyacrylamide gel electrophoresis (PAGE). The proteins were then transferred to a polyvinylidene fluoride (PVDF) membrane, which was sealed with 5% skim milk. The membrane was incubated with primary antibodies and horseradish peroxidase (HRP) -conjugated secondary antibodies. Enhanced Chemiluminescence (ECL) was employed for protein visualization, and images were captured using the XLI Touch Imager (e-BLOT, China). Each band was measured thrice, and results are expressed as the mean ± standard deviation (SD). Glycerol 3- phosphate dehydrogenase (GAPDH) served as the internal control. Primary antibodies used in protein blot analysis were anti-AKT1 (1: 750, ZEN BIO, China), anti-MMP9 (1: 1500, Proteintech, China) and anti-GAPDH (1: 20,000, Proteintech, China).
Statistical analysis
The statistical analysis of experimental data was performed using GraphPad Prism v.9.0.0 software. All measurement data are presented as mean ± SD. Group comparisons were conducted using one-way ANOVA and two-way ANOVA for multiple groups. The experimental data were derived from three independent experiments.
Results
Information of kaempferol
The structure and other basic information about kaempferol are illustrated in Fig. 2. Notably, its oral bioavailability (OB) exceeded the threshold of 30% at 41.88%, while its drug-likeness (DL) value of 0.24 also surpassed the threshold of 0.18. These findings suggest that kaempferol exhibits promising potential for drug activity.
Kaempferol-related targets
A total of 59 kaempferol-related targets were obtained from the TCMSP database, while 103 related targets were retrieved from the SwissTargetPrediction database. After removing duplicates, a final dataset of 153 kaempferol-related targets was compiled by merging the data from both the TCMSP and SwissTargetPrediction databases (Fig. 3A).
OP-related targets and overlapping targets
A total of 4543 OP-related targets were retrieved from the GeneCards database, with an additional 996 targets were obtained from the DisGeNET database. By intersecting these two datasets, a final set of 775 OP-related targets was generated (Fig. 3B). In total, 54 overlapping targets of kaempferol and OP wereidentified through the Venn diagram on the Hiplot platform, as depicted in Fig. 3C. These overlapping targets of kaempferol and OP may signify potential targets of kaempferol in the treatment of OP.
PPI network construction
The above overlapping targets of kaempferol and OP were input into the STRING database to construct the PPI network. Subsequently, the overlapping targets were imported into Cytoscape 3.10.0 for visualization, and the resulting PPI network is displayed in Fig. 4A. The cytoHubba plug-in was utilized to identify the core targets based on the top 10 rankings in MNC, Degree, and Closeness. Notably, the top 10 targets based on the MNC (Fig. 4B), Degree (Fig. 4C), and Closeness (Fig. 4D) parameters consistently included AKT1, MMP9, ESR1, TNF, HSP90AA1, PPARG, SRC, EGFR, JUN, and CASP3.
GO functional annotation and KEGG signaling pathway enrichment analysis
The Hiplot platform was utilized to conduct GO functional annotation analysis on 54 kaempferol-OP overlapping targets (p < 0.05). The GO enrichment analysis included three categories: biological process (BP), cellular components (CC), and molecular function (MF). Figure 5A illustrates the top 10 enrichment items for each category, ranked according to their p-values. In the BP section, the overlapping targets demonstrated enrichment in cellular development oxidative stress, including response to reactive oxygen species, response to oxidative stress, and cellular response to chemical stress (Fig. 5A,B). In the CC section, enrichment was observed in membrane microdomain raft structures, such as membrane raft and membrane microdomain (Fig. 5A,C). Furthermore, the common targets in the MF section were associated with DNA-binding general initiation factors, such as nuclear receptor activity and ligand-activated transcription factor activity (Fig. 5A,D).
KEGG signaling pathway enrichment analysis was performed on 54 kaempferol-OP overlapping targets (p < 0.05) using the Hiplot platform. Figure 6A displays the top 20 signaling pathways identified. The analysis revealed that the overlapping targets were enriched in several pathways related to AGE-RAGE atherosclerosis diabetic complications. These pathways include Fluid shear stress and atherosclerosis, Lipid and atherosclerosis, AGE-RAGE signaling pathway in diabetic complications, IL-17 signaling pathway, and TNF signaling pathway (Fig. 6A,B).
Molecular docking analysis
Among the ten core targets of kaempferol for OP treatment, AKT1, and MMP9 were chosen for molecular docking. The decision to select AKT1 and MMP9 as molecular docking targets is based on two primary reasons. Firstly, AKT1 demonstrates the highest Degree value within the PPI network, signifying its importance in the network. Secondly, AKT1 and MMP9 are both enriched in multiple signaling pathways, such as Fluid shear stress and atherosclerosis, Lipid and atherosclerosis, AGE-RAGE signaling pathway in diabetic complications, and TNF signaling pathway.
To explore the binding of kaempferol with these two targets, MOE was utilized to predict their interactions. The binding energy, indicating the strength of the interaction, was assessed in this study. Generally, a binding energy of less than 0 kcal mol−1 suggests binding activity between molecules, while a binding energy of less than − 5.0 kcal mol−1 indicates strong binding activity. The smaller the binding energy, the stronger the binding ability. The results of molecular docking analysis of kaempferol with AKT1 and MMP9 can be observed in Fig. 7. The binding energies were calculated to be 5.89 kcal mol−1 and 6.51 kcal mol−1, respectively. These values are below − 5 kcal mol−1, indicating that kaempferol exhibited significant and strong binding ability with both AKT1 and MMP9.
Effects of kaempferol on the viability of MC3T3-E1 cells
After treating MC3T3-E1 cells with various concentrations of kaempferol (2.5, 5, 7.5, 10, 12.5, and 15 μM) for 24 h and 48 h, we observed that the concentrations of 5 μM and 7.5 μM of kaempferol had the most significant impact on the activity of MC3T3-E1 cells at 24 h (p ˂ 0.05) compared to the control group (Fig. 8A). When MC3T3-E1 cells were co-cultured with kaempferol concentrations of 5 μM and 7.5 μM for 24 h, the drug significantly promoted cell viability (p < 0.05 and p < 0.01, respectively). Therefore, MC3T3-E1 cells treated with 5 μM and 7.5 μM of kaempferol for 24 h were selected for further experiments.
Kaempferol affects the expression of AKT1 and MMP9 expression. (A) Results of cell viability assay. The statistical analyses were conducted by two-way ANOVA. * p < 0.05, ** p < 0.01 vs. control group. (B, C) Quantitative analysis of AKT1 and MMP9 mRNAs. (D) The analysis of the protein expression of AKT1 and MMP9. * p < 0.05, ** p < 0.01, *** p < 0.001. The statistical analyses were conducted by one-way ANOVA. Results are shown as mean ± SD of three individual experiments.
Effects of kaempferol on the expression of AKT1 and MMP9 in MC3T3-E1 cells
In Fig. 8B, the mRNA expression of AKT1 in MC3T3-E1 cells exhibited a significant increase (p < 0.001) when treated with 5 μM of kaempferol compared to the control group. However, under 7.5 μM of kaempferol, no significant effect was observed on the expression of AKT1 (p > 0.05). Conversely, the mRNA expression of MMP9 in MC3T3-E1 cells significantly decreased (p < 0.05) under both 5 μM and 7.5 μM kaempferol (Fig. 8C). We further analyzed the protein expression of AKT1 and MMP9. As depicted in Fig. 8D, the protein expression of AKT1 was increased in the dosing group compared with that in the control group, while the protein expression of MMP9 was decreased in the dosing group compared to the control group. These findings suggest that kaempferol may play an anti-OP role by up-regulating the expression of AKT1 and down-regulating the expression of MMP9.
Discussion
Network pharmacology is a novel research methodology that combines systems network analysis with pharmacology to elucidate the potential mechanisms of multi-target drugs in the molecular-level treatment of diseases through compound-target and target-disease network analyses34,35,36. The present study employed a network pharmacology approach to investigate the mechanism of action underlying the therapeutic use of kaempferol in treating OP. The findings identified 54 potential therapeutic targets for kaempferol in OP treatment, with AKT1, MMP9, ESR1, TNF, HSP90AA1, PPARG, SRC, EGFR, JUN, and CASP3 identified as the core targets. Moreover, KEGG enrichment analysis indicated that the overlapping targets of kaempferol and OP were predominantly associated with signaling pathways implicated in atherosclerosis, the AGE/RAGE signaling pathway, and the TNF signaling pathway. By integrating the PPI network (with AKT1 having the highest degree value), KEGG signaling pathway enrichment analysis (which identified AKT1 and MMP9 as being enriched in multiple signaling pathways, including those associated with atherosclerosis, AGE-RAGE, and TNF, all of which are closely linked to OP), and molecular docking analysis (which revealed the significant and strong binding ability of kaempferol to AKT1 and MMP9), it becomes apparent that AKT1 and MMP9 play a crucial role in the therapeutic effects of kaempferol against OP.
Exploring the key targets of kaempferol in treating OP is crucial for elucidating its potential mechanism of action. This study highlights the importance of regulating the expression of AKT1 and MMP9 in OP treatment. RAC-alpha serine/threonine-protein kinase (AKT1) is a protein kinase present in osteoblasts and osteoclasts that plays a crucial role in regulating cell proliferation, differentiation, and maintaining bone mass. Studies have shown that a deficiency of AKT1 can lead to bone loss, which is associated with OP37. Yanjun Wang et al. demonstrated that tanshinone may improve OP by targeting and up-regulating AKT1 expression38. Li Ou et al. found that Rehmanniae Radix Preparata could increase bone density and promote bone formation by significantly increasing AKT1 expression in bone tissue39, highlighting the significance of AKT1 in bone health. Matrix metalloproteinase 9 is a Zn2+-dependent proteolytic enzyme that is closely related to the pathogenesis of OP. Evidence shows that serum MMP-9 concentration is negatively correlated with bone mineral density, which is considered a biochemical marker of bone resorption and reconstruction, and an important marker for early diagnosis of OP40,41. Additionally, Guoju Hong et al. showed that Robinin may inhibit osteoclast proliferation to prevent OP by down-regulating the expression of the osteoclast target gene, MMP942. Consistent with our cell experiments, kaempferol significantly increased the expression of AKT1 and decreased the expression of MMP9. Therefore, we hypothesize that the therapeutic effect of kaempferol in OP may be attributed to its ability to modulate the expression levels of AKT1 and MMP9.
KEGG enrichment analysis can identify pathways and functions related to specific diseases or physiological processes, offering insights into potential candidate targets. Our study revealed that common targets of kaempferol and OP are mainly involved in the Atherosclerosis-related signaling pathways, AGE/RAGE signaling pathway, and TNF signaling pathway. Epidemiological studies have demonstrated a strong association between atherosclerosis and OP, suggesting they may share a common pathogenic mechanism43,44. This explains why the common targets of kaempferol and OP are enriched in multiple atherosclerosis-related signaling pathways. Concurrently, numerous studies have shown that the AGE‐RAGE interaction can induce osteoblast apoptosis, inhibit cell proliferation and migration, decrease bone mass, and promote OP in diabetic patients45. Evan G Buettmann et al.46 concluded that bone loss due to aging and disuse is associated with AGE-RAGE signaling pathway. Liang Mo et al.47 found that the AGE-RAGE signaling pathway in diabetic complications may play a significant role in diabetic skeletal fragility, including bone function impairment. Additionally, other clinical symptoms of diabetes, such as decreased bone mineral density, inhibition of bone turnover markers, and bone quality impairment, are all closely related to the AGE-RAGE signaling pathway48. Among the KEGG enrichment results, the TNF signaling pathway was also significantly enriched. TNF has been reported to have a significant impact on bone metabolism, aligning with previous studies49,50. Moreover, TNF-α, primarily produced by activated mononuclear macrophages, is closely associated with OP, with high expression levels detected in OP cases51. Importantly, the key targets AKT1 and MMP9 are also enriched in the aforementioned signaling pathways. Therefore, we propose that kaempferol’s treatment for OP exhibits a multi-pathway effect and might improve OP by regulating the expression of relevant genes, thereby influencing the associated pathways.
The limitation of this study is that the predicted results were mainly validated through cell experiments, and further studies involving animal and clinical trials are lacking. Further exploration is needed to better understand the mechanism of kaempferol in treating OP, thereby strengthening the persuasiveness of our findings.
Conclusion
In conclusion, our study preliminarily elucidated that kaempferol exhibited characteristics of targeting multiple pathways in the treatment of OP, as evidenced by network pharmacology analysis and in vitro experiments. The mechanism of action of kaempferol in treating OP may involve up-regulating the expression of AKT1 and down-regulating the expression of MMP9, while participating in the Atherosclerosis-related signaling pathways, AGE/RAGE signaling pathway, and TNF signaling pathway. This study contributes to the understanding of the mechanism of kaempferol in treating OP and provides a theoretical foundation for future research and development of new drugs.
Data availability
The datasets generated and analyzed during the current study can be found in the Kaempferol-related targets were predicted by the TCMSP (https://old.tcmsp-e.com/index.php) and SwissTargetPrediction (http://www.swisstargetprediction.ch/) database. After screening, targets were inputted into the UniProt database (https: //www.uniprot.org) for standardization to obtain the official names of the targets. With “OP” as the key word and “Homo sapiens” as the species, OP-related targets were collected from the GeneCards (https://www.genecards.org/) and DisGeNET (https://www.disgenet.org/) databases.
References
Cosman, F. et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. U.S.A. 25, 2359–2381. https://doi.org/10.1007/s00198-014-2794-2 (2014).
Salari, N. et al. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 16, 609. https://doi.org/10.1186/s13018-021-02772-0 (2021).
Lin, X. et al. Epidemiology and management of osteoporosis in the People’s Republic of China: Current perspectives. Clin. Interv. Aging 10, 1017–1033. https://doi.org/10.2147/cia.S54613 (2015).
LeBoff, M. S. et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. U.S.A. 33, 2049–2102. https://doi.org/10.1007/s00198-021-05900-y (2022).
Aibar-Almazán, A. et al. Current status of the diagnosis and management of osteoporosis. Int. J. Mol. Sci. 23, 16. https://doi.org/10.3390/ijms23169465 (2022).
Zeng, Q. et al. N-butanol extract of modified You-Gui-Yin attenuates osteoclastogenesis and ameliorates osteoporosis by inhibiting RANKL-mediated NF-κB signaling. Front. Endocrinol. 13, 925848. https://doi.org/10.3389/fendo.2022.925848 (2022).
Wu, Z. et al. Hmga1-overexpressing lentivirus protects against osteoporosis by activating the Wnt/β-catenin pathway in the osteogenic differentiation of BMSCs. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 37, e22987. https://doi.org/10.1096/fj.202300488R (2023).
Park, O. J. et al. Muramyl dipeptide alleviates estrogen deficiency-induced osteoporosis through canonical Wnt signaling. J. Pathol. 260, 137–147. https://doi.org/10.1002/path.6069 (2023).
Gosset, A., Pouillès, J. M. & Trémollieres, F. Menopausal hormone therapy for the management of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 35, 101551. https://doi.org/10.1016/j.beem.2021.101551 (2021).
Dempster, D. W. et al. Early effects of abaloparatide on bone formation and resorption indices in postmenopausal women with osteoporosis. J. Bone Min. Res. Off. J. Am. Soc. Bone Min. Res. 36, 644–653. https://doi.org/10.1002/jbmr.4243 (2021).
Ayers, C. et al. Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: A living systematic review and network meta-analysis for the American college of physicians. Ann. Intern. Med. 176, 182–195. https://doi.org/10.7326/m22-0684 (2023).
Kendler, D. L., Cosman, F., Stad, R. K. & Ferrari, S. Denosumab in the treatment of osteoporosis: 10 years later—A narrative review. Adv. Ther. 39, 58–74. https://doi.org/10.1007/s12325-021-01936-y (2022).
Zeytinoglu, M., Naaman, S. C. & Dickens, L. T. Denosumab discontinuation in patients treated for low bone density and osteoporosis. Endocrinol. Metab. Clin. N. Am. 50, 205–222. https://doi.org/10.1016/j.ecl.2021.03.004 (2021).
Martínez-Reina, J., Calvo-Gallego, J. L. & Pivonka, P. Are drug holidays a safe option in treatment of osteoporosis?—Insights from an in silico mechanistic PK-PD model of denosumab treatment of postmenopausal osteoporosis. J. Mech. Behav. Biomed. Mater. 113, 104140. https://doi.org/10.1016/j.jmbbm.2020.104140 (2021).
Chavassieux, P. et al. Bone-forming and antiresorptive effects of romosozumab in postmenopausal women with osteoporosis: bone histomorphometry and microcomputed tomography analysis after 2 and 12 months of treatment. J. Bone min. Res. Off. J. Am. Soc. Bone Min. Res. 34, 1597–1608. https://doi.org/10.1002/jbmr.3735 (2019).
Beck, B. R. et al. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J. Sci. Med. Sport 20, 438–445. https://doi.org/10.1016/j.jsams.2016.10.001 (2017).
Vandenbroucke, A., Luyten, F. P., Flamaing, J. & Gielen, E. Pharmacological treatment of osteoporosis in the oldest old. Clin. Interv. Aging 12, 1065–1077. https://doi.org/10.2147/cia.S131023 (2017).
Wong, S. K., Chin, K. Y. & Ima-Nirwana, S. The osteoprotective effects of Kaempferol: The evidence from in vivo and in vitro studies. Drug Des. Devel. Ther. 13, 3497–3514. https://doi.org/10.2147/dddt.S227738 (2019).
Zhu, J. et al. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int. Immunopharmacol. 43, 236–242. https://doi.org/10.1016/j.intimp.2016.12.020 (2017).
Suh, K. S. et al. Kaempferol attenuates 2-deoxy-d-ribose-induced oxidative cell damage in MC3T3-E1 osteoblastic cells. Biol. Pharm. Bull. 32, 746–749. https://doi.org/10.1248/bpb.32.746 (2009).
Wu, X. et al. Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways. J. Bone Miner. Res. 27, 1298–1308. https://doi.org/10.1002/jbmr.1576 (2012).
Kim, I. R. et al. The role of kaempferol-induced autophagy on differentiation and mineralization of osteoblastic MC3T3-E1 cells. BMC Complement Altern. Med. 16, 333. https://doi.org/10.1186/s12906-016-1320-9 (2016).
Kim, C. J. et al. The effects of Kaempferol-inhibited autophagy on osteoclast formation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19010125 (2018).
Ru, J. et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13. https://doi.org/10.1186/1758-2946-6-13 (2014).
Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucl. Acids Res. 47, W357-w364. https://doi.org/10.1093/nar/gkz382 (2019).
Wang, Y. et al. A crowdsourcing open platform for literature curation in UniProt. PLoS Biol. 19, e3001464. https://doi.org/10.1371/journal.pbio.3001464 (2021).
UniProt: a worldwide hub of protein knowledge. Nucl. Acids Res. 47: D506–d515. https://doi.org/10.1093/nar/gky1049 (2019).
Dong, Y. et al. Identification and characterization of a novel molecular classification incorporating oxidative stress and metabolism-related genes for stomach adenocarcinoma in the framework of predictive, preventive, and personalized medicine. Front. Endocrinol. 14, 1090906. https://doi.org/10.3389/fendo.2023.1090906 (2023).
Piñero, J., Saüch, J., Sanz, F. & Furlong, L. I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 19, 2960–2967. https://doi.org/10.1016/j.csbj.2021.05.015 (2021).
Zhao, J. et al. Global publication trends and research hotspots of the immune system and osteoporosis: A bibliometric and visualization analysis from 2012 to 2022. Endocr. Metab. Immune Disord. Drug Targets 24, 455–467. https://doi.org/10.2174/0118715303257269231011073100 (2023).
Szklarczyk, D. et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucl. Acids Res. 51, D638-d646. https://doi.org/10.1093/nar/gkac1000 (2023).
Burley, S. K. et al. Protein data bank: A comprehensive review of 3d structure holdings and worldwide utilization by researchers, educators, and students. Biomolecules 12, 1425. https://doi.org/10.3390/biom12101425 (2022).
Kim, S. et al. PubChem in 2021: New data content and improved web interfaces. Nucl. Acids Res. 49, D1388-d1395. https://doi.org/10.1093/nar/gkaa971 (2021).
Jiang, Y. et al. Elucidation of the mechanisms and molecular targets of Yiqi Shexue formula for treatment of primary immune thrombocytopenia based on network pharmacology. Front. Pharmacol. 10, 1136. https://doi.org/10.3389/fphar.2019.01136 (2019).
Zhou, S. et al. Deciphering the pharmacological mechanisms of Taohe-Chengqi decoction extract against renal fibrosis through integrating network pharmacology and experimental validation in vitro and in vivo. Front. Pharmacol. 11, 425. https://doi.org/10.3389/fphar.2020.00425 (2020).
Zhang, J. Y. et al. Target identification of active constituents of Shen Qi Wan to Treat Kidney Yang deficiency using computational target fishing and network pharmacology. Front. Pharmacol. 10, 650. https://doi.org/10.3389/fphar.2019.00650 (2019).
Mukherjee, A. & Rotwein, P. Selective signaling by Akt1 controls osteoblast differentiation and osteoblast-mediated osteoclast development. Mol. Cell Biol. 32, 490–500. https://doi.org/10.1128/mcb.06361-11 (2012).
Wang, Y. et al. Tanshinone ameliorates glucocorticoid-induced bone loss via activation of AKT1 signaling pathway. Front. Cell Dev. Biol. 10, 878433. https://doi.org/10.3389/fcell.2022.878433 (2022).
Ou, L. et al. Investigation of anti-osteoporosis mechanisms of Rehmanniae Radix Preparata based on network pharmacology and experimental verification. J. Orthop. Surg. Res. 16, 599. https://doi.org/10.1186/s13018-021-02751-5 (2021).
Chai, L. J. et al. The antiosteoporosis effects of Zhuanggu Guanjie Pill in vitro and in vivo. Biomed. Res. Int. 2018, 9075318. https://doi.org/10.1155/2018/9075318 (2018).
Zheng, X. et al. Dynamic expression of matrix metalloproteinases 2, 9 and 13 in ovariectomy-induced osteoporosis rats. Exp. Ther. Med. 16, 1807–1813. https://doi.org/10.3892/etm.2018.6356 (2018).
Hong, G. et al. A novel RANKL-targeted flavonoid glycoside prevents osteoporosis through inhibiting NFATc1 and reactive oxygen species. Clin. Transl. Med. 11, e392. https://doi.org/10.1002/ctm2.392 (2021).
Ambrogini, E. et al. Oxidation-specific epitopes restrain bone formation. Nat. Commun. 9, 2193. https://doi.org/10.1038/s41467-018-04047-5 (2018).
Mo, L. et al. Integrated bioinformatic analysis of the shared molecular mechanisms between osteoporosis and atherosclerosis. Front. Endocrinol. 13, 950030. https://doi.org/10.3389/fendo.2022.950030 (2022).
Zhang, M. et al. Blockade of receptors of advanced glycation end products ameliorates diabetic osteogenesis of adipose-derived stem cells through DNA methylation and Wnt signalling pathway. Cell Prolif. 51, e12471. https://doi.org/10.1111/cpr.12471 (2018).
Buettmann, E. G. et al. Similarities between disuse and age-induced bone loss. J. Bone Min. Res. Off. J. Am. Soc. Bone Min. Res. 37, 1417–1434. https://doi.org/10.1002/jbmr.4643 (2022).
Mo, L. et al. Integrated analysis of crucial genes and miRNAs associated with osteoporotic fracture of type 2 diabetes. BioMed. Res. Int. 2022, 3921570. https://doi.org/10.1155/2022/3921570 (2022).
Asadipooya, K. & Uy, E. M. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: Review of the literature. J. Endocr. Soc. 3, 1799–1818. https://doi.org/10.1210/js.2019-00160 (2019).
Seriolo, B., Paolino, S., Sulli, A., Ferretti, V. & Cutolo, M. Bone metabolism changes during anti-TNF-alpha therapy in patients with active rheumatoid arthritis. Ann. N. Y. Acad. Sci. 1069, 420–427. https://doi.org/10.1196/annals.1351.040 (2006).
Hu, Y. et al. Exploring quercetin anti-osteoporosis pharmacological mechanisms with in silico and in vivo models. Life (Basel) 12, 980. https://doi.org/10.3390/life12070980 (2022).
Wang, X. et al. Melatonin reverses the loss of stemness induced by TNF-α in human bone marrow mesenchymal stem cells through upregulation of YAP expression. Stem Cells Int. 2019, 6568394. https://doi.org/10.1155/2019/6568394 (2019).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Q.D., G.R. and Y.L. The first draft of the manuscript was written by Q.D. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dong, Q., Ren, G., Li, Y. et al. Network pharmacology analysis and experimental validation to explore the mechanism of kaempferol in the treatment of osteoporosis. Sci Rep 14, 7088 (2024). https://doi.org/10.1038/s41598-024-57796-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57796-3
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.