Abstract
The present work aimed to investigate the effect of salinity in natural habitats in Egypt on the main secondary metabolites of Rosmarinus officinalis L. and Artemisia monosperma L. plants compared to plants grown at normal conditions. Plants grown under salinity were collected from Egyptian Western Coastal region habitats irrigated with underground water. Results showed that salinity increased the essential oil percentage of R. officinalis L. by 52.7% and A. monosperma L by 0.29% in addition to the total phenolics and flavonoids content in dry leaves compared to control plants. GC/MS analysis of rosemary essential oils revealed that salinity decreased the amount of some major oil monoterpenes component as verbenone, with a slight effect on 1,8 cineole and increased Camphor, endo- Boreneol, and linalool in addition to the appearance of new specific components such as Chrysanthenone monoterpene ketone and Caryophyllene sesquiterpene, while, in the case of Artemisia, the GC/MS showed that Artemisia ketone, Camphor, β -phellandrene monoterpenes andα-Bisabolol sesquiterpenewere the major oil components; salinity decreased Camphor and β -phellandrene content and increased artemisia ketone and α-Bisabolol oil content. About 11 new oil constituents were detected such as ( +)-2-Bornanone and Sesquisabinene hydrate. Mineral ions (N, K+, Ca+2, P, and Mg+2) uptake by R. officinalis and A. monosperma decreased in plants grown under salinity, while Na content increased compared to corresponding controls. Results demonstrated that both plants could tolerate the high salinity level in natural Western Coastal region soil which promoted more production of valuable secondary metabolites. The antimicrobial effect of R. officinalis L. and A. monosperma L. leaf methanolic extracts, results showed that R. officinalis extracts had an inhibitory response against all tested gram-positive and negative bacteria, in addition to the yeast (Candida albicans), whereas there was no any inhibitory effect concerning A. monosperma L extract on the tested species.
Similar content being viewed by others
Introduction
Plant growth and development are adversely affected by many environmental stresses such as salinity, drought, flooding, heat, oxidative stress, and heavy metal toxicity. However, salinity stress is one of the major factors limiting agricultural production. According to recent reports, 20% of land worldwide is subjected to salinity stress1. In recent decades, the increase in salinization of soils and ground waters is considered to be a major problem of agriculture in Egypt and is thought to be a result of the Nile’s weak demineralization of the soil. It is also facilitated by the absorption and accumulation of salts in quantities that are toxic to plants2. Soil salinization suppresses the growth of many economic agricultural plants. Moreover, the availability of non-saline water for irrigation is limited, and its quality continues to decline in arid and semi-arid areas. Therefore, saline water usage in agriculture currently seems to be an urgent solution. Cultivation of resistant medicinal and aromatic plants is an option to utilize these soils. Rosemary and Artemisia are wild plant species originating in the Mediterranean region; they constitute an interesting solution to avoid desertification and rapid soil erosion due to their high tolerance to environmental stresses such as salinity.
An important member of the Lamiaceae family, R. officinalis L., is native to the Mediterranean Sea coasts of Egypt. R. officinalis, are cultivated as a medicinal plantin different regions of the world, such as the Mediterranean, Asia, and Latin America3. It is characterized by high content of aromatic phenolic compounds with nutraceutical and pharmaceutical properties, including ant obesity, anti-inflammatory, antidiabetic, diuretic, antithrombotic, antimicrobial, anticancer, hepatoprotective, and antioxidant4. Numerous studies have highlighted that the majority of these biological activities are correlated with the phenolic composition5. Its essential oil is utilized also for a variety of purposes, including aromatherapy6,7, pest control products, and flavoring and fragrance8,9. 1,8-cineole, camphor, -pinene, -pinene, and borneol are the active components of rosemary essential oils10.
Artemisia monosperma L. is a member of the Asteraceae family. In Chinese herbal medicine (CHM), it is also referred to as Sweet Annie, wormwood, or Qing Hao, and it is used for a variety of ailments including fever and malaria. A. monosperma has been found to have significant bioactive components like artemisinin, endoperoxide sesquiterpene lactone, and essential oil (EO)11. Several Artemisia species grow wildly or as cultivated plants for their use as medication and as a herbal tea preparation in the Mediterranean region12. The leaves of Artemisia have been shown in prior studies to possess some biological activities, including antifungal, antimicrobial, antimalarial, antibacterial, anti-inflammatory, anti-tumor, and antiallergenic qualities13,14.
Aromatic plants respond to stress conditions through different physiological defense mechanisms by secondary metabolites production, which are toxic to insects, micro-organisms and/or herbivore repellent. Essential oil percentage and composition are affected by a range of environmental factors including climate, pollution, and exposure to pests or diseases15. The stress factor resulted in different changes in the essential oil (EO) composition of some aromatic plants such as rosemary, Sweet Annie, mint, oregano, and basil16.
Although salinity is considered an abiotic stress with a negative effect on most plants, it can also function as a promotive and driving force on plant secondary metabolites. The objective of the current study is to investigate the effect of salinity on growth and some secondary metabolites content of two important salt-tolerant medicinal plants R. officinalis L. and A. monosperma L.with high economical valuable secondary metabolites.
Materials and methods
R. officinalis L. and A. monosperma L. plants grown under salinity were collected from Egyptian Western Coastal region 10m Elevation (30 ͦ 51′ 5″ N, 29 ͦ 18′ 25″ E) in which underground water was used for irrigation and the control specimens were collected from commercial farm in Cairo where Nile water was used for irrigation. Soil samples were collected from the two habitats for analysis (Table1).
For secondary metabolites detection, aerial parts of R. officinalis L. and Artemisia monosperma L. plants were collected, air dried to complete dryness.
Leaves essential oil was extracted using hydrodistillator as traditional method for extraction of bioactive compounds, mainly essential oils from plants17. Mohammed et al.18, revealed that the best volatile oil yield by the hydrodistillation procedure of oil extractions. Hydro distillation was carried out for 2h and repeated with total 3 replicates for each. Finally, the essential oils were stored at 4 °C for GC/MS analysis, which was carried out at the Agriculture Research Centre, Cairo, Egypt, as follows.
Gas chromatography–mass spectrometry analysis
(GC–MS) system (Agilent Technologies) was performed using gas chromatograph (7890B) and mass spectrometer detector (5977A) Samples were diluted with hexane (1:19, v/v). The GC was equipped with HP-5MS column (30 m × 0.25 mm internal diameter and 0.25 μm film thickness). Analyses were carried out using hydrogen as the carrier gas at a flow rate of 1.0 ml/min at a split 1:20 of, injection volume of 1 µl and the following temperature program: 40 °C for 1 min; rising at 4 °C /min to 150 °C and held for 6 min; rising at 4 °C/min to 210 °C and held for 1 min. The injector and detector were held at 280 °C and 220 °C, respectively. Mass spectra were obtained by electron ionization (EI) at 70 eV; using a spectral range of m/z 50–550 and solvent delay 4 min. Identification of different constituents was determined by comparing the spectrum fragmentation pattern with those stored in Wiley and NIST Mass Spectral Library data.
Total phenolics and flavonoids estimation were extracted by adding 0.1 g powdered air-dried leaves to 25 ml methanol 80% at 60 °C for 2 days with continuous stirring. After filtration the extract was used for the estimation of total phenolics and flavonoids.
Total phenolic content was determined according to Kujala et al.19, using Folin–Ciocalteu reagent and gallic acid as a standard. Briefly, 0.5 ml of filtered extract was added to 2.5 mL Folin-Ciocalteu’s reagent (diluted with ethanol 1:10), 2 mL of Na2CO3 (7.5%) and mixed well. After 15 min incubation at room temperature, the absorbance of mixtures was recorded by Jenway 6405 UV–Vis spectrophotometer at 765 nm. The total phenolic content was expressed as mg gallic acid equivalent (GAE) per gram of extract (mg GAE/g dry weight of extract).
Total flavonoids content was determined according to Zhishen et al.20 and Paciolla et al.21, where 0.5 ml of the extract was added to 150 µl of 5% sodium nitrate and allowed to stand for 6 min. Then 150 µl of 10% Aluminum chloride solution was added and allowed to stand for 6 min after which 200 µl of 1 M sodium hydroxide was added then the mixture was completed to 5 ml with methanol and mixed well. After incubation for 15 min, the absorbance was measured spectrophotometrically against a blank at 510 nm. The total flavonoids content was expressed in milligrams of quercetin equivalents (QE) per gram extract (mg QE/g).
Antimicrobial activity
Methanolic extracts were prepared from air dried leaves, 4g powdered leaves were extracted by 200 ml methanol 80% at 60 °C for 2 days with continuous stirring then the extracts were allowed to dry at 40 °C until complete dryness and redissolved w/v in methanol for preparation of known concentrations.
Tested microorganisms
Antibacterial activity was estimated in vitro against six pathogenic bacterial strains including Staphylococcus aureus (ATCC 6538), Clostridium perfringens (ATCC 13,124) and Micrococcus leutus, as Gram positive stains while Escherichia coli (ATCC 5739), Shigella sonnei (ATCC 29,930) and Salmonella typhimurium (ATCC 14,028) as Gram negative stains. In addition, the pathogenic yeast Candida albicans was used.
Well diffusion assay
R. officinalis L. and A. monosperma’s antibacterial activity was in vitro investigated using the agar well diffusion method22. 100 µl of 24 h old microbial culture of the tested strains with a concentration equivalent to 0.5 McFarland (1.5 × 108 CFU ml–1) were streaked on the surface of the agar plates (nutrient agar media for bacteria and potato dextrose agar media for yeast). Further, under aseptic conditions, 100 µl of either R. officinalis L. or A. monosperma extracts were inoculated into the 0.8 cm wells at concentration 25 mg ml-1 in methanol. Clindamycin (2.0 mg/disk), chloramphenicol (30 µg/disk) and nystatin (100,000 IU/ml) were used as positive controls, while methanol was used as a negative control. The incubation was carried out at 35–37 °C for 24–48 h to allow the growth of bacteria and yeast. The inhibition zone formation around the wells were recorded and measured in mm. The experiment was carried out in triplicate and the means of inhibition zones were measured in millimeters ± standard deviation.
Results
In the present study, a great decrease in shoot vegetative growth of R. officinalis and A. monosperma grown under salinity in the Egyptian Western Coastal region was noticed (Fig. 1). Data illustrated in Table 2 shows that under salinity stress, the uptake of N, Mg2+, Ca2+, K+, and P decreased while Na+ ion concentration increased in both R. officinalis L. and A.monosperma L. plants significantly compared to corresponding controls. Plant shoot Na+ concentration rises as salinity rises, while shoot K+ and the K+/Na+ ratio decreases.
Salinity increased essential oil (EO) percent on a dry weight basis of R. officinalis and A. monosperma plants more than their relative controls (Fig. 2). The study of mass spectra identified 15 compounds in the essential oils of control rosemary plants while 18 compounds were detected in salt-stressed plants. According to GC/MS analysis of essential oils from dry aerial parts of R. officinalis, the main constituents of R. officinalis oil were found to be Verbenone, α-pinene, 1,8cineole, and Camphor in control plants, whereas in the essential oil of salt-stressed plants, Verbenone, Camphor, endo-Borneol and 1,8cineole were the main components. Salinity decreased Verbenone from 39.48 to 26.15%, α-pinene from 15.52 to 4.16%, with slight effect on 1,8cineole while increased Camphor, endo- Boreneol and linalool from 8.57%, 4.39%, 2.12% to 22.2%, 16.8% and 6.31% respectively in addition to the appearance of new specific components which were Chrysanthenone and Caryophyllene under salinity stress (see Table 3). Simultaneously, the analysis of Artemisia monosperma L essential oil showed that ketone, Camphor, α-Bisabolol, and β -phellandrene were the main oil components. Salinity decreased Camphor and β -phellandrene content yet increased ketone and α-Bisabolol content in Artemisia oil at the same time. About 11 new oil constituents were detected such as ( +)-2-Bornanone and Sesquisabinene hydrate (see Table 3). In comparison to rosemary, A. monosperma plants often contain more phenolics and flavonoids (see Table 4). Salinity increased the total leaves content of phenolics (288.63 and 85.93 mg g–1) and flavonoids (61.6 and 10.5 mg g–1) in both R. officinalis L. and A. monosperma L. plants, respectively, compared to their corresponding controls phenolics (206.25 and 66.60 mg g–1) and flavonoids (40.5 and 9.23 mg g–1).
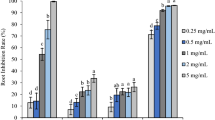
The findings of the antimicrobial assay conducted on R. officinalis L. and A. monosperma L revealed that only the methanolic extracts of R. officinalis L. exhibited inhibitory effects against the tested microorganisms specially the Gram-positive bacteria and the pathogenic yeast. The highest activity was observed against Micrococcus luteus followed by Staphylococcus aureus, and Clostridium perfringens, with recorded inhibition zones of 37 mm, 30 mm, and 16 mm, respectively, using the treated R. officinalis L.extract. These values were higher than those recorded for the control extract, which exhibited inhibition zones of 35 mm, 24 mm, and 13 mm respectively. On the other hand, resistance response was shown by all the tested Gram-negative bacteria. Furthermore, the antimicrobial assay revealed that the treated R. officinalis L extract exhibited a stronger inhibitory response against the pathogenic yeast Candida albicans compared to the control extract with inhibition zones of 18 mm and 14 mm, respectively (see Table 5 & Fig. 3).
Discussion
Arid and semi-arid regions face a high accumulation of salt in soil and underground water. Salinity stress is one of the abiotic stresses that harm agriculture by decreasing plant vegetative growth and productivity. Salinity hampers photosynthetic machinery, transpiration, and gaseous exchange by decreasing the content of chlorophyll and carotenoids, distorting chloroplast ultrastructure and PSII system, and reducing stomatal conductance23.
Salinity leads to an extensive accumulation of ions (Na+ and Cl−) and inhibits K+ and Ca2+ uptake resulting in ionic imbalance24 which enhances reactive oxygen species (ROS) production in plant cells and creates oxidative stress causing uncontrolled damage to cell macromolecules including membranes lipid peroxidation DNA, and protein damage25. By upsetting the equilibrium of nutrient intake that the plant system maintains, salinity directly affects plant growth. The most significantly impacted are nutrient availability, partitioning, and transport due to the competition between the nutritional ions K+, Ca2+, and NO3- and the Na+ and Cl-ions. The excess of Na+ and Cl– ions directly causes these ionic imbalances impacting the biophysical and/or metabolic elements of the plant system. Many plants have shown an increase in Na+ and Cl– during salt stress, while fennel, Trachyspermum ammi, peppermint, lemon verbena, Matricaria recutita, and Achillea fragratissima showed a decline in N, P, K+, Ca2+, and Mg2+ levels26,27,28.
Ali and Hassan29 stated that N, P, Ca2+, and K+ percentages were influenced by different salt concentrations in the leaves of Simmondsia chinensis (jojoba). Salt concentration over 17.2 mM drastically lowered the mentioned elements' uptake and content in the plant. Plant responds to saline conditions in terms of various strategies and approaches such as ion homeostasis and compartmentalization, transport of ions, osmotic adaptation, stimulation of antioxidant machinery, and biosynthesis of secondary metabolites30.
Essential oil is one of the most valuable secondary metabolites in aromatic plants. The essential oil (%) extracted from chamomile, lemon verbena, and peppermint increased under salinity stress levels as reported by Ref.31. Gharib et al.32 also reported a 32.33% increase in the rosemary oil percentage under salinity stress (100 mM NaCl) in irrigation water. Stimulation of essential oil production under a moderate degree of salinity could be due to a higher oil gland density and an increase in the absolute number of glands produced prior to leaf emergence because of a stress-induced reduction in leaf area33 and secondary metabolites synthesis and accumulation as self-defense components to cope with stressful conditions32. The increase in the essential oil content in some of the salt-stressed plants might be also attributed to a decline in the primary metabolites, causing intermediary products to become available for secondary metabolite synthesis. Despite the bulky structures, caryophyllene easily penetrates cell membranes34, which determines their bioavailability and a variety of biological properties including antioxidant and anti-inflammatory properties. R. officinalis essential oil also exhibits free radical scavenging activity and excellent hepatoprotective properties by limiting the lipid peroxidation. The 1,8-cineole from R. officinalis essential oil is a lipid-soluble compound that facilitates passage across the blood brain barrier, producing neuronal effect on the receptor sites or impacting enzyme activity of neurons35. Mohammed et al.18, stated that the major rosemary oil constituents were 1,8-cineole, camphor, and camphene, these three major constituents is about 50.9% of the total oil compositions. As the time of harvesting also affects the oil content he also reported that the yields of camphene, β-pinene, α-terpineol, bornyl acetate, β-caryophyllene, and d-germacrene increased in the two- and three-weeks dried herbs-based volatile oils as compared to the fresh and one-week dried herbs-based oils. Sarmoum et al.36 reported that 1,8cineole content in rosemary essential oil decreased up to 50% with increasing NaCl concentrations (from 25 to 200 mM). This agrees with Langroudi et al.37 who recorded those different levels of salinity decreased 1,8-cineole, borneol, camphor, and α-pinene the main constituents of R. officinalis essential oils in Iran. Salinity decreased monoterpene hydrocarbons including very low α-pinene and camphene, whereas oxygenated monoterpenes recorded the highest value including high camphor and borneol yet resulted in lower values of 1,8-cineol as compared with control rosemary plants32. The phytochemical analysis of Artemisia judaica essential oil revealed the dominance of the highly active antioxidant volatile compounds, oxygenated monoterpenes, and cinnamic acid derivatives in the essential oil constituents of the plant. Such classes of compounds were reflected in the in vitro and in vivo potential antioxidant activity of essential oil14. Artemisia monosperma L. plants adapted to salinity produced higher values of EO (%) and EO constituents [α-pinene, camphene, coumarin, and dihydro-epi-deoxyarteannuin B38,39. To reduce damage, plants have evolved complex antioxidative systems, involving antioxidant enzymes and secondary metabolites like phenolic compounds40. Mehrizi et al.41 and Abd EL Azim et al.27 reported an increase in phenolics and flavonoids under salinity from 50 mM to below 100 mM in R. officinalis and Achillea fragratissima.
Free radicals can be effectively eliminated by flavonoids and other phenolics42,43 improving plant tolerance to salinity stress. Antioxidants inhibit the oxidation of lipids, proteins, and DNA; hence, tolerant plants tend to increase the production of phenolics under salinity stress. Phenols increased in Achillea fragratissima with increased salinity levels27 the same result was reported in R. officinalis44. Salinity usually causes a reduction in growth providing an additional carbon skeleton for phenols indicating that salinity is a more effective factor in increasing phenols i.e., the natural antioxidants. An increase in the total phenols content of tolerant genotypes could be an adaptive mechanism for preventing damage during stress45. The GC-Mass analysis results showed that α-Terpineol and Terpinen-4-ol, represented major constituents of the R. officinalis as similarly reported by Ref.46. These compounds are known for their antibacterial activity due to their lipophilic nature which causes the disruption of the lipopolysaccharide in the bacterial membrane, causing cell disruption47. Interestingly, the need for new antimicrobial agents has become increasingly crucial due to the rise of microbial resistance. Fortunately, the present results showed the promising antimicrobial activity of the methanolic R. officinalis extract specially against Micrococcus luteus, Staphylococcus aureus, Clostridium perfringens and Candida albicans.
Manilal et al.48 investigated the antibacterial activity of R. officinalis extract against multidrug-resistant clinical isolates and meat-borne pathogens, and reported that Salmonella sp. and S. aureus were the most sensitive clinical isolates.
According to Gomez-Estaca et al.49, rosemary oil prevented the growth of typical food bacteria that cause food to spoil. Burt50 has demonstrated the rosemary essential oil's antibacterial efficacy against S. aureus, Bacillus cereus, and E. coli. Additionally, Sirocchi et al.'s research51 demonstrated that Brochothrix thermosphacta and Enterobacteriaceae growth was suppressed by rosemary essential oil. Moreover, Jafari-Sales & Hossein-Nezhad (2020) reported the antibacterial activity of the methanolic R. officinalis extract against S. aureus, B. cereus, E. coli and Pseudomonas aeruginosa. Also, Mattazi et al.52 reported the antibacterial activity of R. officinalis EO against Micrococcus luteus.
R. officinalis extract components act in synergy and interact with the bacterial cell membrane, affecting the generation of fatty acids, genetic material, and nutrients as well as the transfer of electrons, cellular component leakage, and fatty acid transport. Additionally, it caused a protein interaction with the membrane that resulted in the loss of membrane structure and functionality53.
The results also showed a promising antifungal activity of the rosemary extract against Candida albicans. This may be due to the presence of various compounds in R. officinalis extract which had antifungal properties such as 1,8 cineole and camphor as well as phenolics and flavonoids54. Similarly, Saeidi et al.55 reported the inhibitory effect of R. officinalis extract in concentration of 100 µg/mL on Candida albicans.According to Zaouali et al.’s findings, the effectiveness of R. officinalis bioactive compounds is related to the combined action of the various minor components present in its volatile and nonvolatile fractions and should not be associated with the action of any particular component56.
Conclusion
In conclusion, aromatic plants are a safe and rich source of natural secondary metabolites with many reported biological activities. R. officinalis and A. monosperma can be planted in moderate saline soil where stressed plants tend to increase essential oil percentage and total phenolics content compensating for the decrease in growth. This may help in making use of many salt-affected arid and semiarid land areas. The results showed that rosemary methanolic extract could be a promising alternative antimicrobial source against various pathogenic bacteria and yeast, helping in drug resistance issues which is a global health problem. Further optimization studies could be performed to enhance the antimicrobial potency and for the best exploitation of R. officinalis.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Experimental research and field studies on plants, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation. Identification of the plant material used in the study was performed by Prof. Dr. Mohamed Soliman and Prof. Dr. Loutfy Mohsen at Faculty of Science- Helwan University – Botany and Microbiology department. Some of collected samples was kept in Helwan University herbarium (vouchers no 00000897 and 00000999).
References
Langroudi, M. E., Sedaghathoor, S. & Bidarigh, S. Effect of different salinity levels on the composition of rosemary (Rosmarinus officinalis) essential oils. Am. Eur. J. Agric. Environ. Sci. 13, 68–71 (2013).
Conde-Hernández, L., Espinosa-Victoria, J., Trejo, A. & Guerrero-Beltran, J. CO2-Supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. https://doi.org/10.1016/j.jfoodeng.2016.12.022 (2016).
Fernández-Ochoa, Á. et al. Phenolic compounds in Rosemary as potential source of bioactive compounds against colorectal cancer: In situ absorption and metabolism study. J. Funct. Foods 33, 202–210. https://doi.org/10.1016/j.jff.2017.03.046 (2017).
Kol, O., Walia, S. & Dhaliwal, G. S. Essential oils as green pesticides: Potential and constraints. Biopest. Int. 4, 63–84 (2008).
Borrás Linares, D., Arráez-Román, M., Herrero, E., Ibáñez, A. & Segura-Carretero, A.-G. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time. J. Chromatogr. A 1218(42), 7682–7690 (2011).
Kalkhan, M.A., Stohlgren, T.J., Chong, G.W., et al. A predictive spatial model of plant diversity: Integration of remotely sensed data, GIS and spatial statistics. In Proc. 8th Biennial Remote Sensing Application (Conf. RS, 2000), Albuquerque, pp. 1–8 (2000).
Almela, L., Sánchez-Muñoz, B., Fernández-López, J. A., Roca, M. J. & Rabe, V. Liquid chromatographic-mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J. Chrom. A 1120, 221–229 (2006).
Mizrahi, I., Juarez, M. A. & Bandoni, A. L. The essential oil of Rosmarinus officinalis growing in Argentina. J. Essent. Oil Res. 3, 11–15 (1991).
Lee, C. J. et al. The correlation between skin-care effects and phytochemical contents in Lamiaceae plants. Food Chem. 124, 833–841 (2011).
Castilho, P. C., Gouveia, S. C. & Rodrigues, A. I. Quantification of artemisinin in Artemisia annua extracts by1H-NMR. Phytochem. Anal. 19, 329–334 (2008).
Bora, K. S. & Sharma, A. The genus Artemisia: Acomprehensive review. Pharm. Biol. 49, 101–109 (2011).
Vouillamoz, J. F., Carlen, C., Taglialatela-Scafati, O., Pollastro, F. & Appendino, G. The génépi Artemisia species. Ethnopharmacology, cultivation, phytochemistry, and bioactivity. Fitoterapia 106, 231–241 (2015).
Cavar, S., Maksimovic, M., Vidic, D. & Paric, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 37, 479–485 (2012).
Mohammed, H. A. et al. Bio-evaluation of the wound healing activity of Artemisia judaica L. as part of the plant’s use in traditional medicine; phytochemical, antioxidant, anti-inflammatory, and antibiofilm properties of the plant’s essential oils. Antioxidants 11, 332. https://doi.org/10.3390/antiox11020332 (2022).
Badawy, E.-S.M., Khalid, K. A., Heikal, A.A.-E.M. & Nagdy, M. M. Effect of salinity stress and soil types on growth, photosynthetic pigments and essential oil of Artemisia annua L. Asian J. Crop Sci. 10, 40–47 (2018).
Khadhri, A., Neffati, M., Smiti, S., Nogueira, J. M. F. & Araujo, M. E. M. Influence of salt stress on essential oil yield and composition of lemon grass (Cymbopogon schoenanthus L. Spreng. ssp. Laniger (Hook) Maire et Weil). Nat. Prod. Res. Former. Nat. Prod. Lett. 25, 108–177 (2011).
Azmir, J. et al. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 117(4), 426–436. https://doi.org/10.1016/J.JFOODENG.2013.01.014 (2013).
Mohammed, H. A. et al. Drying induced impact on composition and oil quality of rosemary herb, Rosmarinus Officinalis Linn. Molecules 25, 2830. https://doi.org/10.3390/molecules25122830 (2020).
Kujala, T. S., Loponen, J. M., Klika, K. D. & Pihlaja, K. Phenolics and beta cyanins in red beet root (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 48, 5338–5342 (2000).
Zhishen, J., Mengcheng, T. & Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559 (1999).
Paciolla, C., De Leonardis, S. & Dipierro, S. Effects of selenite and selenate on the antioxidant systems in Senecio scandens L. Plant Biosyst. 145, 253–259 (2011).
Balakumar, R. et al. Antibacterial and antifungal activity of fruit bodies of Phellinus mushroom extract. Int. J. Biosci. 1, 72–77 (2011).
Pan, T. et al. Non-stomatal limitation of photosynthesis by soil 779 salinity. Crit. Rev. Environ. Sci. Technol. https://doi.org/10.1080/10643389.2020.1735231 (2020).
Isayenkov, S. V. & Maathuis, F. J. M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 10, 80. https://doi.org/10.3389/fpls.2019.00080 (2019).
El Ghazali, G. Suaeda vermiculata Forssk. ex J.F. Gmel.: Structural characteristics and adaptations to salinity and drought: A review. Int. J. Sci. 9, 28–33. https://doi.org/10.18483/ijSci.2268 (2020).
Abd El-Wahab, M. A. The efficiency of using saline and fresh water irrigation as alternating methods of irrigation on the productivity of Foeniculum vulgare Mill subsp. vulgare var. vulgare under North Sinai conditions. Res. J. Agric. Biol. Sci. 2, 571–577 (2006).
Abd El-Azim, W. M. & Ahmed, S. T. Effect of salinity and cutting date on growth and chemical constituents of Achillea fragratissima Forssk, under Ras Sudr conditions. Res. J. Agric. Biol. Sci. 5, 1121–1129 (2009).
Queslati, S. et al. Physiological and antioxidant responses of Mentha pulegium (Pennyroyal) to salt stress. Acta Physiol. Plant. 32, 289–296 (2010).
Ali, E. F. & Hassan, F. A. S. Salt effects on growth and leaf chemical constituents of Simmondsia chinensis (Link) Schneider. J. Med. Plant Stud. 1, 22–34 (2013).
de Freitas, P. A. F. et al. Salt acclimation in sorghum plants by exogenous proline: Physiological and biochemical changes and regulation of proline metabolism. Plant Cell Rep. 38, 403–416. https://doi.org/10.1007/s00299-019-02382-5 (2019).
Tabatabaie, J. & Nazari, J. Influence of nutrient concentrations and NaCl salinity on the growth, photosynthesis and essential oil content of peppermint and lemon verbena. Turk. J. Agric. For. 31, 245–253 (2007).
Gharib, F., Zeid, I., Salem, O. & Zakaria, E. Effects of Sargassum latifolium extract on growth, oil content and enzymatic activities of rosemary plants under salinity stress. Life Sci. J. 11, 933–945 (2014).
Ezz El-Din, A. A., Aziz, E. E., Hendawy, S. F. & Omer, E. A. Response of Thymus vulgaris L. to salt stress and alar (B9) in newly reclaimed soil. J. Appl. Sci. Res. 5(12), 2165–2170 (2009).
Sarpietro, M. G., Di Sotto, A., Accolla, M. L. & Castelli, F. Interaction of β-caryophyllene and β-caryophyllene oxide with phospholipid bilayers: Diferential scanning calorimetry study. Thermochim. Acta 600, 28–34. https://doi.org/10.1016/j.tca.2014.11.029 (2015).
Kumar, V., Marković, T., Emerald, M. & Dey, A. Herbs: Composition and dietary importance. In Encyclopedia of Food and Health (eds Caballero, B. et al.) 332–337 (Academic Press, 2016). https://doi.org/10.1016/B978-0-12-384947-2.00376-7.
Sarmoum, R. et al. Effect of salinity and water stress on the essential oil components of rosemary (Rosmarinus officinalis L.). Agronomy 9(5), 214. https://doi.org/10.3390/agronomy9050214 (2019).
Langroudi, M. E., Sedaghathoor, S. & Bidarigh, S. Effect of different salinity levels on the composition of rosemary (Rosmarinus officinalis) essential oils. Am.-Eurasian J. Agric. Environ. Sci. 13(1), 68–71 (2013).
Khalid, A. K. & Shedeed, M. R. GC-MS analyses of black cumin essential oil produces with sodium chloride. Int. Food Res. J. 23, 832–836 (2016).
Ahmed, A. M. A., Talaat, I. M. & Khalid, A. K. Soil moisture and glutamic acid affect yield, volatile oil and proline contents of oregano herb Origanum vulgare L.). Int. J. Bot. 13, 43–51 (2017).
Posmyk, M. M., Kontek, R. & Janas, K. M. Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol. Environ. Saf. 72, 596–602 (2009).
Mehrizi, M. H., Shariatmadari, H., Khoshgoftarmanesh, A. H. & Dehghani, F. Copper effects on growth, lipid peroxidation, and total phenolic content of Rosemary leaves under salinity stress. J. Agric. Sci. Tech. 14, 205–212 (2012).
Wang, Y. & Nii, N. Changes in chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J. Hortic. Sci. Biotechnol. 75, 623–627 (2000).
Rice-Evans, C. A., Miller, N. J. & Paganaga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 2, 152–159 (1997).
Mohammad Khani, N. Effects of salinity on phenolic compounds in tolerant and sensitive grapes. Poljopr. Sumar. https://doi.org/10.17707/AgricultForest.64.2.05 (2018).
Realini, C. E. & Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 98, 404–419 (2014).
Ultee, A., Bennik, M. H. J. & Moezelaar, R. the phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus Cereus. Appl. Environ. Microbiol. 68, 1561–1568 (2002).
Hussain, A. I. et al. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 41(4), 1070–8. https://doi.org/10.1590/S1517-838220100004000027 (2010).
Manilal, A. et al. Antibacterial activity of Rosmarinus officinalis against multidrug-resistant clinical isolates and meat-borne pathogens. Evid.-Based Complement. Altern. Med. 2021, 1–10 (2021).
Gómez-Estaca, J., López de Lacey, A., López-Caballero, M. E., Gómez-Guillén, M. C. & Montero, P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 27, 889–896. https://doi.org/10.1016/j.fm.2010.05.012 (2010).
Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 94, 223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022 (2004).
Sirocchi, V. et al. Biogenic amines as freshness index of meat wrapped in a new active packaging system formulated with essential oils of Rosmarinus officinalis. Int. J. Food Sci. Nutr. 64, 921–928. https://doi.org/10.3109/09637486.2013.809706 (2013).
Mattazi, N. E. Z. H. A., Farah, A. B. D. E. L. L. A. H., Fadil, M. O. U. H. C. I. N. E., Chraibi, M. A. R. W. A. & Benbrahim, K. F. Essential oils analysis and antibacterial activity of the leaves of Rosmarinus officinalis, Salvia officinalis and Mentha piperita cultivated in Agadir (Morocco). Int. J. Pharm. Pharm. Sci. 7(9), 73–79 (2015).
Fung, D. Y. C., Taylor, S. & Kahan, J. Effect of butylated hydroxyanisole (BHA) and buthylated hydroxytoluebe (BHT) on growth and aflatoxin production of Aspergillus flavus. J. Food Saf. 1, 39–51. https://doi.org/10.1111/j.1745-4565.1977.tb00258.x (1977).
Meccatti, V. M. et al. Rosmarinus officinalis L. (rosemary) extract has antibiofilm effect similar to the antifungal nystatin on Candida samples. Anais Acad. Bras. Ciênc. 93, e20190366 (2021).
Saeidi, S., Forgani, F., Javadian, F. & Javadian, E. Effects of Rosmarinus officinalis plant extract on Trichomonas vaginalis parasites and Candida albicans under laboratory conditions: an experimental study. Gene Cell Tissue https://doi.org/10.5812/gct.92867 (2019).
Tornuk, F. et al. Efficacy of various plant hydrosols as natural food sanitizers in reducing Escherichia coli O157:H7 and Salmonella typhimurium on fresh cut carrots and apples. Int. J. Food Microbiol. 148, 30–35. https://doi.org/10.1016/j.ijfoodmicro.2011.04.022 (2011).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All the authors participated in practical work, writing and revising manuscript. Prof Dr. Soliman suggested the research point, collected plants, soil and water samples and participated in collected samples analysis in addition to writing and revising the manuscript. Dr. Marwa participated in essential oil analysis, plant, soil, and water chemical analysis and participated in writing and revising the manuscript. Dr. Eman participated in essential oil extraction, extracts preparation, evaluation of secondary metabolites in plants extracts and participated in writing and revising manuscript. Dr. Yasmin was responsible for antimicrobial evaluation of plants extracts and participated in writing and revising the manuscript. All authors have read and agreed on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soliman, M.M., Elsaba, Y.M., Soliman, M.S.A. et al. Composition and antimicrobial activity of Rosmarinus officinalis L. and Artemisia monosperma L. leaf essential oils and methanolic extracts from plants grown in normal and saline habitats in Egypt. Sci Rep 14, 7342 (2024). https://doi.org/10.1038/s41598-024-57301-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57301-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.