Abstract
Anemia is common in critically ill patients undergoing continuous renal replacement therapy (CRRT). We investigated the impact of anemia requiring red blood cell (RBC) transfusion or erythropoiesis-stimulating agents (ESAs) on patient outcomes after hospital discharge in critically ill patients with acute kidney injury (AKI) requiring CRRT. In this retrospective cohort study using the Health Insurance Review and Assessment database of South Korea, 10,923 adult patients who received CRRT for 3 days or more between 2010 and 2019 and discharged alive were included. Anemia was defined as the need for RBC transfusion or ESAs. Outcomes included cardiovascular events (CVEs) and all-cause mortality after discharge. The anemia group showed a tendency to be older with more females and had more comorbidities compared to the control group. Anemia was not associated with an increased risk of CVEs (adjusted hazard ratio [aHR]: 1.05; 95% confidence interval [CI]: 0.85–1.29), but was associated with an increased risk of all-cause mortality (aHR: 1.41; 95% CI 1.30–1.53). For critically ill patients with AKI requiring CRRT, anemia, defined as requirement for RBC transfusion or ESAs, may increase the long-term risk of all-cause mortality.
Similar content being viewed by others
Introduction
Anemia is a very common condition in critically ill patients, and approximately 30–50% require a red blood cell (RBC) transfusion1,2. Various factors can contribute to anemia in critically ill patients, including major bleeding, significant hemolysis, insufficient erythropoietin (EPO) production, impaired bone marrow response to EPO, nutritional deficiencies, obscure blood loss from frequent blood sampling, occult gastrointestinal bleeding, or invasive procedures3,4,5,6. Inflammatory cytokines can disrupt iron homeostasis, hinder EPO production, and suppress bone marrow erythropoiesis7,8,9.
EPO is an essential hormone for RBC production, primarily secreted by renal interstitial fibroblasts10,11. Kidney dysfunction can inevitably aggravate insufficient production of EPO12. Therefore, critically ill patients with acute kidney injury (AKI) are at a higher risk of developing anemia due to both kidney dysfunction and the severity of their illness. Renal replacement therapy, including continuous renal replacement therapy (CRRT), can further exacerbate anemia because of blood loss from hemofilter clotting that cannot be returned13 as well as bleeding associated with catheters or anticoagulation14.
Anemia has been associated with poor outcomes in various clinical settings15,16,17. AKI is recognized as a long-term risk factor for cardiovascular events and mortality18. One study demonstrated that the substantial impact of AKI on long-term outcomes was, in part, attributed to the development of anemia following AKI19. However, the influence of anemia on long-term outcomes in patients with AKI requiring CRRT remains unclear. Therefore, we investigated the impact of anemia during hospitalization on cardiovascular outcomes and all-cause mortality following hospital discharge among critically ill patients with AKI requiring CRRT.
Results
Patient characteristics
Of 10,923 patients who survived until hospital discharge, 78% (N = 8,495) had anemia during their hospitalization (RBC transfusion in 5,948, erythropoiesis- stimulating agent [ESA] in 270, and RBC transfusion and ESA in 2,227 patients). The anemia group exhibited a higher average age, greater number of comorbidities (except for diabetes mellitus), higher proportion of septic shock, increased mechanical ventilation usage, and a longer stay in the intensive care unit (ICU) compared to the control group (Table 1).
Cardiovascular events after hospital discharge
There were 622 cardiovascular events, of which 141 occurred in the control group and 481 in the anemia group (control group vs. anemia group; 1.6 vs. 2.0 per 100 person-years) (Table 2 and Fig. 1A). Multivariable analysis revealed that the risk of cardiovascular events in the control and anemia groups was similar (adjusted HR: 1.05; 95% CI 0.85–1.29). Stroke was the most frequent type of cardiovascular event in both control and anemia groups (1.0 vs. 1.2 per 100 person-years, respectively). However, the difference between the two groups was not statistically significant (adjusted HR: 0.97; 95% CI 0.75–1.27). Risks of other cardiovascular events, including heart failure, acute myocardial infarction (MI), and revascularization were also comparable between the control and anemia groups.
All-cause mortality after hospital discharge
During the follow-up, 5,094 deaths occurred after hospital discharge. The anemia group exhibited a significantly higher risk of all-cause mortality compared to the control group (control group vs. anemia group; 8.4 vs. 17.3 per 100 person-years; adjusted HR: 1.41; 95% CI 1.30–1.53; Table 2 and Fig. 1B).
Subgroup analysis
The association between anemia and cardiovascular events was not statistically significant in various subgroups, except for age (Fig. 2A). In patients aged ≥ 65 years, there was no difference in cardiovascular events between the control and anemia groups. However, among patients aged < 65 years, the risk of cardiovascular events was significantly higher in the anemia group compared to that in the control group (adjusted HR: 1.50; 95% CI 1.10–2.05). Patient characteristics between the control and anemia groups were comparable in both subgroups, for those aged ≥ 65 years and those < 65 years (Supplementary Table 1).
Subgroup analysis stratified according to age, sex, and comorbidities for cardiovascular events and all-cause mortality. (A) Cardiovascular events and (B) all-cause mortality. A cardiovascular event was defined as a composite outcome of heart failure, acute myocardial infarction, revascularization, or stroke.
The association between anemia and an increased risk of all-cause mortality remained consistently significant in all subgroups, including patients with cancer (adjusted HR: 1.40; 95% CI 1.17–1.67 and 1.36) and without cancer (adjusted HR: 1.40; 95% CI 1.25–1.49) (Fig. 2B).
Sensitivity analysis
In the additional analyses conducted according to the definition of anemia, no significant association was observed between anemia defined by RBC transfusion and the risk of cardiovascular events. However, anemia defined by ESA prescription showed a tendency toward an increased risk of cardiovascular events (adjusted HR: 1.23; 95% CI 0.96–1.57).
Anemia defined by RBC transfusion alone (adjusted HR: 1.53; 95% CI 1.40–1.66) and combination of RBC transfusion and ESA prescription (adjusted HR: 1.23; 95% CI 1.13–1.34) also showed an increased risk of all-cause mortality (Table 3). In contrast, anemia defined by ESA alone was associated with a decreased risk of all-cause mortality (adjusted HR: 0.78; 95% CI 0.64–0.97).
Discussion
In this large nationwide population-based cohort study, we demonstrated the association between anemia defined as requirement for RBC transfusion or ESA therapy, during hospitalization and an increased risk of post-discharge all-cause mortality in critically ill patients with AKI requiring CRRT. This increased mortality associated with anemia may not be attributable to an increase in cardiovascular events among the older population. However, among individuals < 65 years old, anemia during hospitalization was associated with an increased risk of post-discharge cardiovascular events. Our findings indicate that anemia can serve as a long-term prognostic factor in critically ill patients who have undergone CRRT.
Recent studies have indicated that anemia can persist for a significant period following critical illness20,21. In a large cohort study, the one-year recovery rates for moderate anemia (hemoglobin [Hb] < 10.0 g/dL) and severe anemia (Hb < 8.0 g/dL) at hospital discharge were merely 39% and 24%, respectively. Moreover, a higher Hb at hospital discharge was associated with decreased mortality in critically ill patients20. Anemia persisting post-critical illness may indicate incomplete recovery from an underlying disease or chronic inflammation21. A new syndrome called “persistent inflammation-immunosuppression and catabolism syndrome” (PICS) was proposed to explain the underlying pathological consequences for the high morbidity and mortality observed in patients with chronic critical illness following sepsis or severe injury22. The primary mechanism of PICS involves the expansion of a heterogeneous population of inducible immature myeloid cells during critical illness, which predominantly exhibit immunosuppressive and inflammatory properties22,23. Therefore, anemia may serve as an indicator for not only the severity of acute illness but also long-term outcomes affected by PICS.
Our study underscores the significance of anemia requiring RBC transfusion during hospitalization as a prognostic factor for long-term mortality after discharge in critically ill patients undergoing CRRT. This aligns with numerous studies that have reported associations between anemia and poor clinical outcomes24,25,26. Notably, there is a lack of evidence supporting the notion that correcting anemia with transfusions leads to improved patient outcomes; rather, transfusions can expose patients to various transfusion-related adverse events27. Therefore, restrictive RBC transfusion strategies have been recommended.28 Nevertheless, the association between anemia and long-term outcomes in critically ill patients has not been fully elucidated through large-scale cohort studies. A recent study showed that anemia (Hb < 10.0 g/dL) in the first week was associated with long-term morality among critically ill patients29, and another study reported that anemia following AKI was associated with long-term adverse outcomes19. We analyzed the association between severe anemia and long-term outcomes focusing on severe AKI patients requiring CRRT in a much larger cohort compared to these previous studies. The National Health Insurance Service (NHIS) database did not allow us to determine when CRRT was applied or when blood transfusions or ESAs were administered during hospitalization. Therefore, comparing results over a short period of time can be unreliable. The cumulative incidence of mortality shows that the difference between the control and anemia groups occurs primarily within 1–2 years. Therefore, despite the lack of consistency at the start of follow-up, we believe that the difference in mortality between the control and anemia groups may be related to anemia during hospitalization rather than to new events after discharge. Our findings strongly suggest the importance of vigilant monitoring and management of ongoing anemia and correctable conditions, even after hospital discharge, particularly for critically ill patients with AKI severe enough to require CRRT.
The increase in new-onset cardiovascular events was evident among patients aged < 65 years in the anemia group compared to the control group. However, for patients aged ≥ 65 years, the incidence of cardiovascular events remained comparable regardless of anemia. In contrast, there were no associations between anemia and cardiovascular events according to sex and comorbidities. This age-dependent difference remains unclear. However, a possible explanation is that older patients at a higher risk of cardiovascular events may have been preemptively excluded from our cohort due to their preexisting cardiovascular diseases. Anemia is recognized as a risk factor for cardiovascular diseases, although the causal relationship remains controversial.15 Anemia may cause or aggravate cardiovascular diseases through hemodynamic effects30 and other mechanisms, such as decreased adiponectin levels and peripheral endothelial progenitor cells.31,32 While our study did not demonstrate that severe anemia during hospitalization increased the overall risk of cardiovascular events in critically ill patients, it did reveal the increased risk in patients aged < 65 years. Further research is needed to clarify the causal relationship between anemia and the risk of cardiovascular events according to age.
In our study, we observed that RBC transfusion during hospitalization was associated with an increased risk of all-cause mortality, while ESA alone was associated with a decreased risk of all-cause mortality. Previous studies have revealed inconsistent results regarding the effects of ESA therapy on mortality in critically ill patients, although a recent meta-analysis suggested that ESA therapy may reduce mortality in critically ill patients33,34,35. Moreover, ESA therapy appeared to reduce the requirement for RBC transfusions in critically ill patients, although the clinical relevance is yet to be determined36. Although recent guideline suggested not using ESAs to prevent RBC transfusion (conditional recommendation, low certainty)37, its use is still controversial, especially in critically ill patients with renal impairment; thus, the application and method of ESA therapy are highly dependent on the preferences of the physician. Since the results of observational studies are inevitably subject to selection bias and confounders, further prospective studies are required to elucidate the impact of ESA therapy on mortality or requirement of RBC transfusion in critically ill patients with AKI.
We aimed to evaluate the effect of anemia on long-term outcomes by assuming that patients receiving RBC transfusion or ESAs without history of surgery or intervention for bleeding control are likely to have significant anemia. However, our study could not distinguish whether adverse outcomes were related to anemia or RBC transfusion per se. Beyond short-term adverse effects, transfusions can also exert long-term effects by mechanisms such as transfusion-related immunomodulation or iron overload38,39. Furthermore, white blood cells are known to release several pro-inflammatory mediators during storage40. Previous studies reported the association of transfusions with long-term adverse outcomes in various clinical settings such as surgery or myocardial infarction41,42,43. Therefore, further studies that comprehensively evaluate long-term outcomes according to hemoglobin level as well as transfusion are required.”
Our study has several limitations. First, the causal relationship between anemia and mortality or cardiovascular outcomes was not fully elucidated due to the retrospective nature of the study. Additionally, the database of KNIHS had limitations in providing a full comprehensive picture of patient conditions, especially the diagnoses at the time of ICU admission and detailed laboratory findings. Although kidney function plays an important role in the development or persistence of anemia, serum creatinine or cystatin C was not available in this study. Second, as mentioned earlier, the definition of anemia was determined according to RBC transfusion or ESA administration because hemoglobin or hematocrit levels were not available in the KNIHS database. Although a restrictive strategy of RBC transfusion has been widely accepted in clinical practice28, the strategy for RBC transfusion and ESA administration can vary among physicians and may be influenced by underlying diseases or personal convictions, including religious considerations. However, we consider that RBC transfusion can reflect anemia in general. Third, the data regarding the persistence or degree of anemia after discharge could not be collected. Fourth, our study focused on patients with AKI who were critically ill enough to require CRRT to reduce heterogeneity in study population, so that the conclusion of our study may be insufficient for generalization to all patients with AKI. Despite these limitations, our study holds clinical significance as it reveals the association between anemia and long-term outcomes in a large cohort of critically ill patients with severe AKI requiring CRRT.
In conclusion, our study demonstrates that anemia, defined as requirement for RBC transfusion or ESA therapy, during hospitalization was associated with an increased risk of post-discharge all-cause mortality in critically ill patients with AKI requiring CRRT. Additionally, the risk of new-onset cardiovascular events was also increased in the patients aged < 65 years in this cohort. Therefore, it is crucial to provide vigilant monitoring and treatment for all correctable conditions, including anemia, to critically ill patients with severe anemia and AKI requiring CRRT, even after hospital discharge. Appropriate ESA therapy with adequate nutritional support including iron supplementation and active rehabilitation after discharge should be considered in patients with persistent anemia and renal impairment.
Materials and methods
Study population and design
This retrospective cohort study obtained data from the Korean National Health Insurance Service (KNHIS) database, which provides comprehensive coverage to approximately 97% of the Korean population. The remaining 3% of individuals who are unable to afford national insurance are covered by the Medical Aid Program44. Therefore, the KNHIS database effectively represents the entirety of the South Korean population and contains the national records of all covered inpatient and outpatient visits, prescriptions, and procedures. The KNHIS data includes modules on insurance eligibility and medical treatment. The insurance eligibility module contains the information on age, sex, residential area, and income level. The medical treatment module contains information related to treatments, including conditions and prescriptions45. This study was conducted with the approval of the KNHIS official review committee (protocol number: NHIS-2022-1-240), and access to the data was granted per approval.
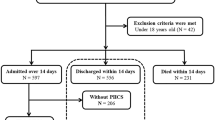
We aimed to evaluate the long-term consequences of anemia in relation to cardiovascular events and mortality after hospital discharge. For this, we included adult patients > 18 years who were admitted to the ICU and received CRRT for ≥ 3 days between January 1, 2010 and December 31, 2019 (N = 119,421). We excluded participants with preexisting end-stage kidney disease (ESKD) (N = 19,605); history of acute MI, stroke, heart failure, or revascularization (N = 43,039); and history of other cardiovascular diseases (N = 28,479). We further excluded patients who received major surgery, endoscopic hemostasis, or vascular embolization (N = 52,946) and those who died during hospitalization (N = 19,294). Thus, a total of 10,923 participants were included in the final analyses.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Samsung Medical Center in compliance with the Declaration of Helsinki (IRB No. 2021-01-052, December 31, 2020, title: “Investigation of Evidence-Based Optimal Management Strategies for Continuous Renal Replacement Therapy”). The study procedures were followed in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Helsinki Declaration of 1975. The Institutional Review Board of Samsung Medical Center waived the requirement for informed consent due to the retrospective nature of the study and deidentified data collection13.
Exposure
Anemia was defined as the requirement for RBC transfusion or the prescription of ESAs. ESA prescription was defined as having at least one prescription of darbepoetin or methoxy polyethylene glycol-epoetin, or a minimum of three prescriptions of short-acting erythropoietin during hospitalization.
Study variables
The KNHIS records for inpatient and outpatient visits, prescriptions, and procedures were coded using the International Classification of Diseases (ICD), 10th Revision46. These data are highly reliable due to routine audits by the KNHIS and have been used in numerous peer-reviewed publications47,48. In a validation study, the diagnostic accuracy for MI in KNHIS data was reported to be 93%49.
We collected claim codes that encompassed information regarding comorbidities, management procedures during ICU admission, prescriptions, and demographic characteristics. Comorbidities, such as chronic liver disease, diabetes mellitus, chronic kidney disease, and cancer diagnosed within the year preceding hospitalization, were summarized using the Charlson index50,51. Additionally, we included in our analysis hypertension (ICD-10 codes: I10, I13, I15) and septic shock (defined as ≥ 2 days of vasopressor and ≥ 1 week of antibiotics administration), despite not being covered by the Charlson index. Management procedures included CRRT (Korean NHI procedure codes O7031-O7035, O7051-O7055) and mechanical ventilation (Korean NHI procedure codes M5857, M5858, or M5860).
Outcomes
The primary outcomes were cardiovascular events and all-cause mortality after hospital discharge. Cardiovascular events were defined as hospitalization for heart failure (ICD-10 codes I110, I130, I132, I255, I420, I425-I429, I43, I50, and I971), acute MI (ICD-10 codes I21-I23, and I252), the presence of coronary revascularization codes (percutaneous coronary intervention, Korean NHI codes M6551-M6554, M6561-M6567, and M6571-M6572; coronary artery bypass graft surgery [CABG], O1640-O1642, O1647-O1649, OA640-OA642, and OA647-OA649), or stroke (ICD-10 codes I63, I64, and G45). The data regarding all-cause mortality was obtained from death certificates collected by Statistics Korea, part of the Ministry of Strategy and Finance of South Korea47.
Subgroup analysis
We conducted subgroup analysis to evaluate the association of anemia with post-discharge cardiovascular events based on various preexisting factors. Subgroup analyses were stratified by age (age < 65 vs. ≥ 65 years old), sex, and specific comorbidities, including chronic liver disease, diabetes mellitus, chronic kidney disease, cancer, septic shock, and severe respiratory failure requiring mechanical ventilation.
Sensitivity analysis
We performed additional analysis based on different definitions of anemia, categorizing patients into three groups: those who received only RBC transfusions, those prescribed with ESA alone, and those who received both RBC transfusions and ESA prescriptions.
Statistical analysis
We compared the control and anemia groups using the following methods: continuous variables were presented as mean and standard deviation or as median and interquartile range, and we performed comparisons using the t-test or Mann–Whitney Test. Categorical variables were presented as numbers and proportions and compared using the chi-square test or Fisher’s exact test. Person-time was calculated from the date of hospital discharge to the event date, death, or the last follow-up date. Survival curves were generated by the Kaplan–Meier product-limit method and compared using log-rank tests. We used Cox proportional hazards regression models to estimate hazard ratio (HR) with 95% confidence interval (CI) for cardiovascular events and all-cause mortality, adjusting for age, sex, tertiary hospital, comorbidities, septic shock, CRRT duration, and mechanical ventilation. The proportional hazards assumption was assessed through log–log plots survival function and Schoenfeld residuals. To account for competing risks due to mortality, a proportional sub-distribution hazards regression model was employed for the incidence of cardiovascular events, considering death as a competing event. All analyses were two-sided and P-values < 0.05 were considered statistically significant. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc, Cary, NC, USA) and R software version 3.3.2 (Free Software Foundation, Inc., Boston, MA, USA).
Data availability
The data from this study cannot be freely shared by the authors as it is the property of the Korean National Health Insurance Service (KNHIS). However, the anonymized dataset underlying this article can be obtained upon reasonable request to the National Health Insurance Sharing Service (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do).
References
Corwin, H. L. et al. The CRIT Study: Anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit. Care Med. 32, 39–52. https://doi.org/10.1097/01.Ccm.0000104112.34142.79 (2004).
Thomas, J., Jensen, L., Nahirniak, S. & Gibney, R. T. Anemia and blood transfusion practices in the critically ill: A prospective cohort review. Heart Lung 39, 217–225. https://doi.org/10.1016/j.hrtlng.2009.07.002 (2010).
van Iperen, C. E. et al. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit. Care Med. 28, 2773–2778. https://doi.org/10.1097/00003246-200008000-00015 (2000).
Rodriguez, R. M. et al. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J. Crit. Care 16, 36–41. https://doi.org/10.1053/jcrc.2001.21795 (2001).
Rogiers, P. et al. Erythropoietin response is blunted in critically ill patients. Intensive Care Med. 23, 159–162. https://doi.org/10.1007/s001340050310 (1997).
Jackson Chornenki, N. L. et al. Blood loss from laboratory testing, anemia, and red blood cell transfusion in the intensive care unit: A retrospective study. Transfusion 60, 256–261. https://doi.org/10.1111/trf.15649 (2020).
Jelkmann, W. Proinflammatory cytokines lowering erythropoietin production. J. Interferon Cytokine Res. 18, 555–559. https://doi.org/10.1089/jir.1998.18.555 (1998).
Means, R. T. Jr. & Krantz, S. B. Inhibition of human erythroid colony-forming units by gamma interferon can be corrected by recombinant human erythropoietin. Blood 78, 2564–2567 (1991).
Nemeth, E. et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 113, 1271–1276. https://doi.org/10.1172/jci20945 (2004).
Fisher, J. W. A quest for erythropoietin over nine decades. Annu. Rev. Pharmacol. Toxicol. 38, 1–20. https://doi.org/10.1146/annurev.pharmtox.38.1.1 (1998).
Bachmann, S., Le Hir, M. & Eckardt, K. U. Co-localization of erythropoietin mRNA and ecto-5’-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J. Histochem. Cytochem. 41, 335–341. https://doi.org/10.1177/41.3.8429197 (1993).
Elliot, J. M. et al. Erythropoietin mimics the acute phase response in critical illness. Crit. Care 7, R35-40. https://doi.org/10.1186/cc2185 (2003).
Al-Dorzi, H. M. et al. Anemia, blood transfusion, and filter life span in critically ill patients requiring continuous renal replacement therapy for acute kidney injury: A case-control study. Crit. Care Res. Pract. 2019, 3737083. https://doi.org/10.1155/2019/3737083 (2019).
Akhoundi, A. et al. Incidence of adverse events during continuous renal replacement therapy. Blood Purif. 39, 333–339. https://doi.org/10.1159/000380903 (2015).
Sarnak, M. J. et al. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J. Am. Coll. Cardiol. 40, 27–33. https://doi.org/10.1016/s0735-1097(02)01938-1 (2002).
Sabatine, M. S. et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 111, 2042–2049. https://doi.org/10.1161/01.Cir.0000162477.70955.5f (2005).
Rasmussen, L., Christensen, S., Lenler-Petersen, P. & Johnsen, S. P. Anemia and 90-day mortality in COPD patients requiring invasive mechanical ventilation. Clin. Epidemiol. 3, 1–5. https://doi.org/10.2147/clep.S12885 (2010).
Odutayo, A. et al. AKI and long-term risk for cardiovascular events and mortality. J. Am. Soc. Nephrol. 28, 377–387. https://doi.org/10.1681/asn.2016010105 (2017).
Nishimoto, M. et al. Anemia following acute kidney injury after noncardiac surgery and long-term outcomes: The NARA-AKI cohort study. Clin. Kidney J. 14, 673–680. https://doi.org/10.1093/ckj/sfaa184 (2021).
Warner, M. A. et al. Prevalence of and recovery from anemia following hospitalization for critical illness among adults. JAMA Netw. Open 3, e2017843. https://doi.org/10.1001/jamanetworkopen.2020.17843 (2020).
Bateman, A. P., McArdle, F. & Walsh, T. S. Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit. Care Med. 37, 1906–1912. https://doi.org/10.1097/CCM.0b013e3181a000cf (2009).
Mira, J. C. et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit. Care Med. 45, 253–262. https://doi.org/10.1097/ccm.0000000000002074 (2017).
Bronte, V. Myeloid-derived suppressor cells in inflammation: Uncovering cell subsets with enhanced immunosuppressive functions. Eur. J. Immunol. 39, 2670–2672. https://doi.org/10.1002/eji.200939892 (2009).
Du Pont-Thibodeau, G., Harrington, K. & Lacroix, J. Anemia and red blood cell transfusion in critically ill cardiac patients. Ann. Intensive Care 4, 16. https://doi.org/10.1186/2110-5820-4-16 (2014).
Abdullah, H. R. et al. Association between preoperative anaemia with length of hospital stay among patients undergoing primary total knee arthroplasty in Singapore: A single-centre retrospective study. BMJ Open 7, e016403. https://doi.org/10.1136/bmjopen-2017-016403 (2017).
Musallam, K. M. et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet 378, 1396–1407. https://doi.org/10.1016/s0140-6736(11)61381-0 (2011).
Mirski, M. A., Frank, S. M., Kor, D. J., Vincent, J. L. & Holmes, D. R. Jr. Restrictive and liberal red cell transfusion strategies in adult patients: Reconciling clinical data with best practice. Crit. Care 19, 202. https://doi.org/10.1186/s13054-015-0912-y (2015).
Carson, J. L. et al. Red blood cell transfusion: A clinical practice guideline from the AABB*. Ann. Intern. Med. 157, 49–58. https://doi.org/10.7326/0003-4819-157-1-201206190-00429 (2012).
Lin, I. H. et al. Anaemia in the first week may be associated with long-term mortality among critically ill patients: Propensity score-based analyses. BMC Emerg. Med. 23, 32. https://doi.org/10.1186/s12873-023-00806-w (2023).
Nelson, A. H., Fleisher, L. A. & Rosenbaum, S. H. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit. Care Med. 21, 860–866. https://doi.org/10.1097/00003246-199306000-00013 (1993).
Hara, K. et al. Determinants of serum high molecular weight (HMW) adiponectin levels in patients with coronary artery disease: Associations with cardio-renal-anemia syndrome. Intern. Med. 50, 2953–2960. https://doi.org/10.2169/internalmedicine.50.5926 (2011).
Solomon, A. et al. Endothelial progenitor cells are suppressed in anemic patients with acute coronary syndrome. Am. J. Med. 125, 604–611. https://doi.org/10.1016/j.amjmed.2011.10.025 (2012).
Corwin, H. L. et al. Efficacy and safety of epoetin alfa in critically ill patients. N. Engl. J. Med. 357, 965–976. https://doi.org/10.1056/NEJMoa071533 (2007).
Corwin, H. L. et al. Efficacy of recombinant human erythropoietin in critically ill patients: A randomized controlled trial. JAMA 288, 2827–2835. https://doi.org/10.1001/jama.288.22.2827 (2002).
Litton, E., Latham, P., Inman, J., Luo, J. & Allan, P. Safety and efficacy of erythropoiesis-stimulating agents in critically ill patients admitted to the intensive care unit: A systematic review and meta-analysis. Intensive Care Med. 45, 1190–1199. https://doi.org/10.1007/s00134-019-05686-y (2019).
Wijnberge, M. et al. Erythropoiesis-stimulating agents as replacement therapy for blood transfusions in critically ill patients with anaemia: A systematic review with meta-analysis. Transfus. Med. 30, 433–441. https://doi.org/10.1111/tme.12715 (2020).
Vlaar, A. P. et al. Transfusion strategies in non-bleeding critically ill adults: A clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 46, 673–696. https://doi.org/10.1007/s00134-019-05884-8 (2020).
Refaai, M. A. & Blumberg, N. Transfusion immunomodulation from a clinical perspective: an update. Expert. Rev. Hematol. 6, 653–663. https://doi.org/10.1586/17474086.2013.850026 (2013).
Dasararaju, R. & Marques, M. B. Adverse effects of transfusion. Cancer Control 22, 16–25. https://doi.org/10.1177/107327481502200104 (2015).
Nielsen, H. J. et al. Time-dependent, spontaneous release of white cell- and platelet-derived bioactive substances from stored human blood. Transfusion 36, 960–965. https://doi.org/10.1046/j.1537-2995.1996.36111297091738.x (1996).
Morris, F. J. D., Fung, Y. L., Craswell, A. & Chew, M. S. Outcomes following perioperative red blood cell transfusion in patients undergoing elective major abdominal surgery: A systematic review and meta-analysis. Br. J. Anaesth. 131, 1002–1013. https://doi.org/10.1016/j.bja.2023.08.032 (2023).
Chen, F. T. et al. Effect of massive blood transfusion on late outcomes after surgical repair of acute type A aortic dissection. Medicine (Baltimore) 98, e17816. https://doi.org/10.1097/md.0000000000017816 (2019).
Shishehbor, M. H. et al. Impact of blood transfusion on short- and long-term mortality in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2, 46–53. https://doi.org/10.1016/j.jcin.2008.09.011 (2009).
Kim, D. et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: Nationwide cohort study. BMJ 373, n991. https://doi.org/10.1136/bmj.n991 (2021).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. https://doi.org/10.1093/ije/dyv319 (2016).
Chun, C. B., Kim, S. Y., Lee, J. Y. & Lee, S. Y. Republic of Korea health system review. Health Syst. Transit. 11, 1–184 (2009).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort profile: The national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 46, e15. https://doi.org/10.1093/ije/dyv319 (2017).
Shin, D. W., Cho, B. & Guallar, E. Korean national health insurance database. JAMA Intern. Med. 176, 138. https://doi.org/10.1001/jamainternmed.2015.7110 (2016).
Kimm, H., Yun J. E., Lee S. H., Jang Y., & Jee S. H. Validity of the diagnosis of acute myocardial infarction in korean national medical health insurance claims data: the korean heart study. Korean Circ J. 42, 10–15. https://doi.org/10.4070/kcj.2012.42.1.10 (2012).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383. https://doi.org/10.1016/0021-9681(87)90171-8 (1987).
Kim, K. H. Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients. J. Prevent. Med. Public 43, 42–49. https://doi.org/10.3961/jpmph.2010.43.1.42 (2010).
Acknowledgements
We would like to thank the Editage for the English language editing.
Funding
This study received support from the Korean Health Technology Research and Development Project (no. HC20C0085) through the Korean Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea. The funding entity had no role in the study design, data collection, analysis, reporting, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
H.R.J. conceptualized the study and obtained the funding. H. P. and D. K. were responsible for data curation. J.J., K.L., D.K., J.C., J.E.L., W.H., and H.R.J. were responsible for the investigation. H.P. and D.K. were responsible for the formal analysis. J.J., H.P., D.K., J.C., and H.R.J. were responsible for the methodology. H.P. and D.K. were responsible for visualization. J.J. and D.K. wrote the initial draft. J.J. and H.R.J. reviewed and edited the manuscript. J.C., J.E.L., and W.H. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeon, J., Kang, D., Park, H. et al. Impact of anemia requiring transfusion or erythropoiesis-stimulating agents on new-onset cardiovascular events and mortality after continuous renal replacement therapy. Sci Rep 14, 6556 (2024). https://doi.org/10.1038/s41598-024-56772-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56772-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.