Abstract
Ehlers–Danlos syndrome spondylodysplastic type 3 (EDSSPD3, OMIM 612350) is an inherited recessive connective tissue disorder that is caused by loss of function of SLC39A13/ZIP13, a zinc transporter belonging to the Slc39a/ZIP family. We previously reported that patients with EDSSPD3 harboring a homozygous loss of function mutation (c.221G > A, p.G64D) in ZIP13 exon 2 (ZIP13G64D) suffer from impaired development of bone and connective tissues, and muscular hypotonia. However, whether ZIP13 participates in the early differentiation of these cell types remains unclear. In the present study, we investigated the role of ZIP13 in myogenic differentiation using a murine myoblast cell line (C2C12) as well as patient-derived induced pluripotent stem cells (iPSCs). We found that ZIP13 gene expression was upregulated by myogenic stimulation in C2C12 cells, and its knockdown disrupted myotubular differentiation. Myocytes differentiated from iPSCs derived from patients with EDSSPD3 (EDSSPD3-iPSCs) also exhibited incomplete myogenic differentiation. Such phenotypic abnormalities of EDSSPD3-iPSC-derived myocytes were corrected by genomic editing of the pathogenic ZIP13G64D mutation. Collectively, our findings suggest the possible involvement of ZIP13 in myogenic differentiation, and that EDSSPD3-iPSCs established herein may be a promising tool to study the molecular basis underlying the clinical features caused by loss of ZIP13 function.
Similar content being viewed by others
Introduction
Zinc is an essential trace element and its homeostasis is tightly regulated mainly by two types of zinc transporters: Solute carrier 39A (SLC39A)/zrt and irt-like proteins (ZIPs) and solute carrier 30A (SLC30A)/zinc transporters (ZnTs)1. SLC39As/ZIPs transport zinc into the cytosol from the extracellular environment or intracellular organelles, while SLC30As/ZnTs transport zinc in the opposite direction1. Recent studies have uncovered that these transporters are involved in many cellular and signaling events, such as growth factor-, cytokine-, and transcription factor-mediated signaling2 and their dysfunction has been noted in several human diseases2, indicating that zinc transporters could be considered potential therapeutic targets3.
SLC39A13/ZIP13 is a representative ZIP transporter that is associated with human diseases. Namely, Ehlers–Danlos syndrome spondylodysplastic type 3 (EDSSPD3, OMIM 612350)4,5,6,7 is caused by homozygous mutations in SLC39A13/ZIP13 gene and is characterized by severe connective tissue impairments, such as short stature, skeletal dysplasia, spine deformity, fragile skin, and hypodontia. This is a relatively rare disease and approximately ten individual patients have been reported so far6,7. We previously reported two siblings with EDSSPD3 harboring a homozygous point mutation (c.221G > A, p.G64D) in exon 2 of ZIP13 (ZIP13G64D)4, whose clinical features highly correlated with the phenotype of Zip13-deficient (KO) mice4. ZIP13G64D protein is readily degraded via the valosin-containing protein-linked ubiquitin–proteasome pathway, which is attributed as the main cause of EDSSPD38; i.e., loss of functional ZIP13 protein is the molecular mechanism underlying the pathogenesis of EDSSPD3. Patients with EDSSPD3 suffer from muscular symptoms including muscular hypotonia, reduction of muscular strength4 and myopathy9, a wide spectrum of muscular diseases comprising muscle weakness, muscle hypotonia, atrophies and/or myalgias, muscle stiffness, and cramps10. Myopathies are thought to be caused by perturbations in myogenesis as a result of functional abrogation of myogenic regulatory factors (MRFs) such as myogenic differentiation 1 (MyoD)11, myogenin12, and myogenic factor 5 (Myf5)13. Although the molecular mechanism through which ZIP13 regulates myogenesis remains to be elucidated, it can be hypothesized that ZIP13 regulates the expression and/or functions of MRFs during myogenic differentiation.
Human induced pluripotent stem cells (hiPSCs) are well-recognized as beneficial tools that present unique research opportunities not only in regenerative medicine but also in drug discovery as an approach to treat intractable and rare diseases14. Consistently, hiPSCs have been used for treatment of Duchenne muscular dystrophy (DMD)15,16,17, Miyoshi myopathy (MM)18,19, and Pompe disease20. In vitro disease models of skeletal muscles using iPSCs have been established by ectopic introduction of MyoD21, and these patient-derived iPSCs can be further differentiated into cells that exhibit clinical features of diseases, such as impairment of dystrophin expression, Ca2+ influx, and contractile performance15,17,19; thus, patient-derived iPSCs can be used to establish in vitro disease models. However, to the best of our knowledge, there are no reports on the generation and application of patient-derived iPSCs for EDSSPD3. Therefore, establishment of iPSCs derived from patients with EDSSPD3 (EDSSPD3-iPSCs) will provide opportunities to elucidate the pathogenic mechanisms underlying the loss of ZIP13 functions, and can potentially have clinical and pharmaceutical applications.
Herein, we report that ZIP13 is involved in myogenic differentiation. We found that ZIP13 gene expression was upregulated during myogenic differentiation and its knockdown resulted in failed myogenic differentiation in C2C12 mouse myoblast cells. Additionally, we established EDSSPD3-iPSCs that successfully differentiated into myoblasts, and further demonstrated that the EDSSPD3-iPSC-derived myocytes exhibited attenuated expression of myogenic differentiation markers, which was restored by genetic editing to correct the pathogenic mutation, indicating the possible involvement of ZIP13 in myogenic differentiation. Therefore, our findings suggest that ZIP13 may be a potential novel MRF that regulates normal myogenesis. Further, EDSSPD3-iPSCs have applications not only in the elucidation of mechanisms underlying ZIP13-mediated biological events, but also in regenerative studies and pharmaceutical applications as a treatment approach for EDSSPD3.
Methods
Culture and myogenic differentiation of C2C12 cells
C2C12 cells were cultured and maintained in Dulbecco’s modified Eagle complete medium (DMEM; WAKO, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and antibiotic–antimycotic mixture containing 100 units/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B (Nacalai Tesque, Kyoto, Japan) at 37 °C in the presence of 5% CO2. Myogenic differentiation of C2C12 cells was performed as described previously22. A schematic depiction of the myogenic differentiation protocol of C2C12 cells is shown in Fig. 1a. Briefly, C2C12 cells were seeded at a density of 1 × 105 cells/dish in 6 cm-dish with 4 mL of DMEM (WAKO) supplemented with 10% FBS (Thermo Fisher Scientific) and the antibiotic–antimycotic mixture at 37 °C in the presence of 5% CO2. The medium was replaced the next day with DMEM containing 2% horse serum (Sigma-Aldrich, St. Louis, MO, USA) and the antibiotic–antimycotic mixture. After 5 days of incubation, myogenic differentiation was evaluated by microscopic observation based on the formation of myotubes.
ZIP13 is required for myogenic differentiation of C2C12 cells. (a) Schematic of the protocol for horse serum (HS)-induced myogenic differentiation of C2C12 cells. C2C12 cells were cultured for 24 h in Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS); thereafter, the medium was changed to DMEM containing 2% HS and cultured for 5 days. The cells were harvested at the indicated time points for the indicated analyses. (b–d) Gene expression profiles of HS-induced mZip13 (b) and myogenic differentiation markers, mMyoD (c) and mMyogenin (d), (n = 3 each) analyzed by qPCR. C2C12 cells were harvested at the indicated time points. Data are presented as the mean ± standard error of mean (SEM) of three independent experiments. *P < 0.05 and **P < 0.01 relative to the data on Day 0. (e) The expression of Zip13 mRNA in Scramble 1 and two clones of Zip13-KD (clone 6 and 7) of C2C12 cells (n = 4 each), developed using the Zip13 shRNA plasmid was examined by qPCR. Data are presented as the mean ± SEM of three independent experiments. **P < 0.01 relative to the values of Scramble C2C12 cells. (f–k) Scramble and two clones of Zip13-KD C2C12 cells (6 and 7) were differentiated into skeletal muscle cells by HS treatment as described in Fig. 1a. (f) Microscopic evaluation of the morphological changes in Scramble and Zip13-KD C2C12 cells (clone 7) on Days 0 and 3 of myogenic differentiation. The yellow arrows indicate myotube formation. Scale bar, 50 μm. (g–i) Gene expression profiles of mMyoD (g), mMyogenin (h), mMyf5 (i), and mMyh2 (j) (n = 4 each) in Zip13-KD C2C12 cells during myogenic differentiation was analyzed by qPCR at the indicated time points (Days 0–3). Data are presented as the mean ± SEM of three independent experiments. *P < 0.05 and **P < 0.01 versus Scramble C2C12 cells. (k) MYH protein expression in Scramble and two clones of Zip13-KD C2C12 cells on Days 0 and 3 of myogenic differentiation were analyzed by western blotting. All data were collected from 3–4 independent experiments.
Establishment of Zip13-knockdown (KD) C2C12 cells
Either mouse Zip13-shRNA or non-specific (Scramble) DNA (Supplementary Table S1) were inserted into the pSUPER.retro.puro vector plasmid (OligoEngine, Seattle, WA, USA) according to the manufacturer’s instructions to generate Zip13-shRNA or Scramble plasmids. C2C12 cells were transfected with either Zip13-shRNA or Scramble plasmids using Lipofectamine LTX (Thermo Fisher Scientific) according to the manufacturer’s instructions and cultured in a medium containing 5 µg/mL puromycin for 48 h. Cells were then collected, and the KD efficiency of Zip13 mRNA was examined by reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) analysis. The remaining cells were seeded by limiting dilution to generate a monoclonal cell line for both Zip13-KD and Scramble C2C12 cells. We examined Zip13 mRNA expression in the twelve monoclonal cell lines and selected two clones (clone 6 and 7) based on the KD efficiency of Zip13, and four clones of Scramble C2C12 cells (clone number: 1, 5, 9, and 13) were used as the experimental control.
Ethical approval
All experimental procedures used in the study were approved by the Ethics Committee of Tokushima Bunri University (approval number R2-19 and R3-16) and performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from the two patients with EDSSPD3 (one female and one male: siblings), harboring a homozygous point mutation ZIP13G64D,4 who were included in the present study, for the use of their human dermal fibroblasts (HDFs).
Culture of HDFs
Healthy 1 (H1, female, Caucasian, 36 years old) or healthy 2 (H2, male, Caucasian, 49 years old) HDFs were purchased from Cell Applications, Inc. (San Diego, CA, USA). HDFs from patient 1 (EDSSPD3-P1, female) and patient 2 (EDSSPD3-P2, male) with EDSSPD34,8 were isolated. HDFs were cultured and maintained in DMEM (WAKO) supplemented with 10% FBS (Hyclone Laboratories, South Logan, UT, USA), 1 × GlutaMAX (Thermo Fisher Scientific), and 100 units/mL penicillin and 100 µg/mL streptomycin (P/S; Thermo Fisher Scientific) at 37 °C in the presence of 5% CO2.
Generation of hiPSCs using an episomal vector
hiPSCs were generated in an integration-free manner with episomal plasmid vectors (http://www.cira.kyoto-u.ac.jp/e/research/protocol.html)23. Briefly, H1-, H2-, EDSSPD3-P1-, and EDSSPD3-P2-DFs were cultured in DMEM supplemented with 10% FBS (Hyclone Laboratories), 1 × GlutaMAX (Thermo Fisher Scientific), and P/S (Thermo Fisher Scientific). Episomal plasmid vectors pCXLE-hOCT3/4-shp53-F, pCXLE-hUL, and pCXLE-hSK, which express human OCT3/4 and shRNA against p53, human L-MYC and LIN28, and human SOX2 and KLF4, respectively, were purchased from Addgene Inc. (Watertown, MA, USA) and used for generation of integration-free hiPSCs. Three micrograms of the episomal plasmid mixture (1 µg of each plasmid) were electroporated into 3 × 105 HDFs using the Neon transfection system (MPK5000; Thermo Fisher Scientific). The electroporation conditions were 1650 V, 10 ms, and 3 time pulses. The cells were cultured for 7 days after transduction at 37 °C in the presence of 5% CO2, and 5 × 104 cells were replated onto mitomycin-C (Kyowa Hakko Kirin, Tokyo, Japan)-treated SNL76/7 (SNL) feeder cell (DS pharma Biomedical, Osaka, Japan) layer in 60-mm dishes. The next day, the culture medium was replaced with primate embryonic stem cell (ESC) medium (ReproCELL, Kanagawa, Japan) supplemented with 4 ng/mL recombinant human basic fibroblast growth factor (WAKO) and P/S (Thermo Fisher Scientific). Colonies were generated 20–30 days after plating, and colonies with morphology similar to hESCs were selected and grown on mitomycin-C-treated SNL feeder cell layer.
Feeder-free culture of hiPSCs
The selected iPSCs were maintained under feeder-free condition in an uncoated manner using laminin fragments as described previously24. Briefly, iPSC colonies derived from HDFs of healthy individuals (H1 and H2) or patients with EDSSPD3 (P1 and P2) were dissociated with a detachment solution comprising 0.5 × TrypLE select (Thermo Fisher Scientific) and 0.75 mM EDTA in Dulbecco's phosphate-buffered saline (DPBS) (−) (Nacalai Tesque) for 10 min at 25 °C after the removal of SNL feeder cells using CTK solution (2.5% trypsin [Thermo Fisher Scientific], 1 mg/mL collagenase IV [Thermo Fisher Scientific], 0.1 M CaCl2, and 20% knockout serum replacement [KSR; Thermo Fisher Scientific] in H2O). Colonies consisting of single cells were suspended in StemFit medium (AK02N; Ajinomoto, Tokyo, Japan) supplemented with 10 µM Y27632 (WAKO), Rho-associated, coiled-coil containing protein kinase inhibitor, and P/S (Thermo Fisher Scientific). The cells were then seeded at a density of 2 × 104 cells/cm2 onto cell culture plates with StemFit medium (Ajinomoto) supplemented with 0.25 µg/cm2 iMatrix-511 (Nippi, Tokyo, Japan), laminin-511 E8 fragment, 10 µM Y27632 (WAKO), and P/S (Thermo Fisher Scientific). The culture medium was replaced the next day with StemFit medium (Ajinomoto) supplemented with P/S (Thermo Fisher Scientific). After 4 days of incubation, the culture medium was changed daily. hiPSCs at 95% confluency were passaged in an uncoated manner using iMatrix-511 (Nippi). Then, each hiPSC line was maintained as a feeder-free culture on iMatrix-511 (Nippi).
For sequencing analysis of ZIP13-exon2 mRNA of EDSSPD3-iPSCs, total RNA was extracted from lysates of H1 and H2 or EDSSPD3-P1 and -P2 HDFs and iPSCs, respectively, using a RNeasy Mini Kit (Qiagen, GmbH, Germany). Total RNA was used to synthesize cDNA using SuperScript IV (Thermo Fisher Scientific) according to the manufacturer’s instructions. The synthesized cDNA was used as a template for PCR using human ZIP13-exon2 mRNA primers (Supplementary Table S1). Each PCR amplicon was analyzed by Sanger sequencing.
Establishment of hiPSC lines expressing tetracycline-inducible human MyoD1 (hMyoD)
MyoD1 is the master transcriptional factor that regulates the differentiation of various cell types into myocytes through its enforced expression25,26. For the myogenic differentiation of hiPSCs, tetracycline-inducible hMyoD-expressing iPSC lines (iPSCsMYOD) were established by transduction of its gene cassette-inserted piggyBac (PB)-based vector as described previously15,18,20. Briefly, the plasmids for tetracycline-inducible hMyoD and mCherry and the neomycin resistant gene were constitutively prepared in PB vector (PB-hMyoD) or EF1a promoter-driven PB transposase gene in pHL vector (pHL-EF1a-hcPBase)15,18,20. The feeder-free maintained hiPSCs were treated with 10 µM Y27632 (WAKO) at least 2 h prior to transduction. hiPSCs were dissociated into single cells using a detachment solution, and the cells were resuspended in resuspension buffer R (Thermo Fisher Scientific). Two micrograms of PB-hMyoD and pHL-EF1a-hcPBase mixtures (1 µg each) were electroporated into 5 × 105 cells using the Neon transfection system (Thermo Fisher Scientific). Electroporation conditions were 1200 V, 20 ms, and 2 time pulses. Transfected hiPSCs were incubated as a feeder-free culture on iMatrix-511 (Nippi). After 2 days, the culture medium was replaced with StemFit medium (Ajinomoto) supplemented with 2–4 mg/mL neomycin (Nacalai Tesque), and the cells were subjected to antibiotic selection. Each neomycin-selected hiPSC was cloned by limiting dilution. The established hMyoD-expressing hiPSC lines, named iPSCsMYOD, were confirmed based on the expression of mCherry in the presence of 1 µg/mL doxycycline (DOX; LKT Laboratories, Saint Paul, MN, USA). The obtained clones and 253G4MYOD #35 which is a healthy-hiPSCs clone 253G427,28 expressing tetracycline-inducible human MyoD118 were maintained as a feeder-free culture on iMatrix-511 (Nippi).
Correction of ZIP13 G64D in EDSSPD3-iPSCsMYOD by Cas9/sgRNA and single-stranded oligodeoxynucleotide (ssODN) electroporation
ZIP13G64D in EDSSPD3-P1-iPSCsMYOD was edited using homology-directed repair (HDR) combined with the CRISPR-Cas9 system and ssODNs29. We designed ssODN containing silent mutations at amino acid positions 65 (TCC < TCA, Ser) and 66 (CTC < CTA, Leu) to prevent Cas9 from recutting the ZIP13-exon2 in the genome. GGG site showed the sequence of protospacer adjacent motif for Cas9 nuclease target. The day before transduction, EDSSPD3-P1-iPSCsMYOD were treated with 10 µM Y27632 (WAKO). hiPSCsMYOD were dissociated into single cells using a detachment solution, and the cells were resuspended in resuspension buffer R (Thermo Fisher Scientific). For ribonucleotide protein (RNP) electroporation, 2 µg TrueCut Cas9 protein v2 (Thermo Fisher Scientific) and 500 ng scaffold-modified ZIP13G64D_sgRNA (Thermo Fisher Scientific; Supplementary Table S2) were incubated for 5 min at 25 °C, and then 8 × 104 cells in resuspension buffer R (Thermo Fisher Scientific), 3 µg Alt-R HDR-modified ZIP13G64D-corrected_ssODN (IDT, Newark, NJ, USA; Supplementary Table S2), and 3 µM electroporation enhancer (IDT) were added. Cas9/sgRNA complex and ssODN mixture was electroporated into iPSCsMYOD using the Neon transfection system (Thermo Fisher Scientific). Electroporation conditions were 1050 V, 20 ms, and 2 time pulses. Transfected EDSSPD3-P1-iPSCsMYOD were incubated as a feeder-free culture on iMatrix-511 (Nippi) with StemFit medium (Ajinomoto) supplemented with 30 µM Alt-R HDR enhancer (IDT). The culture medium was replaced daily with StemFit medium (Ajinomoto). After 3 days of incubation, iPSCsMYOD were cloned by limiting dilution. The obtained clones were maintained as a feeder-free culture on iMatrix-511 (Nippi).
For sequencing analysis of human ZIP13-exon2, genomic DNA was extracted from lysates of transfected EDSSPD3-P1-iPSCMYOD clones using Geno Plus Mini kit (VIOGENE, New Taipei, Taiwan). The genomic DNA was used as a template for PCR using human ZIP13-exon2 genome primers (Supplementary Table S1). Each PCR amplicon was analyzed by Sanger sequencing. For separate sequencing analysis of individual alleles in a genome, genomic DNA of ZIP13G64D-corrected EDSSPD3-P1-iPSCMYOD clone (repaired clone; RC) 1–3 was used as a template for PCR using human ZIP13-exon2 genome primers for In-Fusion_pGEM (Supplementary Table S1). Their PCR amplicons were inserted in pGEM-T easy vector (Promega, Madison, WI, USA) using In-Fusion cloning kit (Takara Bio, Siga, Japan) according to the manufacturer’s instructions. The PCR amplicon-inserted plasmids were cloned and analyzed by Sanger sequencing.
Myogenic differentiation of iPSCsMYOD
Differentiation of EDSSPD3-iPSCsMYOD into myocytes was induced by forced hMyoD expression under the control of DOX as described previously19,30. A scheme for myogenic differentiation protocol of feeder-free iPSCsMYOD with DOX-inducible hMyoD expression is shown in Supplementary Figure S5a. Myogenic differentiation using H1-iPSCsMYOD and EDSSPD3-P1-iPSCsMYOD, H2-iPSCsMYOD and EDSSPD3-P2-iPSCsMYOD, or H1-iPSCsMYOD, H2-iPSCsMYOD, and 253G4MYOD #35 were performed simultaneously, respectively. Briefly, a 24-well cell culture plate was coated with Matrigel Growth Factor Reduced Basement Membrane Matrix (Corning, Corning, NY, USA) at least 2 h prior to cell seeding. Feeder-free cultured iPSCsMYOD were dissociated into single cells using a detachment solution, and 0.6 × 105 cells/well were seeded on the Matrigel-coated 24-well plate with StemFit medium (Ajinomoto) supplemented with 10 µM Y27632 (WAKO). The culture medium was replaced the next day with primate ESC medium (ReproCELL). On Day 2, 1 µg/mL DOX (LKT Laboratories) was added to the same medium, while on Day 3 the latter was replaced with minimum essential medium Eagle, alpha modification (Nacalai Tesque) supplemented with 5% KSR (Thermo Fisher Scientific), 200 µM 2-mercaptoethanol (Thermo Fisher Scientific), and 1 µg/mL DOX (LKT Laboratories). mCherry expression in differentiated cells on Day 3 was observed using a fluorescence microscope (BIOREVO BZ-9000; Keyence, Osaka, Japan). The medium was changed on Days 4, 5, and 7. Myogenic differentiation was performed at 37 °C in the presence of 5% CO2 until Day 8.
RT-qPCR
Total RNA of myogenic differentiated C2C12 cells or iPSCsMYOD was extracted from cell lysates using a Sepazol (Nacalai Tesque) or RNeasy Mini kit (Qiagen) and was used to synthesize cDNA with the PrimeScript RT Reagent Kit (Takara Bio) or SuperScript VILO (Thermo Fisher Scientific) according to the manufacturer’s instructions. The synthesized cDNA was used as a template for qPCR, which was performed using the SYBR Green real-time PCR Master Mix (TOYOBO, Osaka, Japan). The gene-specific primers used for qPCR are provided in Supplementary Table S1. PCR and data analyses were performed using a QuantStudio3 Applied Biosystems instrument (Thermo Fisher Scientific) or an Applied Biosystems StepOnePlus real-time PCR system (Thermo Fisher Scientific). The expression of each mRNA was normalized to that of mouse β-actin or human glyceraldehyde 3-phosphate dehydrogenase genes (GAPDH).
Western blot (WB) analysis
Cells were lysed with RIPA buffer (Nacalai Tesque) supplemented with 0.1% bromophenol blue and 10% β-mercaptoethanol and denatured for 5 min. Cell lysates were then loaded onto a 6–10% polyacrylamide gel and subjected to SDS–PAGE. The separated proteins were transferred onto a polyvinylidene fluoride microporous membrane (Millipore, Burlington, MA, USA), and the membrane was blocked with 5% skim milk in tris-buffered saline with Tween 20. Respective primary and secondary antibodies (Supplementary Table S3) were used to detect specific proteins. The signals were detected using Immobilon Western Chemiluminescent Horseradish Peroxidase Substrate (Millipore). Signal intensities were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA), and target protein levels were normalized to that of β-ACTIN and relative protein levels were calculated. Uncropped images of the western blotting membranes are shown in Supplementary Figures S10–S14.
Alkaline phosphatase (ALP) staining
hiPSC colonies were fixed using 4% paraformaldehyde in DPBS(−) (Nacalai Tesque) for 10 min at 25 °C. After washing with water, and ALP staining was performed using an Alkaline Phosphatase Kit (Sigma-Aldrich) according to the manufacturer's instructions. ALP-positive hiPSC colonies stained red, and the stained colonies were photographed under phase contrast using a fluorescence microscope (BIOREVO BZ-9000; Keyence) without a fluorescent laser.
Immunofluorescence (IF) staining
Cells were fixed using 4% paraformaldehyde for 30 min at 4 °C and subsequently permeabilized by the addition of 0.3% Triton X-100 for 20 min at 25 °C. The cells were then washed with DPBS(−) and blocked using a stain buffer supplemented with FBS (BD Biosciences, Franklin Lakes, NJ, USA). Respective primary and secondary antibodies (Supplementary Table S3) were used to detect specific proteins. Cell nuclei were then stained using diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific). Wells were photographed using a fluorescence microscope (BIOREVO BZ-9000; Keyence).
Flow cytometry (FC)
Feeder-free maintained hiPSCs were dissociated into single cells using a detachment solution for 10 min at 25 °C, and then suspended in a stain buffer with FBS (BD Biosciences). The cells were stained with primary antibodies for 2 h at 4 °C, followed by a secondary antibody for 1 h at 4 °C. The antibodies used for FC analysis are listed in Supplementary Table S3. Dead cells were excluded based on 7-amino-actinomycin D (BioLegend, San Diego, CA, USA) staining. Finally, the stained cells were analyzed using the BD FACSMelody cell sorter (BD Biosciences), and the data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
All results are presented as mean ± standard error of the mean. The statistical significance between two groups was analyzed by Student’s t-test, whereas that between more than two groups was analyzed by one-way analysis of variance with the post-hoc Tukey’s test. Results were considered statistically significant at P < 0.05.
Results
Role of ZIP13 in the normal myogenic differentiation of C2C12 cells
To clarify the involvement of ZIP13 in skeletal muscle differentiation in vitro, we used C2C12 cells, which are commonly used for studying myotube differentiation (Fig. 1a). We found that Zip13 mRNA expression was upregulated by myogenic stimulation (Fig. 1b), which occurred after upregulation of MRFs such as MyoD and Myogenin (Fig. 1c and d; Supplementary Fig. S1). Silencing of Zip13 resulted in the suppression of myogenic differentiation (Fig. 1e–k; Supplementary Fig. S1–S3). These results suggest the possible involvement of ZIP13 in myogenic differentiation in C2C12 cells.
Generation of iPSCs from HDFs of patients with EDSSPD3
To further evaluate the indispensable role of ZIP13 in human myogenic differentiation, we generated iPSCs from HDFs of patients with EDSSPD3. First, we generated iPSCs from HDFs of H1, H2, EDSSPD3-P1, and EDSSPD3-P2 by transfecting HDFs with episomal plasmid vectors. The morphological characteristics and ALP activity of undifferentiated EDSSPD3-iPSCs were equivalent to those of control iPSCs (Fig. 2a,b), both of which exhibited the same features of previously reported iPSCs27. We further confirmed the expression of undifferentiated-cell surface markers SSEA4 (Fig. 2c,e) and TRA-1-81 (Fig. 2d,f) in all hiPSCs and confirmed that all EDSSPD3-iPSCs harbored the original mutation ZIP13G64D (Supplementary Fig. S4).
Generation and characterization of undifferentiated induced pluripotent stem cells (iPSCs) from healthy controls and patients with Ehlers–Danlos syndrome spondylodysplastic type 3 (EDSSPD3) harboring ZIP13G64D mutation. iPSCs derived from healthy controls (H1 and H2) and patients with EDSSPD3 harboring ZIP13G64D mutation (EDSSPD3-P1 and EDSSPD3-P2) were generated from dermal fibroblasts in an integration-free manner using episomal plasmid vectors. Each hiPSC line was maintained as a feeder-free culture on iMatrix-511. Undifferentiated H1, H2, EDSSPD3-P1, and EDSSPD3-P2 iPSC colonies were assessed by morphological examination (a) or alkaline phosphatase staining (b). Scale bar, 100 µm. (c,d) The surface expression of undifferentiated stem cell markers, SSEA4 (c) and TRA-1-81 (d) (green), in H1, H2, EDSSPD3-P1, and EDSSPD3-P2 iPSC colonies were analyzed by immunofluorescence (IF) staining. Cell nuclei were stained using DAPI (blue). Scale bar, 100 µm. (e,f) Flow cytometric analysis of SSEA4 and TRA-1-81 in H1, H2, EDSSPD3-P1, and EDSSPD3-P2. Mouse IgG3 antibody was used as an isotype control. Flow cytometric histograms were overlayed with data of SSEA4 (e) or TRA-1-81 (f) and mouse IgG3 isotype control. Dead cells were excluded using 7-amino-actinomycin D staining. Data are representative of three independent experiments.
Next, we generated iPSCsMYOD and differentiated them into myocytes under the control of DOX (Supplementary Fig. S5). The expression of exogenous genes in these cells was monitored through the expression of mCherry and Exo-MYOD, which showed equivalent expression, suggesting that iPSCsMYOD exhibited dosage-dependent responses similar to DOX treatment (Supplementary Fig. S5b–g), whose expression were gradually downregulated during DOX-induced myogenic stimulation (Supplementary Fig. S5d–g) as previously reported30,31. The expression patterns of mCherry and Exo-MYOD mRNA indicate DOX-induced myogenic stimulation occurred in iPSCsMYOD, suggesting that we established iPSCMYOD lines of healthy controls (H1-iPSCsMYOD and H2-iPSCsMYOD) and patients with EDSSPD3 (EDSSPD3-P1-iPSCsMYOD and EDSSPD3-P2-iPSCsMYOD).
Characterization of myocytes differentiated from EDSSPD3-iPSCs
To assess whether the pathogenic ZIP13G64D mutation has an impact on myogenic processes, we induced myogenic differentiation in iPSCsMYOD through DOX-inducible hMyoD overexpression (Supplementary Fig. S5). First, to examine variabilities on myogenic differentiation between healthy controls, all the healthy control iPSCsMYOD clones, H1-iPSCsMYOD, H2-iPSCsMYOD, and 253G4MYOD #35 were simultaneously differentiated into myocytes with the similar trends by the same dose of DOX treatment (Supplementary Fig. S6). The expression of exogenous genes in these cells were monitored through the expression of mCherry and Exo-MYOD (Supplementary Fig. S6a, c, and d). They exhibited comparable spindle shape and expression level of myosin heavy chain (MYH) protein as noted through IF staining (Supplementary Fig. S6b). They enhanced the expression of hZIP13 during myogenic differentiation (Supplementary Fig. S6e), and the same was also true for the expression changes of myogenic differentiation marker mRNAs, such as human endogenous-MYOD (hEndo-MYOD) and hMYOGENIN (Supplementary Fig. S6f. and g), and their proteins (Supplementary Fig. S6h–j). Considering that 253G4MYOD #35 is the representative healthy control hiPSCs clone, these results suggest that H1-iPSCsMYOD and H2-iPSCsMYOD that we established could be used as healthy controls for experiments of myogenic differentiation.
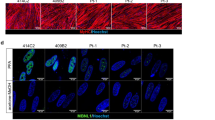
There were few apparent differences between the cells differentiated from patient-iPSCs and control iPSCs; both exhibited comparable spindle shape and expressed MYH protein (Fig. 3a,b). However, WB analysis revealed that MYH protein levels were lower in cells differentiated from EDSSPD3-P2-iPSCsMYOD than in those differentiated from control iPSCs (Supplementary Fig. S7r and s). Next, we analyzed mRNA and protein expressions of myogenesis-related molecules. The relative mRNA expression of normal hZIP13 or pathogenic hZIP13G64D was significantly upregulated during DOX-induced myogenic differentiation in healthy controls iPSCsMYOD or in EDSSPD3-iPSCsMYOD, respectively (Fig. 3c,f). Further, we assessed the expression of myogenic differentiation markers, and found that the mRNA and protein expression of hEndo-MYOD (Fig. 3d and g, i and l, j and m) and hMYOGENIN (Fig. 3e and h, i and l, k and n) were significantly downregulated in EDSSPD3-iPSCsMYOD. We also clarified the relative mRNA expressions of hEndo-MYOD and hMYOGENIN in myocytes differentiated from three healthy controls iPSCsMYOD and two EDSSPD3-iPSCsMYOD by using raw data of Fig. 3d, e, g, and h and Supplementary Figure S6f. and g, which showed that the mRNA expression levels of hEndo-MYOD and hMYOGENIN in patients were significantly decreased as compared with those of healthy controls when the data were averaged (Supplementary Fig. S8), thereby suggesting that the impairment of myogenic development in EDSSPD3-iPSCs were likely due to the biological failure by loss of function of ZIP13, rather than random effects by such as technical or environmental issues. Therefore, these results indicate that ZIP13G64D mutation may be involved in the possible impairment of myogenic differentiation of iPSCs.
Characterization of myocytes differentiated from EDSSPD3-iPSCsMYOD harboring the ZIP13G64D mutation. Differentiations of H1-iPSCsMYOD and H2-iPSCsMYOD or EDSSPD3-P1-iPSCsMYOD and EDSSPD3-P2-iPSCsMYOD into myocytes were induced by hMyoD overexpression under the control of DOX as depicted in Supplementary Figure S5a. Myogenic differentiation using H1-iPSCsMYOD and EDSSPD3-P1-iPSCsMYOD or H2-iPSCsMYOD and EDSSPD3-P2-iPSCsMYOD was performed simultaneously, respectively. (a,b) IF staining of MYH in differentiated iPSCsMYOD from H1-iPSCsMYOD and EDSSPD3-P1-iPSCsMYOD (a) or H2-iPSCsMYOD and EDSSPD3-P2-iPSCsMYOD (b). The images of MYH—(green) and DAPI-stained (blue) differentiated cells as observed on Day 8. Scale bar, 500 µm and 100 µm for × 4 and × 20 magnified images, respectively. (c–h) Relative mRNA expression analysis for human ZIP13 (hZIP13) gene and myogenic differentiation markers in differentiated H1-iPSCsMYOD and EDSSPD3-P1-iPSCsMYOD (c–e) or H2-iPSCsMYOD and EDSSPD3-P2-iPSCsMYOD (f–h). mRNA expression of hZIP13 (c,f) (n = 9) was determined by RT-qPCR on Days 0, 3, 6, and 8, and expressed in relation to the levels in healthy-iPSCsMYOD on Day 0 (set as 1). mRNA expressions of myogenic differentiation markers hEndo-MYOD (d,g) and hMYOGENIN (e,h) (n = 9 each) were determined by RT-qPCR on Days 3, 6, and 8, and expressed in relation to the levels in healthy-iPSCsMYOD on Day 3 (set as 1). mRNA expression was normalized to that of human GAPDH. Data represent the mean ± SEM and are representative of three independent experiments. **P < 0.01 and ***P < 0.001 versus healthy-iPSCsMYOD on Days 0 or 3. †P < 0.05 and †††P < 0.001 versus EDSSPD3-iPSCsMYOD on Days 0 or 3. (i–n) Relative protein expression analysis for myogenic differentiation markers in differentiated H1-iPSCsMYOD and EDSSPD3-P1-iPSCsMYOD (i–k) and H2-iPSCsMYOD and EDSSPD3-P2-iPSCsMYOD (l–n). Protein levels of hMYOD and hMYOGENIN in cell lysates were analyzed by western blotting on Day 8 (i,l). β-ACTIN was used as an internal control. Signal intensities were measured using ImageJ software. Protein levels of hMYOD (j,m) and hMYOGENIN (k,n) (n = 5 each) were normalized to that of β-ACTIN and were expressed relative to the levels in healthy-iPSCsMYOD on Day 8 (set as 1). Data represent the mean ± SEM and are representative of five independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 versus healthy-iPSCsMYOD.
To investigate whether loss of ZIP13 effects on intracellular zinc levels in these cells, we assessed the mRNA expression of human metallothionein 1H (hMT1H), which is well-correlated with intracellular zinc levels32. We also analyzed the mRNA expression of skeletal muscle factors, hMYH, human glucose transporter type 4 (hGLUT4), and human creatine kinase, M-type (hCK-M), as well as myogenic precursor factors, hMYF5, human paired box 3 (hPAX3), and hPAX7 (Supplementary Fig. S7). We found that the expression of hMT1H in the iPSCs of both patients on Day 0 was significantly higher than that of healthy controls, and its expression in iPSCs from healthy controls and patients on Day 0 was significantly higher than those on Days 3 to 8 (Supplementary Fig. S7a and h), indicating that the upregulation of hMT1H in patient iPSCs might reflect a dysregulation of intracellular zinc status by the ZIP13G64D mutation. On the other hand, hMT1H expression in EDSSPD3-P1-iPSCsMYOD on Day 3 was lower than that in H1-iPSCs MYOD (Supplementary Fig. S7a), whereas its expression in EDSSPD3-P2-iPSCsMYOD on Days 3 was higher than that in H2-iPSCsMYOD (Supplementary Fig. S7h). As the expression level of hMT1H after Day 3 became much lower than that on Day 0, it is difficult to judge whether such minor differences in hMT1H expression after Day 3 have any biological relevance.
In addition, we found that the hCK-M and hPAX3 were significantly downregulated in myocytes derived from EDSSPD3-iPSCsMYOD on Day 8 (Supplementary Fig. S7d and k, f, and m), and hMYH, hGLUT4, and hPAX7 in EDSSPD3-P1-iPSCsMYOD and EDSSPD3-P2-iPSCsMYOD significantly differed with respect to that in healthy controls during myogenic differentiation (Supplementary Fig. S7b and i, c and j, g and o), although it is currently unclear whether statistically significant in changes of those genes would have biological relevance at this point.
Effect of genomic correction of ZIP13 G64D mutation on myogenic differentiation
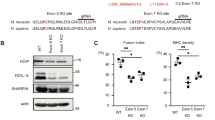
Next, to confirm the requirement of ZIP13 for myogenic differentiation, we corrected the ZIP13G64D mutation in EDSSPD3-P1-iPSCsMYOD by genome editing using HDR combined with the CRISPR-Cas9 system and ssODNs (Fig. 4a upper). ZIP13G64D-corrected EDSSPD3-P1-iPSCsMYOD, designated “RC1–3”, showed allelic knockin patterns as assessed by Sanger sequencing (Fig. 4a, lower). We further separately analyzed individual alleles in the genome of RC1–3 by In-Fusion cloning using the ZIP13-exon2 PCR amplicons and found that RC1–3 had one of the corrected allele 2 (C2) (Fig. 4b). These results demonstrated that the ZIP13G64D mutation in EDSSPD3-P1-iPSCsMYOD was substituted resulting in the change of homogenous alleles to heterogeneous alleles, which are similar to the genotype of the parents of the patients with EDSSPD3 included in the present study. The parents exhibited no clinical issues because EDSSPD3 is a recessive disorder4. Subsequently, we induced myogenic differentiation in the corrected clones (RC1–3) using DOX treatment, and observed similar spindle shape and high MYH expression as that in the control cells (Fig. 4c). Although the relative mRNA expression of hZIP13 in EDSSPD3-P1-iPSCsMYOD and RC1–3 was significantly upregulated during DOX-induced myogenic differentiation (Supplementary Fig. S9b), mRNA expressions of Exo-MYOD and hMT1H were altered on Day 3 (Supplementary Fig. S9a and c). Each iPSC clone expressed Exo-MYOD mRNA with DOX supplementation, which was followed by myogenic differentiation30. The relative expression levels of hMYOGENIN (Fig. 4d) and hMYOGENIN protein (Supplementary Fig. S9d and e) were increased in RC1–3 on Day 8 relative to that in EDSSPD3-P1-iPSCsMYOD. These results indicate that genome editing of the ZIP13G64D mutation resulted in normalized expression of myogenic differentiation markers, so that ZIP13 is possibly involved in the regulation of myogenic differentiation.
Characterization of myocytes differentiated from ZIP13G64D-corrected EDSSPD3-iPSCsMYOD. (a) We corrected ZIP13G64D in EDSSPD3-P1-iPSCsMYOD by genome editing using HDR combined with CRISPR-Cas9 system and single-stranded oligodeoxynucleotides (ssODNs). For the correction from ZIP13G64D of EDSSPD3-P1-iPSCsMYOD, we used the synthesized single guide RNA (sgRNA) and ssODN (Supplementary Table S2) (a, upper part). After electroporation of precomplexed Cas9/sgRNA with ssODN, subclones were analyzed, and three clones of ZIP13G64D-corrected EDSSPD3-P1-iPSCsMYOD, designated “RC1–3”, showed allelic knockin by Sanger sequencing (R = G or A, Y = A or C) (a, lower part). (b) To sequence individual alleles separately in RC1–3, we performed In-Fusion cloning using the ZIP13-exon2 PCR amplicons and analyzed them by Sanger sequencing. (c,d) Differentiation of EDSSPD3-P1-iPSCsMYOD and RC1–3 into myocytes was induced by hMyoD overexpression under the control of DOX. A schematic depicting the protocol of myogenic differentiation of feeder-free hiPSCsMYOD with DOX-inducible hMyoD expression is shown in Supplementary Figure S5a. EDSSPD3-P1-iPSCsMYOD with homogenous alleles of ZIP13G64D mutation was used as an experimental control. (c) IF staining of MYH in differentiated EDSSPD3-P1-iPSCsMYOD and RC1–3. The images of MYH—(green) and DAPI-stained (blue) differentiated cells were observed on Day 8. Scale bar, 500 µm and 100 µm for × 4 and × 20 magnified images, respectively. (d) The relative mRNA expression of myogenic differentiation marker hMYOGENIN in myocytes derived from EDSSPD3-P1-iPSCsMYOD and RC1–3 was determined by RT-qPCR, and normalized to that of human GAPDH. The relative expression of hMYOGENIN with respect to Exo-MYOD expression (hMYOGENIN/Exo-MYOD) (n = 12 each) was calculated using that of Exo-MYOD, as described in Supplementary Figure S9a and expressed relative to the levels in EDSSPD3-P1-iPSCsMYOD on Day 0 (set as 1). Data represent the mean ± SEM and are representative of four independent experiments. *P < 0.05 and **P < 0.01 versus EDSSPD3-P1-iPSCsMYOD on Day 8.
Discussion
In the present study, using pathogenic ZIP13G64D mutation-harboring iPSCs established from patients with EDSSPD3, we demonstrated that ZIP13 is possibly involved in myogenic differentiation.
Skeletal muscles accumulate the largest proportion of total zinc in body, which is about 60% of the total levels in the body33,34; however, physiological roles of zinc transporters in skeletal muscle are not fully understood. Patients with EDSSPD3 suffer from muscular hypotonia or myopathy, implicating the role of ZIP13 in skeletal muscles4,6,9. In the present study, we found that Zip13 was upregulated in mouse C2C12 cells and human iPSCs during myogenic differentiation along with myogenic differentiation markers MyoD and Myogenin. Additionally, Zip13 silencing suppressed myogenic differentiation. The same was also observed with EDSSPD3-iPSCs, which suggests that ZIP13 is involved in myogenic differentiation. However, we could not exclude the involvement of other factors such as culture conditions and passage numbers, which may have affected myogenesis along with the loss of ZIP13 in our experimental conditions. Intriguingly, another ZIP family member, ZIP14, reportedly acts on muscle homeostasis in a manner different to ZIP1335. ZIP14 was reported to be upregulated in skeletal muscles of mice and patients with metastatic cancers, while ZIP14-mediated zinc uptake in muscle progenitor cells and differentiated myocytes repressed the expression of MyoD and MYH, respectively, promoting muscle wasting in cancer cachexia35. These reports together with the present findings suggest that a functional balance between ZIP family members is required for muscle homeostasis, and that ZIP13 may play a role in the early stages of myogenesis.
How does ZIP13 regulate molecular events during myogenic differentiation? ZIP13 is reportedly involved in BMP/TGF-β-mediated signaling pathways and is responsible for various phenotypes and symptoms in Zip13-KO mice and patients with EDSSPD3 harboring ZIP13G64D mutation4,8. Interestingly, Zip13-KO mice and patients with EDSSPD3 exhibit reduced white fat mass4. This may be because Zip13-KO mice showed accelerated adipocyte browning in association with increased protein levels of CCAAT/Enhancer Binding Protein β (C/EBP-β), suggesting an important role of ZIP13 in suppressing adipocyte browning through the downregulation of C/EBP-β protein levels36. Several studies have reported that both BMP signaling and C/EBP-β play important roles in myogenic differentiation. BMP signaling maintains the proliferative status of muscle progenitors and increases muscle mass37,38, while C/EBP-β plays essential roles in repressing myogenesis39,40. Notably, C/EBP-β is rapidly degraded upon activation by the ubiquitin–proteasome system to facilitate myogenic differentiation39. Consistently, C/EBP-β overexpression in myoblasts inhibits their differentiation, whereas loss of its expression promotes myogenic differentiation and myofiber development41. C/EBP-β expression also inhibits the transcription of MyoD and Myogenin40,41. In the present study, we demonstrated that loss of ZIP13 attenuated the expression of myogenic differentiation markers MyoD and Myogenin in C2C12 cells and EDSSPD3-iPSCs. Therefore, the balance between impaired BMP signaling and C/EBP-β-mediated cascades can potentially be impaired by loss of ZIP13, which may in turn result in abnormal myogenic differentiation.
To further clarify the role of ZIP13 in early skeletal muscle development, especially in humans, we derived iPSCs from HDFs of patients with EDSSPD3 harboring ZIP13G64D mutation because using a patient-derived disease cell model is beneficial for elucidating the underlying pathogenetic mechanisms and has potential applications in regenerative medicine14. In vitro disease models using patient-derived iPSCs can also be used for high-throughput screening of drugs. In fact, in vitro models of various muscle-related diseases, such as DMD15,16,17, MM18,19, and Pompe disease20, have been used for elucidating their pathogenetic mechanisms and for drug screening. Herein, we generated iPSCs from patients with EDSSPD3 harboring ZIP13G64D mutation, which were further differentiated into myoblasts by hMyoD overexpression. We showed that these myocytes exhibited attenuated expression of myogenic differentiation markers as compared to that in healthy controls. Previous studies have reported that myopathies may impair myogenesis via the expression of myogenic differentiation markers such as MyoD11,42,43,44 and Myogenin12,45,46,47. Taken together, these results indicate that myopathies caused by loss of ZIP13 function may initiate attenuation of the expression of myogenic differentiation markers in EDSSPD3-iPSC-derived myocytes.
To precisely elucidate the phenotypic abnormalities of EDSSPD3-iPSCs-derived myocytes and to further confirm the requirement of ZIP13 for myogenic differentiation, we performed genome editing using ssODN-mediated HDR combined with CRISPR-Cas9 system to rectify the ZIP13G64D mutation. This genome editing system is widely utilized for precise genome editing at a desired locus29 and has been applied previously to study muscle diseases, such as DMD48 or MM29. Herein, we showed that genomic editing of the ZIP13G64D mutation in EDSSPD3-iPSCsMYOD recovered the expression of myogenic differentiation marker Myogenin in myocytes differentiated from EDSSPD3-iPSCsMYOD. These findings indicate that ZIP13 is indispensably involved in the early stages of myogenic differentiation.
It has been established that the alternation of MRFs, such as MyoD and Myogenin impacts skeletal development and/or functions49,50, since they are integral to the development of normal skeletal muscle through regulation of proliferation and myogenic fusion during development51. Indeed, Myogenin is necessary for both early muscle development45,46 and for myofiber growth in adults12. In the future, genetic murine models of the disease such as Zip13-deficient mice and muscle specific Zip13-conditional knock out mice should be employed to clearly elucidate the mechanisms underlying the pathophysiological abnormalities observed in patients with EDSSPD3. In this study, we found that the attenuated expression of myogenic differentiation markers similarly occurred in myocytes differentiated from the iPSCs of two patients with EDSSPD3 harboring the ZIP13G64D mutation. However, variations in the expression levels of several muscle-associated genes were observed between the two patients (Supplementary Fig. S7), which makes it difficult to conclude whether such statistically significant data indicate any biological relevance that could define and characterize these cells. Thus, further research is necessary to fully clarify the role of ZIP13 in myogenesis.
In conclusion, we demonstrated that ZIP13 is involved in myogenic differentiation (Fig. 5). Our findings suggest that ZIP13 may be a novel MRF that regulates the expression of MyoD and Myogenin to facilitate accurate myogenesis. Recently, we reported the possible involvement of ZIP13 in the control of cardiovascular system52. Although further research is necessary to clarify the role of ZIP13 in the physiological functions of affected tissues including the heart and skeletal muscles, the patient-derived iPSCs established in the present study could be useful not only to uncover the mechanisms underlying ZIP13-mediated biological functions, but can also facilitate regenerative studies and pharmaceutical applications for the treatment of EDSSPD3.
ZIP13 is involved in normal myogenic differentiation. ZIP13 is upregulated under myogenic stimulation, most likely through myogenic regulatory factors (MRFs) including MyoD and Myogenin. In addition, ZIP13 and MRFs, namely MyoD and Myogenin, are required for induction with each other, implying that ZIP13 might have a role to amplify the expression loop of MRFs leading the accurate myogenic differentiation.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files. Raw sequencing data described in Supplementary Figure S4 and Fig. 4a and b were deposited in DNA Data Bank of Japan (DDBJ) that belongs to the International Nucleotide Sequence Database Collection (INSDC) under accession numbers LC741039–42 and LC741043–46 and LC741047–50 and LC74104351–56, respectively. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kambe, T., Taylor, K. M. & Fu, D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 296, 100320. https://doi.org/10.1016/j.jbc.2021.100320 (2021).
Hara, T. et al. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 67, 283–301. https://doi.org/10.1007/s12576-017-0521-4 (2017).
Hara, T., Yoshigai, E., Ohashi, T. & Fukada, T. Zinc transporters as potential therapeutic targets: An updated review. J. Pharmacol. Sci. 148, 221–228. https://doi.org/10.1016/j.jphs.2021.11.007 (2022).
Fukada, T. et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS One 3, e3642. https://doi.org/10.1371/journal.pone.0003642 (2008).
Giunta, C. et al. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome–an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am. J. Hum. Genet. 82, 1290–1305. https://doi.org/10.1016/j.ajhg.2008.05.001 (2008).
Kumps, C. et al. The connective tissue disorder associated with recessive variants in the SLC39A13 Zinc transporter gene (Spondylo-Dysplastic Ehlers-Danlos Syndrome Type 3): Insights from four novel patients and follow-up on two original cases. Genes (Basel) 11, 420. https://doi.org/10.3390/genes11040420 (2020).
Agrawal, P., Kaur, H., Kondekar, A. & Rathi, S. A case of Ehlers-Danlos syndrome presenting as short stature: A novel mutation in SLC39A13 causing spondylodysplastic Ehlers-Danlos syndrome. Oxf. Med. Case Rep. 2023, omac107. https://doi.org/10.1093/omcr/omac107 (2023).
Bin, B. H. et al. Molecular pathogenesis of spondylocheirodysplastic Ehlers-Danlos syndrome caused by mutant ZIP13 proteins. EMBO Mol. Med. 6, 1028–1042. https://doi.org/10.15252/emmm.201303809 (2014).
Dusanic, M. et al. Novel nonsense mutation in SLC39A13 initially presenting as myopathy: Case report and review of the literature. Mol. Syndromol. 9, 100–109. https://doi.org/10.1159/000485881 (2018).
Janssen, L. et al. Muscle toxicity of drugs: When drugs turn physiology into pathophysiology. Physiol. Rev. 100, 633–672. https://doi.org/10.1152/physrev.00002.2019 (2020).
Megeney, L. A., Kablar, B., Garrett, K., Anderson, J. E. & Rudnicki, M. A. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 10, 1173–1183. https://doi.org/10.1101/gad.10.10.1173 (1996).
Ganassi, M., Badodi, S., Wanders, K., Zammit, P. S. & Hughes, S. M. Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis. Elife 9, e60445. https://doi.org/10.7554/eLife.60445 (2020).
Gayraud-Morel, B. et al. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev. Biol. 312, 13–28. https://doi.org/10.1016/j.ydbio.2007.08.059 (2007).
Tiscornia, G., Vivas, E. L. & Izpisua Belmonte, J. C. Diseases in a dish: Modeling human genetic disorders using induced pluripotent cells. Nat. Med. 17, 1570–1576. https://doi.org/10.1038/nm.2504 (2011).
Shoji, E. et al. Early pathogenesis of Duchenne muscular dystrophy modelled in patient-derived human induced pluripotent stem cells. Sci. Rep. 5, 12831. https://doi.org/10.1038/srep12831 (2015).
Zhao, M. et al. Induced fetal human muscle stem cells with high therapeutic potential in a mouse muscular dystrophy model. Stem Cell Rep. 15, 80–94. https://doi.org/10.1016/j.stemcr.2020.06.004 (2020).
Uchimura, T., Asano, T., Nakata, T., Hotta, A. & Sakurai, H. A muscle fatigue-like contractile decline was recapitulated using skeletal myotubes from Duchenne muscular dystrophy patient-derived iPSCs. Cell Rep. Med. 2, 100298. https://doi.org/10.1016/j.xcrm.2021.100298 (2021).
Tanaka, A. et al. Efficient and reproducible myogenic differentiation from human iPS cells: Prospects for modeling Miyoshi Myopathy in vitro. PLoS One 8, e61540. https://doi.org/10.1371/journal.pone.0061540 (2013).
Kokubu, Y. et al. Phenotypic drug screening for dysferlinopathy using patient-derived induced pluripotent stem cells. Stem Cells Transl. Med. 8, 1017–1029. https://doi.org/10.1002/sctm.18-0280 (2019).
Yoshida, T. et al. A skeletal muscle model of infantile-onset pompe disease with patient-specific iPS cells. Sci. Rep. 7, 13473. https://doi.org/10.1038/s41598-017-14063-y (2017).
Chal, J. & Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 144, 2104–2122. https://doi.org/10.1242/dev.151035 (2017).
Sin, J. et al. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 12, 369–380. https://doi.org/10.1080/15548627.2015.1115172 (2016).
Okita, K. et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 8, 409–412. https://doi.org/10.1038/nmeth.1591 (2011).
Miyazaki, T., Isobe, T., Nakatsuji, N. & Suemori, H. Efficient adhesion culture of human pluripotent stem cells using laminin fragments in an uncoated manner. Sci. Rep. 7, 41165. https://doi.org/10.1038/srep41165 (2017).
Davis, R. L., Weintraub, H. & Lassar, A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000. https://doi.org/10.1016/0092-8674(87)90585-x (1987).
Gianakopoulos, P. J. et al. MyoD directly up-regulates premyogenic mesoderm factors during induction of skeletal myogenesis in stem cells. J. Biol. Chem. 286, 2517–2525. https://doi.org/10.1074/jbc.M110.163709 (2011).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. https://doi.org/10.1016/j.cell.2007.11.019 (2007).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106. https://doi.org/10.1038/nbt1374 (2008).
Kagita, A. et al. Efficient ssODN-mediated targeting by avoiding cellular inhibitory RNAs through precomplexed CRISPR-Cas9/sgRNA ribonucleoprotein. Stem Cell Rep. 16, 985–996. https://doi.org/10.1016/j.stemcr.2021.02.013 (2021).
Uchimura, T., Otomo, J., Sato, M. & Sakurai, H. A human iPS cell myogenic differentiation system permitting high-throughput drug screening. Stem Cell Res. 25, 98–106. https://doi.org/10.1016/j.scr.2017.10.023 (2017).
Rodriguez-Polo, I. et al. A piggyBac-based platform for genome editing and clonal rhesus macaque iPSC line derivation. Sci. Rep. 11, 15439. https://doi.org/10.1038/s41598-021-94419-7 (2021).
Debabrata Chowdhury, G. S. D. J., Kavitha Subramanian Vignesh. The Metallothionein-Zinc Landscape: How It Shapes Antimicrobial Immunity. Second edn, 57–77 (Springer Singapore, 2019).
Prasad, A. S. Discovery of human zinc deficiency and studies in an experimental human model. Am. J. Clin. Nutr. 53, 403–412. https://doi.org/10.1093/ajcn/53.2.403 (1991).
Takagishi, T., Hara, T. & Fukada, T. Recent advances in the role of SLC39A/ZIP Zinc transporters in vivo. Int. J. Mol. Sci. 18, 2708. https://doi.org/10.3390/ijms18122708 (2017).
Wang, G. et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med. 24, 770–781. https://doi.org/10.1038/s41591-018-0054-2 (2018).
Fukunaka, A. et al. Zinc transporter ZIP13 suppresses beige adipocyte biogenesis and energy expenditure by regulating C/EBP-beta expression. PLoS Genet 13, e1006950. https://doi.org/10.1371/journal.pgen.1006950 (2017).
Borok, M. J., Mademtzoglou, D. & Relaix, F. Bu-M-P-ing Iron: How BMP signaling regulates muscle growth and regeneration. J. Dev. Biol. 8, 4. https://doi.org/10.3390/jdb8010004 (2020).
Shirakawa, T. et al. Factors regulating or regulated by myogenic regulatory factors in skeletal muscle stem cells. Cells 11, 1493. https://doi.org/10.3390/cells11091493 (2022).
Fu, D., Lala-Tabbert, N., Lee, H. & Wiper-Bergeron, N. Mdm2 promotes myogenesis through the ubiquitination and degradation of CCAAT/enhancer-binding protein beta. J. Biol. Chem. 290, 10200–10207. https://doi.org/10.1074/jbc.M115.638577 (2015).
AlSudais, H., Lala-Tabbert, N. & Wiper-Bergeron, N. CCAAT/enhancer binding protein beta inhibits myogenic differentiation via ID3. Sci. Rep. 8, 16613. https://doi.org/10.1038/s41598-018-34871-0 (2018).
Marchildon, F. et al. CCAAT/enhancer binding protein beta is expressed in satellite cells and controls myogenesis. Stem Cells 30, 2619–2630. https://doi.org/10.1002/stem.1248 (2012).
Braun, T., Rudnicki, M. A., Arnold, H. H. & Jaenisch, R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71, 369–382. https://doi.org/10.1016/0092-8674(92)90507-9 (1992).
Rudnicki, M. A., Braun, T., Hinuma, S. & Jaenisch, R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 71, 383–390. https://doi.org/10.1016/0092-8674(92)90508-a (1992).
Sabourin, L. A., Girgis-Gabardo, A., Seale, P., Asakura, A. & Rudnicki, M. A. Reduced differentiation potential of primary MyoD-/- myogenic cells derived from adult skeletal muscle. J. Cell Biol. 144, 631–643. https://doi.org/10.1083/jcb.144.4.631 (1999).
Hasty, P. et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364, 501–506. https://doi.org/10.1038/364501a0 (1993).
Nabeshima, Y. et al. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 364, 532–535. https://doi.org/10.1038/364532a0 (1993).
Higashioka, K., Koizumi, N., Sakurai, H., Sotozono, C. & Sato, T. Myogenic differentiation from MYOGENIN-mutated human iPS cells by CRISPR/Cas9. Stem Cells Int. 2017, 9210494. https://doi.org/10.1155/2017/9210494 (2017).
Chemello, F., Bassel-Duby, R. & Olson, E. N. Correction of muscular dystrophies by CRISPR gene editing. J. Clin. Investig. 130, 2766–2776. https://doi.org/10.1172/JCI136873 (2020).
Zammit, P. S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 72, 19–32. https://doi.org/10.1016/j.semcdb.2017.11.011 (2017).
Hernandez-Hernandez, J. M., Garcia-Gonzalez, E. G., Brun, C. E. & Rudnicki, M. A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 72, 10–18. https://doi.org/10.1016/j.semcdb.2017.11.010 (2017).
Buckingham, M. & Rigby, P. W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 28, 225–238. https://doi.org/10.1016/j.devcel.2013.12.020 (2014).
Hara, T. et al. Role of Scl39a13/ZIP13 in cardiovascular homeostasis. PLoS One 17, e0276452. https://doi.org/10.1371/journal.pone.0276452 (2022).
Acknowledgements
We would like to thank the patients with EDSSPD34 included in this study for providing their dermal fibroblasts to generate iPSCs. This study was supported by grants from the Japan Society for the Promotion of Science KAKENHI (20K07457), The Nakatomi Foundation, The Uehara Memorial Foundation, Tokushima Bunri University for Educational Reform and Collaborative Research (TBU2022-2-2), and YOKOYAMA Foundation for Clinical Pharmacology (YRY-1815) (to MS), as well as the Japan Society for the Promotion of Science KAKENHI (20H03409), research grant of the Princess Takamatsu Cancer Research Fund, SECOM Science and Technology Foundation, Terumo Life Science Foundation, The Uehara Memorial Foundation, Astellas Foundation for Research on Metabolic Disorders, the joint research program of the Institute for Molecular and Cellular Regulation, Gunma University (to TF), and Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan (to TO). We would like to thank Editage (www.editage.com) and Dr. Akiko H. Popiel for English language editing.
Author information
Authors and Affiliations
Contributions
M.S. designed the study, performed the experiments with iPSCs derived from healthy controls and patients with EDSSPD3, analyzed the data, and wrote the manuscript. T.O., Y.Na, Y.No, S.K., H.M., and T.T. performed the experiments with C2C12 cells and analyzed the data. S.N., H.Y., and K.I. supported the experiments involving iPSCs derived from healthy controls and patients with EDSSPD3. A.F., Y.F., and H.S. provided the study materials and supervised the research. T.H. and E.Y. supervised the research. H.G.S. diagnosed the patients with EDSSPD3 harboring ZIP13G64D mutation. T.F. and T.K. designed this research, wrote the manuscript, and supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shoji, M., Ohashi, T., Nagase, S. et al. Possible involvement of zinc transporter ZIP13 in myogenic differentiation. Sci Rep 14, 8052 (2024). https://doi.org/10.1038/s41598-024-56912-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56912-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.