Abstract
We investigated the relationship between dietary phytochemical index (DPI) and migraine headaches in Iranian patients, analyzing both clinical and psychological traits. A cross-sectional study was conducted using non-obese adults aged 20–50 years who were diagnosed with migraine. The study used a validated 168-item food frequency questionnaire to assess the usual dietary intake of participants. The DPI was calculated using the following formula: [daily energy derived from phytochemical-rich foods (in kJ)/total daily energy intake (in kJ)] × 100. Clinical outcomes of migraine including frequency, duration, and severity of headaches, as well as migraine-related disability were obtained using relevant questionnaires. Moreover, the mental health profile of patients including depression, anxiety, and stress, as well as serum levels of nitric oxide (NO) were measured. A Poisson regression was used for headache frequency. Linear regression analyzed migraine-related outcomes including duration, severity, migraine-related disability, and serum NO levels. In addition, psychological traits were analyzed via logistic regression. A total of 262 individuals (85.5% females) with a mean age of 36.1 years were included in the analysis. The frequency of migraine attacks was lower in patients in the last DPI tertile compared to those in the first DPI tertile both in the crude [incidence rate ratio (IRR) = 0.70, 95% confidence interval (CI) 0.63, 0.78, Ptrend < 0.001] and fully-adjusted models (IRR = 0.84, 95% CI 0.74, 0.96, Ptrend = 0.009). After controlling for potential confounders, an inverse relationship was observed between higher adherence to DPI and migraine-related disability (β = − 2.48, 95% CI − 4.86, − 0.10, P trend = 0.046). After controlling for potential confounders, no significant relationship was observed between DPI and depression (OR = 0.79, 95% CI 0.42, 1.47, Ptrend = 0.480), anxiety (OR = 1.14, 95% CI 0.61, 2.14, Ptrend = 0.655), and stress (OR = 1.04, 95% CI 0.57, 1.90, Ptrend = 0.876). Higher intakes of phytochemical-rich foods may be associated with lower migraine frequency and improved daily activities among patients. Further studies should confirm our observations and delineate the biological pathways linking phytochemicals and migraine headaches.
Similar content being viewed by others
Introduction
Migraine is a recurrent neurovascular disorder defined by episodic and unilateral pulsatile headache accompanied by nausea, vomiting, phonophobia, and/or photophobia, and disturbance in quality of life, usual patient activity, and psychological status1,2. This leads to a significant economic burden for both individuals and healthcare systems3. The prevalence of migraine in the general population has been estimated to be 2.89% in 2008 and 14.0% in 20224. The exact pathophysiology of migraine has not been fully discovered. In addition, current medication therapies for migraine management have limited effectiveness and often come with adverse effects5,6,7. Therefore, it is crucial to identify the determinants of a migraine headache to develop effective management strategies based on its pathophysiology.
Besides genetic predisposition, sex, and age, modifiable risk factors such as diet, obesity, mental health, and physical activity are known to contribute to migraine8,9. Namely, dietary behaviors are crucial in migraine management. Previous epidemiological studies have demonstrated the link between healthy eating patterns rich in fruits, vegetables, whole grains, nuts, and olive oil, including the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean eating plans, and migraine-related clinical outcomes10,11,12,13,14,15,16. Plant-based foods are rich sources of phytochemicals, and their health-related efficacy has been proposed17,18. Only a few studies have examined the relationship between dietary phytochemicals and migraine headaches19,20, despite the fact that there is biological evidence to suggest a relationship21,22,23. However, these previous studies were limited in scope and size, and have produced contradictory findings. Therefore, it is still unclear whether there is a definitive link between phytochemicals and migraines.
Hence, we leveraged the dietary phytochemical index (DPI), a validated tool, to enhance our understanding of the relationship between phytochemicals and migraine24. The DPI represents the percentage of energy intake derived from phytochemical-rich foods, reflecting the total content of phytochemicals in the diet. The primary purpose of our study was to detect a relationship between DPI and clinical outcomes of migraine headaches in a sample of Iranian patients. We evaluated the link between DPI and mental health including depression, anxiety, and stress as our secondary aim. We hypothesized that higher consumption of phytochemical-rich foods could be related with favorable outcomes in patients with migraine.
Methods
Study design and participants
This cross-sectional study was conducted between August 2019 and June 2020 at two neurology clinics affiliated with Isfahan University of Medical Sciences, Isfahan, Iran. Together, 265 adults aged 20–50 years with a clinical diagnosis of migraine were included. A board-certified neurologist (F.K) confirmed migraine diagnosis using the International Classification of Headache Disorders-3 (ICHD-3) criteria25. A convenience sampling method was used to recruit patients. Convenience sampling is a non-probability sampling method that involves selecting participants based on their easy availability or accessibility to the researcher26. In this regard, we selected our participants according to predefined inclusion and exclusion criteria from those who were admitted to both neurology clinics.
Patients were excluded if they (1) had a history of conditions overlapping or interfering with migraine complications, including cardiovascular disease, hypertension, diabetes, cancer, hepatic, renal, thyroid, and other neurological disorders; (2) had a body mass index (BMI) of < 18.5 or ≥ 30 kg/m227; or, (3) reported daily energy intakes < 800 kcal/d or > 4200 kcal/d. The risk of migraine is strongly associated with body composition28,29; therefore, only normal-weight and overweight participants were enrolled to increase the internal validity of our work. Moreover, the history of the disease was assessed through a face-to-face interview with a physician and an assessment of the patient’s medical documents.
The study protocol was approved by the research ethics committee of Isfahan University of Medical Sciences (IR.MUI.RESEARCH.REC.1398.352). All procedures were conducted in compliance with the Declaration of Helsinki. All patients provided written informed consent before enrollment.
Dietary assessment
A 168-item semi-quantitative food frequency questionnaire (FFQ) was used to evaluate the usual dietary intake of patients in the preceding year through face-to-face interviews with a registered dietitian. The reliability and validity of FFQ for the Iranian population have been established previously30,31,32. Patients were instructed to report their daily, weekly, and monthly food intake. All portion sizes were converted into grams using household measures33. Daily intake of energy and nutrients was subsequently calculated using the Nutritionist IV software (First Databank, Hearst Corp, San Bruno, CA, USA).
DPI calculations
The DPI was calculated based on the equation suggested by McCarty et al.24 as follows: [DPI = energy intake from phytochemical-rich foods (kJ/d)/total energy intake (kJ/day) × 100]. Legumes, nuts, seeds, fruits and vegetables, fruits and vegetable juices, tomato sauces, whole grains, olive, olive oil, and soy sources were included in the calculation. Due to the limited content of phytochemicals in potatoes, we did not consider this item in the DPI calculation.
Assessments of migraine clinical features
All patients were instructed to complete a 30-day diary to record their migraine clinical traits, including the frequency, duration, and severity of headaches in the upcoming month. Migraine severity was evaluated using the visual analog scale (VAS), wherein the severity was ranked between zero and 10, with “0” indicating no pain and “10” the worst imaginable pain. The validity and reliability of this questionnaire have been approved previously34. Further, the Headache Impact Test (HIT-6) was used to evaluate the impact of migraine on the patients’ usual daily activities. The HIT-6 represented a self-administered questionnaire containing six questions. Each question included five response items (never, rarely, sometimes, very often, always), with assigned scores of six, eight, 10, 11, and 13, respectively. The total HIT-6 scores ranged between 36 and 78. The score ranges 36–49, 50–55, 56–59, and ≥ 60 reflected that migraine had minimal, moderate, substantial, or severe impact on patients’ daily activities35. It has been confirmed that this questionnaire is valid and reliable among the Iranian population36.
Assessment of psychological characteristics
The Depression, Anxiety, and Stress Scale (DASS-21), a 21-item questionnaire, was used to assess psychological traits. The validity and reliability of the scale among the Iranian population have been previously approved37. This questionnaire consisted of seven questions for each subscale of Depression, Anxiety, and Stress. Responses to each question were scored between zero (never) and three (most of the time). The total scores calculated for each subscale were multiplied by two to interpret scores on the original DASS-42 questionnaire. The total score for each subscale of DASS-21 ranged between zero and 42, with higher scores representing higher psychological distress38.
Assessment of other variables
General information including age, sex, marital status, tobacco smoking, family size, migraine type (episodic or chronic), migraine features (with or without aura), family history of migraine diagnosis, time since migraine diagnosis, and medication were collected through face-to-face interviews. To evaluate the physical activity status of patients, a validated International Physical Activity Questionnaire (IPAQ) designed for the Iranian population was used39. The questionnaire consisted of seven questions that distinguished the frequency and duration of mild, moderate, and intensive physical activity, as well as inactivity, over seven days. The overall score was reported as MET.h/d. Blood pressure was measured in the sitting position twice using a mercury sphygmomanometer (Riester, Germany) after 10 min of rest, and the average of two measurements was used for further analysis. A digital scale [Omron BF511 (Omron Corp, Kyoto, Japan)] was used for evaluating the patient’s body weight to the nearest 100 g with minimal clothing and no shoes. Height was measured using upstretched tape to the nearest 1 cm in a standing position with relaxed shoulders and no shoes. BMI was calculated using a formula: weight (kg)/height2 (m2).
A 5 mL fasting blood sample was collected for all patients. Specimens were immediately centrifuged (Avanti J-25, Beckman, Brea, CA, USA) at 3500 rpm for 10 min, and serum was stored at − 80 °C before biochemical analyses. Nitric oxide (NO) levels were assayed using the Griess method and available commercial kits (Kiazist Life Sciences, Iran).
Statistical analysis
The study sample size was calculated based on the suggested formula for a cross-sectional design consistent with previous studies with α = 0.05, β = 0.95, and r = 0.25. After accounting for an estimated 10% of the drop-out rates, a sample size of n = 265 was deemed adequate40. Before data analysis, the normal distribution of continuous variables was examined using Q-Q plots, histogram charts, and skewness statistics. For the skewness statistic, values outside the range of − 2 to + 2 are considered a departure from normality. Since the missing data percentage was small, we restricted analyses to individuals with complete data on all variables. Quantitative and qualitative variables were expressed as mean ± standard error (SE) and frequency (percentage). The difference of continuous variables across DPI tertiles was evaluated by one-way analysis of variance (ANOVA) and Tukey test for post hoc analysis, while the Chi-squared or Fisher’s exact test was utilized for proportions. The relationship between DPI and headache frequency was evaluated using the Poisson regression analysis and an incidence rate ratio (IRR) with a 95% confidence interval (CI) was reported while the other clinical and biochemical outcomes including headache duration, severity, HIT-6, and serum levels of NO were analyzed using linear regression analysis and beta (β) and 95% CI was presented. In addition, the link between psychological traits (depression, anxiety, stress) and DPI was explored using logistic regression analysis, and odds ratio (OR) with 95% CI was reported. All analyses were done in the crude and adjusted models. The first adjusted model accounted for age (continuous), sex (male, female), and total energy intake (continuous). The next model was additionally adjusted for marital status (single, married), current smoking status (yes, no), migraine type (with or without aura), family history (yes, no), mean arterial pressure (continuous), medication use (yes, no), and physical activity (continuous). The last model was additionally adjusted for BMI (continuous). Data were analyzed using SPSS version 21 (IBM Corp, Armonk, NY, USA). All tests were two-tailed and statistically significant at alpha = 0.050.
Results
Participants
Between August 2019 and June 2020, a total of 770 participants were admitted to neurology clinics, of which 476 were excluded. Of the remaining 294 participants, 265 consented to participate in the current study of which an additional three subjects were excluded because their total energy intake, based on FFQ, was outside the range of 800 to 4200 kcal/day (Fig. 1). Overall, 262 patients with migraine, including 224 females and 38 males, were included in the present study. The mean age of the patients was 36.1 (SE: 0.5) years, and the mean BMI was 25.55 (SE: 0.21) kg/m2.
Descriptive data
General characteristics
The baseline characteristics of migraine patients across DPI tertiles are summarized in Table 1. The mean age of patients in the third DPI tertile was substantially higher than in the first tertile (P = 0.008). Additionally, patients in the highest DPI tertile were more likely females (P = 0.010), current smokers (P = 0.023), reported migraine headaches with aura (P = 0.001), and used benzodiazepines (P = 0.037). No significant differences were evident across DPI tertiles for other evaluated measures (all P > 0.05; Table 1).
Dietary intakes
Participants’ dietary intakes, including macronutrients and food groups across DPI tertiles, are shown in Table 2. Patients in the highest DPI tertile had a significantly higher carbohydrate intake (P = 0.019) and protein (P = 0.031) than those in the lowest DPI tertile. In contrast, fat intake was lower in the third DPI tertile than in the first DPI tertile (P = 0.043). Also, higher intakes of vegetables, fruits, whole grains, total fruits (fruits plus fruit juices), nuts and legumes, and seafood or plant proteins were observed in patients in the last DPI vs. the first tertile (P < 0.001). Nevertheless, lower intakes of refined grains and added sugar were evident in patients in the third DPI vs. the first tertile (P < 0.001). Total dairy (P = 0.648) and red and processed meat (P = 0.845) intakes were comparable across the tertiles (Table 2).
Clinical characteristics
The mean HIT-6 score (P = 0.029) and frequency of migraine attacks (P = 0.038) were significantly lower in patients in the highest DPI tertile compared to patients in the lowest tertile. However, no other significant differences were observed in terms of psychological traits and headache duration, severity, and serum levels of NO (Table 3).
Main results
Clinical outcomes of migraine
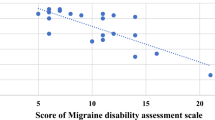
The frequency of migraine attacks was lower in patients in the last DPI tertile compared to those in the first DPI tertile both in the crude (IRR = 0.70, 95% CI 0.63, 0.78, Ptrend < 0.001) and fully-adjusted models (IRR = 0.84, 95% CI 0.74, 0.96, Ptrend = 0.009). A non-significant relationship was found between DPI and HIT-6 in the crude model (β = − 2.05, 95% CI − 4.15, 0.04, Ptrend = 0.056). However, the relationship became significant after adjustment for confounders (β = − 2.48, 95% CI − 4.86, − 0.10, Ptrend = 0.046). No significant relationship was found between DPI and NO, migraine headache duration, and severity in the crude or adjusted models (Table 4).
Physiological traits
Crude and multivariate-adjusted OR and 95% CIs for depression, anxiety, and stress are shown in Table 5. After controlling for potential confounders, no significant relationship was observed between DPI and depression (OR = 0.79, 95% CI 0.42, 1.47, Ptrend = 0.480), anxiety (OR = 1.14, 95% CI 0.61, 2.14, Ptrend = 0.655), and stress (OR = 1.04, 95% CI 0.57, 1.90, Ptrend = 0.876).
Discussion
In this study, we aimed to evaluate the relationship between DPI and clinical, psychological, and biochemical traits of migraine. The most significant finding of the present work was that patients with higher intakes of phytochemical-rich foods presented with lower migraine frequency and improved migraine-related disability, as assessed by the HIT-6. Nevertheless, we observed no relationship between DPI and other clinical outcomes (duration and severity of headaches), psychological characteristics, and serum NO levels. It is important to note the possibility of bidirectionality when interpreting our findings. Migraine sufferers with higher headache frequencies may omit more food triggers due to nausea and vomiting accompanying their migraines. This could result in lower DPI scores for these patients. Therefore, future longitudinal and prospective studies are necessary to investigate this correlation.
Previous studies have demonstrated an inverse relationship between dietary patterns rich in phytochemicals, such as Mediterranean and DASH diet, and some migraine symptoms, such as severity, frequency, and duration of migraine attacks41,42. Moreover, two recent cross-sectional studies have investigated the relationship between DPI and migraine headaches19,20, of which results of a survey by Askarpour and co-workers on 66 women aged 18–50 years showed 33% lower odds of headache severity in patients with higher DPI scores. However, no significant relationship was found between DPI and migraine attack duration19. Results of the other study on 90 patients with episodic migraines revealed a negative correlation between DPI and the severity of migraine attacks, without relationship between DPI scores and disability as evidenced by the Migraine Disability Assessment Score (MIDAS) or the duration of migraine attacks20. Contrary to the results of the studies mentioned above, we observed no relationship between DPI and the severity of migraine headaches. The conflicting results may be partially explained by differences in the studied population, statistical methods used, and covariates considered in the analyses, warranting further investigations to confirm the link and delineate the underlying explanatory mechanism.
Several studies have examined the relationship between DPI and mental health disorders. However, to our knowledge, none have targeted patients with migraine43,43,45. Accordingly, a lower risk of depression, anxiety, and stress has been reported in individuals with the highest vs. lowest DPI scores. We were unable to find studies that investigated the relationship between DPI and psychiatric outcomes in patients with migraine, highlighting a persistent research gap in this critical area. The null findings in our work may be explained, at least in part, by the marginal effect estimates because our study was designed to examine psychological indices as a secondary outcome. Future studies are required to evaluate the link between dietary intake of phytochemicals and psychological trials in patients with migraine.
Several potential mechanisms may underlie the relationship between phytochemicals and migraine headaches. Recent evidence from animal models and human studies suggests that gut microbiota dysbiosis contributes to the pathophysiology of migraine headaches46. Therefore, the therapeutic effects of improved gut microbiota, secondary to dietary modifications, on migraine outcomes have biological plausibility. Some reports indicate that there is a favorable link between dietary intakes of phytochemical-rich foods and gut microbiota. Subsequently, these natural bioactive compounds potentially alleviate migraine symptoms by maintaining gut microbiota homeostasis. However, the underlying molecular mechanisms are not well understood23. Furthermore, an increased vulnerability to oxidative stress, which results from the synthesis of oxidants, has been implicated in patients with migraine47,48. Plant-based foods’ antioxidant properties are mainly due to phytochemicals such as beta-carotene and polyphenols49. Phytochemicals may improve migraine symptoms by scavenging excess reactive oxygen (ROS) and nitrogen (RNS) spices and reducing the risk of oxidative stress22.
This study has certain limitations that require consideration. Due to the cross-sectional nature of the study, it was not feasible to establish a causal relationship between DPI and the outcomes under consideration. As a result, future large-scale prospective studies are necessary to investigate the causal link between the intake of foods high in phytochemicals and the clinical outcomes of migraine. In addition, because the DPI was calculated based on the energy provided by phytochemical-rich foods and not all phytochemical-rich foods provide energy, certain foods with high phytochemical content, such as tea and coffee, were excluded from the study. Despite using a validated FFQ to evaluate dietary intake, recall bias may still influence the findings. Finally, the study’s small sample size limits its generalizability to patients in other regions of Iran or other countries.
In conclusion, we found that adherence to a phytochemical-rich diet could be associated with favorable clinical outcomes for migraine patients including headache frequency and migraine-related disability. Owing to the cross-sectional nature of the current study, we cannot provide any practical implications regarding phytochemicals and migraine. These findings can be used by other researchers to conduct further well-designed longitudinal studies to confirm our findings and also elaborate our understanding regarding the causality between phytochemicals and migraine. Moreover, further studies are also needed to investigate underlying mechanisms regarding phytochemicals and migraine.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- DPI:
-
Dietary phytochemical index
- NO:
-
Nitric oxide
- SQ-FFQ:
-
Semi-quantitative food frequency questionnaire
- HIT-6:
-
Headache impact test-6
- CI:
-
Confidence interval
- ICHD-3:
-
The third edition of International Classification for Headache Disorders
- BMI:
-
Body mass index
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- VAS:
-
Visual analog scale
- PA:
-
Physical activity
- IPAQ:
-
International Physical Activity Questionnaire
- ANOVA:
-
Analysis of variance
- DASS:
-
Depression, Anxiety, and Stress Scale
References
Ashina, M. et al. Migraine: Epidemiology and systems of care. Lancet 397, 1485–1495 (2021).
Ashina, M. et al. Migraine: Integrated approaches to clinical management and emerging treatments. Lancet 397, 1505–1518 (2021).
Radat, F. & Swendsen, J. Psychiatric comorbidity in migraine: A review. Cephalalgia 25, 165–178 (2005).
Stovner, L. J., Hagen, K., Linde, M. & Steiner, T. J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 23, 34 (2022).
Géraud, G., Keywood, C. & Senard, J. M. Migraine headache recurrence: Relationship to clinical, pharmacological, and pharmacokinetic properties of triptans. Headache J. Head Face Pain 43, 376–388 (2003).
Cortelli, P. et al. Frovatriptan versus other triptans in the acute treatment of migraine: Pooled analysis of three double-blind, randomized, cross-over, multicentre, Italian studies. Neurol. Sci. 32, 95–98 (2011).
Lipton, R. B., Reed, M. L., Kurth, T., Fanning, K. M. & Buse, D. C. Framingham-based cardiovascular risk estimates among people with episodic migraine in the US population: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache J. Head Face Pain 57, 1507–1521 (2017).
Cho, S.-J. & Chu, M. K. Risk factors of chronic daily headache or chronic migraine. Curr. Pain Headache Rep. 19, 1–9 (2015).
Bigal, M. E. & Lipton, R. B. Modifiable risk factors for migraine progression. Headache J. Head Face Pain 46, 1334–1343 (2006).
Arab, A., Khorvash, F., Karimi, E., Hadi, A. & Askari, G. Associations between adherence to Mediterranean dietary pattern and frequency, duration, and severity of migraine headache: A cross-sectional study. Nutr. Neurosci. 26, 1–10 (2023).
Bakırhan, H., Yıldıran, H. & Uyar Cankay, T. Associations between diet quality, DASH and Mediterranean dietary patterns and migraine characteristics. Nutr. Neurosci. 25, 2324–2334 (2022).
Mirzababaei, A. et al. Associations between adherence to dietary approaches to stop hypertension (DASH) diet and migraine headache severity and duration among women. Nutr. Neurosci. 23, 335–342 (2020).
Hajjarzadeh, S. et al. The relation of adherence to the DASH diet with migraine attack frequency and pain intensity in Iranian women: A cross-sectional study. Nutr. Neurosci. https://doi.org/10.1080/1028415X.2023.2193766 (2023).
Balali, A. et al. Associations between diet quality and migraine headaches: A cross-sectional study. Nutr. Neurosci. https://doi.org/10.1080/1028415X.2023.2244260 (2023).
Karimi, E. et al. Is there an association between a plant-based eating pattern and clinical findings of a migraine headache?. Front. Nutr. 10, 1117740. https://doi.org/10.3389/fnut.2023.1117740 (2023).
Tirani, S. A., Askari, G., Khorvash, F., As’habi, A. & Arab, A. Associations between dietary diversity score and migraine headaches: The results from a cross-sectional study. Front. Nutr. 10, 1206278 (2023).
Dillard, C. J. & German, J. B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 80, 1744–1756 (2000).
Leitzmann, C. Characteristics and health benefits of phytochemicals. Complement. Med. Res. 23, 69–74 (2016).
Askarpour, M., Yarizadeh, Y., Khorsha, F., Mirzaei, K. & Togha, M. The association of dietary phytochemical index and migraine headaches. J. Iran. Med. Counc. 3, 99–105 (2020).
Bakırhan, H., Pehlivan, M., Uyar Cankay, T. & Kocak, M. Migraine severity, disability, and duration: Is a good diet quality, high intake of phytochemicals and polyphenols important?. Front. Nutr. 9, 1041907 (2022).
Zhu, F., Du, B. & Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 58, 1260–1270 (2018).
Guan, R. et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 271, 129499 (2021).
Yin, R. et al. Gut microbiota, dietary phytochemicals, and benefits to human health. Curr. Pharmacol. Rep. 5, 332–344 (2019).
McCarty, M. F. Proposal for a dietary “phytochemical index”. Med. Hypotheses 63, 813–817 (2004).
Arnold, M. Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia 38, 1–211 (2018).
Sedgwick, P. Convenience sampling. BMJ 347, f6304 (2013).
Estruch, R. et al. Effects of dietary fibre intake on risk factors for cardiovascular disease in subjects at high risk. J. Epidemiol. Community Health 63, 582–588 (2009).
Gelaye, B. et al. Body composition status and the risk of migraine: A meta-analysis. Neurology 88, 1795–1804 (2017).
Ornello, R. et al. Migraine and body mass index categories: A systematic review and meta-analysis of observational studies. J. Headache Pain 16, 1–14 (2015).
Davson, H. & Pollay, M. The turnover of 24Na in the cerebrospinal fluid and its bearing on the blood–brain barrier. J. Physiol. 167, 247 (1963).
Kawano, Y. et al. Sodium and noradrenaline in cerebrospinal fluid and blood in salt-sensitive and non-salt-sensitive essential hypertension. Clin. Exp. Pharmacol. Physiol. 19, 235–241 (1992).
Pirahanchi, Y. & Aeddula, N. R. Physiology, sodium potassium pump (Na+ K+ pump). (2019).
Zhang, J. Basic neural units of the brain: neurons, synapses and action potential. arXiv preprint arXiv:1906.01703 (2019).
Hajihashemi, P., Askari, G., Khorvash, F., Reza Maracy, M. & Nourian, M. The effects of concurrent Coenzyme Q10, L-carnitine supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Cephalalgia 39, 648–654 (2019).
Martynowicz, H. et al. Evaluation of relationship between sleep bruxism and headache impact Test-6 (HIT-6) scores: A Polysomnographic study. Front. Neurol. 10, 487 (2019).
Zandifar, A. et al. Reliability and validity of the Persian HIT‐6 questionnaire in migraine and tension‐type headache. Pain Pract. 14(7):625–631 (2014).
Samani, S. & Joukar, B. A study on the reliability and validity of the short form of the depression anxiety stress scale (DASS-21) (2007).
Henry, J. D. & Crawford, J. R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 44, 227–239 (2005).
Moghaddam, M. B. et al. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: Content and construct validity, factor structure, internal consistency and stability. World Appl. Sci. J. 18, 1073–1080 (2012).
Khorsha, F. et al. Association between diet and migraine characteristics: The role of dietary inflammatory index. Curr. J. Neurol. 19, 67 (2020).
Bakırhan, H., Yıldıran, H. & Uyar Cankay, T. Associations between diet quality, DASH and Mediterranean dietary patterns and migraine characteristics. Nutr. Neurosci. 25, 1–11 (2021).
Arab, A., Khorvash, F., Karimi, E., Hadi, A. & Askari, G. Associations between adherence to Mediterranean dietary pattern and frequency, duration, and severity of migraine headache: A cross-sectional study. Nutr. Neurosci. 26, 1–10 (2021).
Mofrad, M. D., Siassi, F., Guilani, B., Bellissimo, N. & Azadbakht, L. Association of dietary phytochemical index and mental health in women: A cross-sectional study. Br. J. Nutr. 121, 1049–1056 (2019).
Noruzi, Z., Shiraseb, F., Mirzababaei, A. & Mirzaei, K. Association of the dietary phytochemical index with circadian rhythm and mental health in overweight and obese women: A cross-sectional study. Clin. Nutr. ESPEN 48, 393–400 (2022).
Hosseinzadeh, M. Dietary Phytochemical Index and Psychological Disorders in a large sample of Iranian adults: A population-based study. J. Health Popul. Nutr. 42(1), 126 (2020).
Crawford, J., Liu, S. & Tao, F. Gut microbiota and migraine. Neurobiol. Pain 11, 100090. https://doi.org/10.1016/j.ynpai.2022.100090 (2022).
Tuncel, D., Tolun, F. I., Gokce, M., İmrek, S. & Ekerbiçer, H. Oxidative stress in migraine with and without aura. Biol. Trace Elem. Res. 126, 92–97 (2008).
Eren, Y., Dirik, E., Neşelioğlu, S. & Erel, Ö. Oxidative stress and decreased thiol level in patients with migraine: Cross-sectional study. Acta Neurol. Belg. 115, 643–649 (2015).
Zhang, Y.-J. et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20, 21138–21156 (2015).
Acknowledgements
The authors thank the participants for their cooperation in the present study.
Funding
The study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran.
Author information
Authors and Affiliations
Contributions
Conception and Design: A.A, and G.A. Acquisition of Data: A.A, F.K, G.A. Analysis and Interpretation of Data: A.A. Drafting the Manuscript: A.A., SA.T, and A.B. Revising It for Intellectual Content: M.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amani Tirani, S., Balali, A., Kazemi, M. et al. The predictive role of the dietary phytochemical index in relation to the clinical and psychological traits of migraine headaches. Sci Rep 14, 6886 (2024). https://doi.org/10.1038/s41598-024-57536-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57536-7
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.