Abstract
This study used longitudinal data from CHARLS 2011–2018 for cross-sectional and longitudinal analyses to investigate the relationship between sarcopenia and hearing impairment in middle-aged and elderly adults in China. The study selected 9723 participants aged 45 years and older from CHARLS 2011 and followed up in 2015 and 2018. Binary logistic regression and cox proportional risk regression models were used for testing. The results of the study showed that in the cross-sectional analysis, probable sarcopenia was significantly associated with hearing impairment compared with the group without sarcopenia [OR (95% CI) 0.342 (1.187, 1.669), p < 0.001], but sarcopenia was not significantly associated with hearing impairment. In the longitudinal analysis, middle-aged and elderly adults with sarcopenia [HR (95% CI) 0.354 (1.043, 1.945), p < 0.01] were more likely to have hearing impairment than those with probable sarcopenia and without sarcopenia. Probable sarcopenia was strongly associated with hearing impairment in middle-aged and elderly adults, whereas sarcopenia was a strong predictor of hearing impairment over the next 7 years. The results of this study emphasize the urgent need for measures to address sarcopenia in order to prevent and delay the decline in hearing function.
Similar content being viewed by others
Introduction
The trend of global aging is intensifying, and China is no exception. As of 2022, there are 600 million people over the age of 45 in China, accounting for about 41.97% of the total population1. The rapid increase of the middle-aged and elderly has significantly influenced various aspects of healthcare, employment, and social construction, greatly increasing the social burden of elderly care and healthcare demands. In 2015, China’s expenditure on elderly care, medical treatment, nursing, and welfare accounted for 7.33% of GDP, and it is projected to increase to 26.24% by 20502. With age, the physical functions of middle-aged and elderly individuals gradually decline, posing threats and hazards to their physical and mental health. Therefore, timely attention, diagnosis, and intervention in their physical health conditions are essential. It can not only enhance the health awareness of the middle-aged population, prevent and delay diseases and functional impairments, but also improve their quality of life and promote healthy aging.
Among the functional impairments of the middle-aged and elderly, hearing impairment is the most common3. Age-related hearing impairment (ARHI) is the decline in auditory function due to the aging of the auditory system4. WHO estimates that more than 1.5 billion people (one in five) worldwide have varying degrees of hearing impairment5. It is expected that by 2050, 2.5 billion people worldwide will have a hearing impairment5. According to data from China’s sixth population census, 30% of the population aged 60 years and older suffers from hearing impairment6. In 2020, the percentage of hearing impairment among people aged 65 years and older is already close to 50%6. Age-related hearing impairment is the leading cause of hearing disability in China7. Hearing impairment causes middle-aged and elderly people to be less sensitive to sound, which in turn affects their daily communication and cognitive abilities, hinders social participation and information exchange, and reduces the quality of life3,8. In addition, hearing impairment affects the physical activities of middle-aged and elderly people, leading to negative emotions such as loneliness, anxiety, and depression, and affecting mental health3. Previous studies have shown that hearing impairment in middle-aged and elderly adults is affected by genetic factors, smoking, occupational noise, and ototoxic drugs9,10,11. Recent studies have found that chronic diseases such as diabetes, cardiovascular disease, and metabolic syndrome also affect hearing function in middle-aged and elderly adults12,13,14,15. Among them, high BMI and high waist circumference are considered important risk factors for hearing impairment11,16. The pathophysiologic mechanisms of sarcopenia and metabolic syndrome are similar. Therefore, it can be assumed that sarcopenia may be associated with hearing impairment in middle-aged and elderly people.
Sarcopenia is a common and widespread case or physiological phenomenon characterized by a decrease in appendicular skeletal muscle mass (ASM), muscle strength and physical performance17. Globally, 10–27% of adults aged 60 years and older have sarcopenia18 and the prevalence of sarcopenia in people aged 80 years and older approaches 50%19. Sarcopenia has adverse effects on the physical and mental health of adults, such as falls, decreased function, cardiovascular disease, depression, cognitive impairment, death, and hospitalization17,20,21,22. Studies have found that adults with sarcopenia spend an average of $2315.7 more per year on medical care compared to adults without sarcopenia23. And the more severe the sarcopenia, the more money is spent on medical care24. There has been an ongoing debate about the diagnosis of sarcopenia. Diagnostic criteria for sarcopenia have been proposed by organizations such as the Asian Working Group on Sarcopenia (AWGS)25, the European Working Group on Sarcopenia in the Elderly (EWGSOP)26, the International Working Group on Sarcopenia (IWGS)27, and the Foundation for the National Institutes of Health (FNIH)28 in the U.S.A. In 2019, the AWGS has developed new diagnostic criteria for sarcopenia, based on AWGS 2014, and proposed “probable sarcopenia”, which provides a basis for diagnosis, prevention, and intervention of sarcopenia in middle-aged and elderly adults in community-based primary care and healthcare settings. According to AWGS 2019, low ASM and/or low muscle strength is diagnosed as probable sarcopenia, low ASM and low muscle strength or low physical performance is diagnosed as sarcopenia, and low ASM, low muscle strength, and low physical performance is diagnosed as severe sarcopenia25. Previous studies have found that both muscle strength and ASM have been associated with hearing impairments29,30. A cross-sectional study including 15,952 participants showed that sarcopenia has an effect on hearing loss31. Some studies have also inferred the effect of sarcopenia on hearing impairment from the perspective of physiological mechanisms32,33.
Currently, only two cross-sectional studies, a Mendelian randomized study and a prospective study, have reported the association between sarcopenia and hearing impairment, and there is no clear information about the effect of sarcopenia on hearing impairment in middle-aged and elderly adults. Therefore, this study investigated the association between sarcopenia and hearing impairment in middle-aged and elderly adults in China using longitudinal data from CHARLS 2011–2018 to provide a theoretical basis for preventing and delaying hearing impairment in middle-aged and elderly adults.
Materials and methods
Participants and procedures
CHARLS is a nationally representative longitudinal survey conducted in 28 provinces in China34. CHARLS pioneered the electronic mapping software (CHALRS-GIS) technology, used the map method to create village-level sampling frames, and adopted the PPS sampling method in multi-stage sampling, mainly surveying middle-aged and elderly people aged 45 years and above. More than 17,000 participants were surveyed at the baseline from 2011, and were followed up every 2 years by completing structured questionnaires mainly through one-on-one interviews. CHARLS questionnaires included personal information, household structure, economic support, health status, physical measurements, etc. CHARLS was approved by the Ethics Review Board of Peking University (No.: IRB00001052-11015) and was conducted in accordance with the Declaration of Helsinki and other relevant guidelines and regulations. All participants provided informed consent. This study follows the STROBE statement.
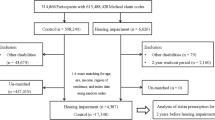
Baseline 2011 and 2018 (Wave 4) data were used in this study. Inclusion criteria were (1) age ≥ 45 years in CHARLS 2011; and (2) availability of data on grip strength, ASM, gait speed, and five chair stand tests. Exclusion criteria were (1) age < 45 years in 2011; and (2) missing data on hearing condition. Multiple imputation was employed to handle the missing data on sarcopenia. Ultimately, based on the inclusion and exclusion criteria, there were a total of 9723 participants in 2011, with 8206 remaining after deleting participants with hearing impairments. In the longitudinal study, we further excluded participants with missing hearing condition data in 2018, resulting in a final residual of 4603 participants. The detailed participant selection process is shown in Fig. 1.
Assessment of sarcopenia status
Sarcopenia was assessed according to the criteria recommended by AWGS 201925 and consisted of three components: muscle strength, ASM and physical performance. Participants performed 2 grip strength measurements (kg) with their dominant and non-dominant hand. Participants were asked to stand with their elbows at a 90-degree angle to the side of the body and hold the Yuejian WL-1000 Grip Strength Device (Nantong Yuejian Physical Fitness Equipment Co.) with maximum force. The average of the maximum values of the two measurements of both hands was taken. The threshold values for assessing low grip strength in men and women were < 28 kg and < 18 kg, respectively. The study showed that the ASM equation modeling was very similar to the results of dual x-ray absorptiometry (DXA) for assessing human limb muscle mass. In this study, ASM in Chinese middle-aged and elderly people was calculated using measurement equations:
where height was measured using Seca™ 213 height meter, weight was measured using Omron™ HN-286 scale and gender was set to 1 (male) and 2 (female). The lowest 20% of ASM/Ht2 values according to gender is the threshold for low muscle mass35,36. In this study, ASM/Ht2 < 5.26 kg/m2 for females and ASM/Ht2 < 6.99 kg/m2 for males were considered as low ASM. Physical performance was assessed in this study using gait speed and five chair stand tests35. Each participant was asked to walk 2 times (back and forth) on a 2.5-m channel and the time for each time was recorded.
The five times chair stand test involved the participant interlocking their arms in front of their chest and sitting down straight from a 47 cm high chair five times as fast as they could without stopping or propping up with their arms in between. The total time taken and the number of times completed are recorded. Gait speed of < 1.0 m/s for a 6-m walk, or ≥ 12 s for 5 chair stand tests was considered low physical performance according to the AWGS 2019 criteria.
The participants in this study were divided into three groups: no sarcopenia (n = 956), possible sarcopenia (n = 3143), and sarcopenia (n = 504). “Possible sarcopenia” was defined as low ASM or low physical performance, while “sarcopenia” was defined as low ASM + low muscle strength or low ASM + low physical performance.
Assessment of hearing impairment
Hearing impairment was assessed by participants’ self-reports, and studies have demonstrated the reliability of self-reported hearing status37,38. We used the CHARLS items “DA038 Now I have some questions about your hearing. Do you ever wear a hearing aid?” and “DA039 Is your hearing very good, good, fair, poor, or very poor ?” to test for hearing impairment. When DA038 selected “Yes” and/or DA039 selected “Poor”, the participant was considered to have a hearing impairment. Otherwise, the participant did not have a hearing impairment.
Covariates
Demographic characteristics, lifestyle variables, physical and mental health indicators and metabolic biomarkers were included as covariates in this study. Demographic characteristics included age, sex, hukou status (birthplace or place of long-term residence), educational level (illiterate, elementary, junior high, high and middle school, college and bachelor’s degree and above), marital status (married, separated, divorced/widowed/unmarried), and annual income (< ¥1000, ¥1000, ¥5000, ¥10,000, ¥20,000). Lifestyle variables included hours of sleep at night, smoking, frequency of drinking (more than once a month, less than once a month, never). Physical and mental health indicators included body mass index (BMI), visual impairment, hypertension, dyslipidemia, diabetes, cancer, and depression. Metabolic biomarkers included mean corpuscular volume (MCV), total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C). Among them, participants were categorized into overweight and obese group (BMI ≥ 24 kg/m2), normal weight group (18.5 kg/m2 ≤ BMI < 24 kg/m2) and underweight group (BMI < 18.5 kg/m2) based on BMI. Depression was scored according to the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10) with a total score of 30 (≥ 10 being depressed).
Statistical analyses
This study contains mainly cross-sectional and longitudinal analyses. We used mean ± standard deviation to describe continuous variables and number and percentage to describe categorical variables. First, according to the category of sarcopenia, comparative analyses were performed using chi-square test and ANOVA to characterize the baseline data in 2011 and the longitudinal data from 2011 to 2018, respectively. Second, binary logistic regression analyses were used to estimate the associations of possible sarcopenia and sarcopenia with hearing impairment in the 2011 cross-sectional data, as indicated by regression coefficients (β), odds ratios (ORs), and 95% confidence interval (95%CI). Then, in longitudinal analyses, we measured follow-up time and used cox proportional risk models to assess the association between baseline sarcopenia status and hearing impairment, expressed using hazard ratios (HR) and 95%CI.
We estimated three models in both cross-sectional and longitudinal analyses, and the three models used different combinations of covariates. Model 1 included age and gender; model 2 included age, gender, marital status, hukou status, educational level, smoking, frequency of drinking, hours of sleep at night and annual income; and model 3 added BMI, visual impairment, hypertension, dyslipidemia, diabetes mellitus, cancer, depression, MCV, TC, TG, HDL-C and LDL-C to model 2. All statistical analyses were performed using the SPSS 26.0 and R version 4.3.1 and were considered statistically significant when p < 0.05.
Ethical approval
CHARLS was approved by the Ethics Review Committee of Peking University (No. IRB00001052-11015).
Results
Basic characteristics of participants
Table S1 in the Appendix shows the baseline characteristics of all participants categorized according to sarcopenia status. The mean age of the participants was 59.59 (± 9.43) years, 5205 (53.5%) were female, and 8206 (84.4%) participants had hearing impairment. The prevalence of hearing impairment was 15.14% for participants without sarcopenia, 13.80% for probable sarcopenia, and 24.04% for sarcopenia. Among participants with possible sarcopenia and sarcopenia, there was a higher proportion of females, those with a rural household registration, lower education levels, married individuals, low incomes, non-smokers, non-drinkers, and those with visual impairments (all p < 0.001). Table S2 in the Appendix shows the baseline characteristics of participants without hearing impairment (n = 8206).
Cross-sectional association of probable sarcopenia and sarcopenia with hearing impairment
In cross-sectional analyses, we used binary logistic regression to analyze the association of probable sarcopenia and sarcopenia with hearing impairment. After adjusting for demographic characteristics and lifestyle variables, both probable sarcopenia and sarcopenia were significantly and positively associated with hearing impairment (p < 0.01). On this basis, after adjusting for physical and mental health indicators and metabolic biomarkers, probable sarcopenia was significantly and positively associated with hearing impairment [0.342 (1.187, 1.669), p < 0.001], but sarcopenia was not significantly associated with hearing impairment [0.159 (0.947, 1.453), p = 0.144] (Table 1). Figure 2 shows the ORs and 95% CIs of sarcopenia status and covariates for hearing impairment in model 3.
ORs and 95% CIs of hearing impairment by sarcopenia status in the cross-sectional analysis. The forest plot shows the ORs and 95% CIs for Model 3, which adjusts for age, gender, marital status, hukou status, educational level, smoking, frequency of drinking, hours of sleep at night, annual income, BMI, visual impairment, hypertension, dyslipidemia, diabetes mellitus, cancer, depression, MCV, TC, TG, HDL-C and LDL-C.
Longitudinal association of probable sarcopenia and sarcopenia with hearing impairment
In a longitudinal analysis of 4603 participants, 524 middle-aged and elderly adults had hearing impairment in 2018. The prevalence of hearing impairment was 13.08% in no sarcopenia, 9.51% in probable sarcopenia, and 19.84% in sarcopenia (see Fig. 3). Table 2 shows the longitudinal associations of probable sarcopenia and sarcopenia with hearing impairment. After adjusting for demographic characteristics, participants with sarcopenia were more likely to have hearing impairment than participants without sarcopenia and probable sarcopenia [0.447 (1.189, 2.057), p < 0.01]. Participants with probable sarcopenia were not significantly associated with an increased risk of hearing impairment compared to participants without sarcopenia. After sequential adjustment for lifestyle variables, physical and mental health indicators and metabolic biomarkers, the longitudinal associations between sarcopenia and hearing impairment remained significant [0.411 (1.143, 1.990), p < 0.01], [0.354 (1.043, 1.945), p < 0.01], and the longitudinal associations between probable sarcopenia and hearing impairment remained nonsignificant. Figure 4 shows the HRs and 95% CIs of sarcopenia status and covariates for hearing impairment in model 3.
Longitudinal association of baseline sarcopenia status with hearing impairment, 2011–2018. The forest plot shows the hazard ratios (HRs) and 95% CIs for Model 3, which adjusts for age, gender, marital status, hukou status, educational level, smoking, frequency of drinking, hours of sleep at night, annual income, BMI, visual impairment, hypertension, dyslipidemia, diabetes mellitus, cancer, depression, MCV, TC, TG, HDL-C and LDL-C.
Discussion
The purpose of this study was to investigate the impact of sarcopenia on hearing impairment in middle-aged and elderly adults in China. It is understood that this is the first study to examine the longitudinal relationship between sarcopenia and hearing impairment in a middle-aged and elderly population using a nationally representative sample. Results of cross-sectional analyses showed that both middle-aged and elderly adults with probable sarcopenia and sarcopenia were significantly positively associated with hearing impairment after adjusting for demographic characteristics and lifestyle variables. After adjusting for physical and mental health indicators and metabolic biomarkers, only the probable sarcopenia was significantly positively associated with hearing impairment. Longitudinal analyses showed that middle-aged and elderly adults with sarcopenia had a higher risk of hearing impairment than middle-aged and elderly adults without sarcopenia and probable sarcopenia, after adjusting for all covariates.
The results of our study are not entirely consistent with those of previous cross-sectional studies30. A study targeting Korean elders aged 60 and over showed that older female adults with greater muscle mass had better hearing, whereas there was no significant connection between muscle mass and hearing in male elders30. Furthermore, another study revealed that older adult women with sarcopenia had a higher incidence of hearing impairment compared to those without sarcopenia39. A Mendelian randomization study found a significant relationship between walking speed, ASM, and grip strength with hearing impairment, and a causal relationship between skeletal muscle mass and hearing impairment in women31. The previous longitudinal study with a follow-up of 5.8 years found a significant negative correlation between muscle mass and BMI with hearing impairment in middle-aged and elderly adults29, revealing the potential link between sarcopenia and hearing impairment. In regards to the inconsistency between the findings of this study and previous studies, we believe that, on one hand, it could be due to the different diagnostic criteria for “sarcopenia” compared to previous research. The diagnostic criteria for “sarcopenia” in this study were based on the latest version of AWGS 2019, categorizing participants into “no sarcopenia”, “possible sarcopenia”, and “sarcopenia”25. Currently, there is no existing literature using AWGS 2019 to investigate the association between possible sarcopenia, sarcopenia, and hearing impairment in middle-aged and elderly adults. On the other hand, this may be predominantly attributed to women experiencing long-term estrogen deficiency after menopause, which may increase the risk of hypertension, cardiovascular diseases, osteoporosis, and the likelihood of developing sarcopenia40, ultimately having a more pronounced impact on auditory function. Additionally, we found that after adjusting for physical and mental health indicators and metabolic biomarkers, the relationship between sarcopenia and hearing impairment was not significant, while the relationship between possible sarcopenia and hearing impairment remained evident. We speculate that some factors within the physical health indicators and metabolic biomarkers may have a more significant influence on hearing impairment, potentially mediating or moderating the association between sarcopenia and hearing impairment. Therefore, future research could further explore this direction.
In the longitudinal analysis, the longitudinal association between sarcopenia and hearing impairment was significantly correlated compared with middle-aged and elderly adults without sarcopenia, and probable sarcopenia and hearing impairment was not significantly correlated. The results are consistent with those of previous studies29,30. Studies in both Korea and Japan have found that sarcopenia increases the risk of hearing loss in older adults, especially in women29,30. This may be due to the fact that, on the one hand, skeletal muscle is able to take up, oxidize, and store fatty acids to participate in lipid metabolism, thereby maintaining the balance of lipid metabolism in the body41. Sarcopenia leads to metabolic disorders, which can cause hyperlipidemia, atherosclerosis, peripheral vascular disease and stroke41. Chronic hyperlipidemia can lead to increased blood viscosity, narrowing of blood vessels, and the formation of microthrombi. More skeletal muscle requires more blood supply, more arterial blood flow and larger arterial diameter42,43. Sarcopenia results in insufficient arterial blood flow and smaller arterial diameter, which leads to reduced blood flow to the cochlea, making hearing function reduced44. On the other hand, cochlear ischemia is associated with damage to the vascular stripe, which leads to hearing impairment45. Metabolic disorders can lead to diabetes46, and in turn, diabetes can exacerbate metabolic disorders and microangiopathy. The most common of these are microvascular lesions in the cochlear vascular stripe33. These lesions cause degeneration of the cochlea and cochlear nerve, which can lead to hearing loss47. In addition, endothelial dysfunction caused by diabetes and hyperglycemia leads to atrophy or damage of the cells of the vascular stripe, which results in disruption of the ion transport system in the vascular stripe and abnormalities in the connecting proteins of the gap junctions, leading to disruption of the formation of the electrical potentials in the cochlea, and to the occurrence of hearing impairment48,49. This study helps to increase the awareness of middle-aged and elderly adults regarding sarcopenia and hearing impairment, allowing family members to pay closer attention to and take care of their relatives’ hearing health in advance. It also helps communities and medical institutions to timely diagnose, prevent, and intervene in sarcopenia in middle-aged and elderly adults, develop new prevention strategies and treatment plans, and reduce and delay the occurrence of hearing impairment. Additionally, this study provides policy makers with an understanding of the latest research progress in hearing impairment, allowing them to formulate more comprehensive hearing health policies.
Strengths and limitations
Our research results not only support the effectiveness of the AWGS 2019 diagnostic algorithm for sarcopenia in Asian older adults but also provide new evidence for the causal relationship between probable sarcopenia, sarcopenia and hearing impairment. The strengths of this study are reflected in the sample selection and prospective study. First, this study used the CHARLS database, which is a nationally representative survey data that can represent the overall level of middle-aged and elderly people in China. Therefore, the study results are more realistic and reliable. Second, our study used longitudinal data with 7 years of follow-up and was able to reveal the causal relationship between sarcopenia and hearing impairment in middle-aged and elderly adults, making it the first study to explore the longitudinal relationship between sarcopenia and possible sarcopenia and hearing impairment in middle-aged and elderly adults in China.
There are also several limitations to this study. First, we used observational data, which may have biased the relationships between variables due to other confounding variables. Therefore, we adjusted for other potential confounders as much as possible, including 21 covariates for demographic characteristics, lifestyle variables, physical and mental health indicators, and metabolic biomarkers. Second, the diagnostic criteria regarding hearing impairment was through participants’ self-report, and no diagnostic records were available in CHARLS. Therefore, there may be some bias in the study results. In addition, the grading and types of hearing impairment were not addressed in CHARLS. Therefore, we could not analyze the relationship between sarcopenia and hearing impairment in depth.
Conclusions
In conclusion, this study evaluated probable sarcopenia and sarcopenia in middle-aged and elderly adults in China using the AWGS 2019 diagnostic criteria and explored the association and impact of probable sarcopenia and sarcopenia with hearing impairment, and found that there was a significant association between probable sarcopenia and hearing impairment, and that sarcopenia increased the risk of developing hearing impairment.
Data availability
The raw data for this study is available from: http://charls.pku.edu.cn/en. The dataset generated from this study is available from the corresponding author.
References
The distribution of the population by age group across the country in 2022. https://zhidao.baidu.com/question/378740092691564124.html (2023).
Liu, Y., Zheng, Z., Rao, K. & Wang, S. Blue Book of Elderly Health: Annual Report on Elderly Health in China (Social Science Academic Press, 2018).
Mick, P., Kawachi, I. & Lin, F. R. The association between hearing loss and social isolation in older adults. Otolaryngol. Head Neck Surg. 150, 378–384 (2014).
Chou, K. L. Combined effect of vision and hearing impairment on depression in older adults: Evidence from the English Longitudinal Study of Ageing. J. Affect. Disord. 106, 191–196 (2008).
Jing, X., Lu, H. & Shusheng, G. WHO: Interpretation and reflections on the world hearing report. Chin. J. Otorhinolaryngol. Head Neck Surg. 56, 1131–1135 (2021).
The data from the sixth national census in China. https://www.stats.gov.cn/sj/pcsj/rkpc/6rp/indexch.htm (2010).
Hearing Health Blue Book: China Hearing Health Report. https://www.pishu.cn/zxzx/xwdt/574203.shtml (2021).
Ye, X., Zhu, D., Chen, S. & He, P. The association of hearing impairment and its severity with physical and mental health among Chinese middle-aged and older adults. Health Qual. Life Outcomes 18, 1–8 (2020).
Kamil, R. J. & Lin, F. R. The effects of hearing impairment in older adults on communication partners: A systematic review. J. Am. Acad. Audiol. 26, 155–182 (2015).
Bainbridge, K. E. & Wallhagen, M. I. Hearing loss in an aging American population: Extent, impact, and management. Annu. Rev. Public Health 35, 139–152 (2014).
Fransen, E. et al. Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: A European population-based multicenter study. J. Assoc. Res. Otolaryngol. 9, 264–276 (2008).
Horikawa, C. et al. Diabetes and risk of hearing impairment in adults: A meta-analysis. J. Clin. Endocrinol. Metab. 98, 51–58 (2013).
Gates, G. A., Cobb, J. L., Dagostino, R. B. & Wolf, P. A. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch. Otolaryngol. Head Neck Surg. 119, 156–161 (1993).
Torre, P., Cruickshanks, K. J., Klein, B. E. K., Klein, R. & Nondahl, D. M. The association between cardiovascular disease and cochlear function in older adults. J. Speech Lang. Hear. Res. 48, 473–481 (2005).
Dhanda, N. & Taheri, S. A narrative review of obesity and hearing loss. Int. J. Obes. 41, 1066–1073 (2017).
von Haehling, S., Morley, J. E. & Anker, S. D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle. 1, 129–133 (2010).
Hwang, J. H., Wu, C. C., Hsu, C. J., Liu, T. C. & Yang, W. S. Association of central obesity with the severity and audiometric configurations of age-related hearing impairment. Obesity 17, 1796–1801 (2009).
Petermann-Rocha, F. et al. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 13, 86–99 (2022).
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet 10191, 2636–2646 (2019).
Cawthon, P. M. et al. Clinical definitions of sarcopenia and risk of hospitalization in community-dwelling older men: The osteoporotic fractures in men study. J. Gerontol. A Biol. Sci. Med. Sci. 10, 1383–1389 (2017).
Kitamura, A. et al. Sarcopenia: Prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J. Cachexia Sarcopenia Muscle 1, 30–38 (2021).
Zhang, X. M. et al. Falls among older adults with sarcopenia dwelling in nursing home or community: A meta-analysis. Clin. Nutr. 1, 33–39 (2020).
Goates, S. et al. Economic impact of hospitalizations in US adults with sarcopenia. J. Frailty Aging 8, 93–99 (2019).
Ye, C. et al. Sarcopenia and catastrophic health expenditure by socio-economic groups in China: An analysis of household-based panel data. J. Cachexia Sarcopenia Muscle 13, 1938–1947 (2022).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 3, 300 (2020).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31 (2019).
Fielding, R. A. et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiologly, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 12, 249–256 (2011).
Studenski, S. A. et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A-Biol. 69, 547–558 (2014).
Kawakami, R. et al. A prospective cohort study of muscular and performance fitness and risk of hearing loss: The Niigata wellness study. Am. J. Med. 134, 235–242 (2021).
Lee, J., Han, K., Song, J. J., Im, G. J. & Chae, S. W. Sarcopenia and hearing loss in older Koreans: Findings from the Korea National Health and Nutrition Examination Survey (KNHANES) 2010. PloS One 11, e0150281 (2016).
Ran, S., Wu, Y. J. & Liu, B. L. Sarcopenia and risk of hearing loss: A 2-sample Mendelian randomization study. Am. J. Med. 136, E102–E103 (2023).
Fetoni, A. R., Picciotti, P. M., Paludetti, G. & Troiani, D. Pathogenesis of presbycusis in animal models: A review. Exp. Gerontol. 46, 413–425 (2011).
Yamasoba, T. et al. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res. 303, 30–38 (2013).
China Health and Retirement Longitudinal Study. http://charls.pku.edu.cn/en
Wu, X. et al. Sarcopenia prevalence and associated factors among older Chinese population: Findings from the China health and retirement longitudinal study. PLoS One 3, e0247617 (2021).
Yang, M. et al. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: A prospective study. J. Cachexia Sarcopenia Muscle 2, 251–258 (2017).
Sindhusake, D. et al. Validation of self-reported hearing loss. The Blue Mountains hearing study. Int. J. Epidemiol. 30, 1371–1378 (2001).
Ferrite, S., Santana, V. S. & Marshall, S. W. Validity of self-reported hearing loss in adults: Performance of three single questions. Rev. Saude. Publ. 45, 824–830 (2011).
Kang, S. H. et al. Association between sarcopenia and hearing thresholds in postmenopausal women. Int. J. Med. Sci. 14, 470–476 (2017).
Petroni, M. L. et al. Prevention and treatment of sarcopenic obesity in women. Nutrients 11, 1302 (2019).
Choi, K. M. Sarcopenia and sarcopenic obesity. Endocrinol. Metab. (Seoul) 28, 86–89 (2013).
Han, K. et al. Sarcopenia as a determinant of blood pressure in older Koreans: Findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008–2010. PLoS One 9, e86902 (2014).
Wilmore, J. H. et al. Heart rate and blood pressure changes with endurance training: The HERITAGE family study. Med. Sci. Sports Exerc. 33, 107–116 (2001).
Oron, Y., Elgart, K., Marom, T. & Roth, Y. Cardiovascular risk factors as causes for hearing impairment. Audiol. Neurootol. 19, 256–260 (2014).
Shi, X. R. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res. 338, 52–63 (2016).
Srikanthan, P. & Karlamangla, A. S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 96, 2898–2903 (2011).
Manouvrier, S. et al. Point mutation of the mitochondrial tRNA (Leu) gene (A3243G) in maternally inherited hypertrophic cardiomyopathy, diabetes-mellitus, renal-failure, and sensorineural deafness. J. Med. Genet. 32, 654–656 (1995).
Akinpelu, O. V., Ibrahim, F., Waissbluth, S. & Daniel, S. J. Histopathologic changes in the cochlea associated with diabetes mellitus–A review. Otol. Neurotol. 35, 764–774 (2014).
Fukushima, H. et al. Cochlear changes in patients with type 1 diabetes mellitus. Otolaryngol. Head Neck Surg. 133, 100–106 (2005).
Wen, X., Wang, M., Jiang, C. M. & Zhang, Y. M. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac. J. Clin. Nutr. 20, 551–556 (2011).
Acknowledgements
I would like to thank the CHARLS research team, the field team, and every respondent for their time and efforts that they have devoted to the CHARLS project.
Author information
Authors and Affiliations
Contributions
Z.Z. is responsible for all the work on this thesis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z. Association between sarcopenia and hearing impairment in middle-aged and elderly people in China: a prospective cohort study. Sci Rep 14, 6061 (2024). https://doi.org/10.1038/s41598-024-56850-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56850-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.