Abstract
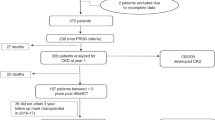
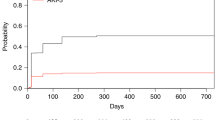
Acute kidney injury (AKI) is a frequent complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT), but few studies have focused on AKI treated with kidney replacement therapy (AKI-KRT), particularly among critically ill patients. We investigated the incidence, risk factors, and 90-day mortality associated with AKI-KRT in 529 critically ill adult allo-HSCT recipients admitted to the ICU within 1-year post-transplant at two academic medical centers between 2011 and 2021. AKI-KRT occurred in 111 of the 529 patients (21.0%). Lower baseline eGFR, veno-occlusive disease, thrombotic microangiopathy, admission to an ICU within 90 days post-transplant, and receipt of invasive mechanical ventilation (IMV), total bilirubin ≥5.0 mg/dl, and arterial pH <7.40 on ICU admission were each associated with a higher risk of AKI-KRT. Of the 111 patients with AKI-KRT, 97 (87.4%) died within 90 days. Ninety-day mortality was 100% in each of the following subgroups: serum albumin ≤2.0 g/dl, total bilirubin ≥7.0 mg/dl, arterial pH ≤7.20, IMV with moderate-to-severe hypoxemia, and ≥3 vasopressors/inotropes at KRT initiation. AKI-KRT was associated with a 6.59-fold higher adjusted 90-day mortality in critically ill allo-HSCT vs. non-transplanted patients. Short-term mortality remains exceptionally high among critically ill allo-HSCT patients with AKI-KRT, highlighting the importance of multidisciplinary discussions prior to KRT initiation.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Zager RA, O’Quigley J, Zager BK, Alpers CE, Shulman HM, Gamelin LM, et al. Acute Renal Failure Following Bone Marrow Transplantation: A Retrospective Study of 272 Patients. Am J Kidney Dis. 1989;13:210–6.
Renaghan AD, Jaimes EA, Malyszko J, Perazella MA, Sprangers B, Rosner MH. Acute Kidney Injury and CKD Associated with Hematopoietic Stem Cell Transplantation. Clin J Am Soc Nephrol. 2020;15:289–97.
Parikh CR, Mcsweeney P, Schrier RW. Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int. 2005;67:1999–2005.
Andronesi A, Sorohan B, Burcea A, Lipan L, Stanescu C, Craciun O, et al. Incidence and Risk Factors for Acute Kidney Injury after Allogeneic Stem Cell Transplantation: A Prospective Study. Biomedicines. 2022;10:262.
Sehgal B, George P, John M, Samuel C. Acute kidney injury and mortality in hematopoietic stem cell transplantation: A single-center experience. Indian J Nephrol. 2017;27:13.
Abramson MH, Gutgarts V, Zheng J, Maloy MA, Ruiz JD, Scordo M, et al. Acute Kidney Injury in the Modern Era of Allogeneic Hematopoietic Stem Cell Transplantation. Clin J Am Soc Nephrol. 2021;16:1318–27.
Hahn T, Rondeau C, Shaukat A, Jupudy V, Miller A, Alam AR, et al. Acute renal failure requiring dialysis after allogeneic blood and marrow transplantation identifies very poor prognosis patients. Bone Marrow Transplant. 2003;32:405–10.
Kanduri SR, Kovvuru K, Cheungpasitporn W, Thongprayoon C, Bathini T, Garla V, et al. Kidney Recovery From Acute Kidney Injury After Hematopoietic Stem Cell Transplant: A Systematic Review and Meta-Analysis. Cureus [Internet]. 2021 Jan [cited 2022 Nov 17]; https://www.cureus.com/articles/47366-kidney-recovery-from-acute-kidney-injury-after-hematopoietic-stem-cell-transplant-a-systematic-review-and-meta-analysis.
Depuydt P, Kerre T, Noens L, Nollet J, Offner F, Decruyenaere J, et al. Outcome in critically ill patients with allogeneic BM or peripheral haematopoietic SCT: a single-centre experience. Bone Marrow Transplant. 2011;46:1186–91.
Miyata M, Ichikawa K, Matsuki E, Watanabe M, Peltier D, Toubai T. Recent Advances of Acute Kidney Injury in Hematopoietic Cell Transplantation. Front Immunol. 2022;12:779881.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter. 2012;2:19–36.
Díaz-Lagares C, Fox L, García-Roche A, Santafe M, Romera I, Barba P, et al. Sequential Organ Failure Assessment Score and the Need for Organ Support Predict Mortality in Allogeneic Stem Cell Transplant Patients Admitted to the Intensive Care Unit. Transplant Cell Ther. 2021;27:865.e1–865.e7.
Saddadi F, Najafi I, Hakemi MS, Falaknazi K, Attari F, Bahar B. Frequency, risk factors, and outcome of acute kidney injury following bone marrow transplantation at Dr Shariati Hospital in Tehran. Iran J Kidney Dis. 2010;4:20–6.
Chapchap EC, Doher MP, Kerbauy LN, Belucci TR, de Santos FPS, Ribeiro AAF, et al. Need for hemodialysis in patients undergoing hematopoietic stem cell transplantation: risk factors and survival in a retrospective cohort. Hematol Transfus Cell Ther. 2022 May;S2531137922000797.
Lengliné E, Chevret S, Moreau AS, Pène F, Blot F, Bourhis JH, et al. Changes in intensive care for allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2015;50:840–5.
Benz R, Schanz U, Maggiorini M, Seebach JD, Stussi G. Risk factors for ICU admission and ICU survival after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49:62–5.
Michel CS, Teschner D, Schmidtmann I, Theobald M, Hauptrock B, Wagner-Drouet EM, et al. Prognostic factors and outcome of adult allogeneic hematopoietic stem cell transplantation patients admitted to intensive care unit during transplant hospitalization. Sci Rep. 2019;9:19911.
Yu ZP, Ding JH, Chen BA, Liu BC, Liu H, Li YF, et al. Risk factors for acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Chin J Cancer. 2010;29:946–51.
Hingorani SR, Guthrie K, Batchelder A, Schoch G, Aboulhosn N, Manchion J, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int. 2005;67:272–7.
Kersting S, Koomans HA, Hené RJ, Verdonck LF. Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant. 2007;39:359–65.
Acknowledgements
No funding was provided for this study. The authors are supported by the following grants from the National Institutes of Health: K23DK125672 (SG); R01HL142093-01 (RMB); R01DK130839 (MES); and R01HL144566, R01DK125786, and R01DK126685 (DEL).
Author information
Authors and Affiliations
Contributions
Research idea and study design: HK, RA, RS, RMB, SG, MS, DEL; Data acquisition: HK, RA, SS, LD, SAK, SK, OY, CT, DM, RN, RS, ZD, IP, TS, AEM; Data analysis/interpretation: HK RA, DEL; Statistical analysis HK, RA, DEL; Supervision or mentorship: HK, RA, DEL. Each author contributed important intellectual content during paper drafting.
Corresponding author
Ethics declarations
Competing interests
The authors report no competing financial interests in relation to this work. DEL received research support from BioPorto, BTG International, and Metro International Biotech LLC, and has received consulting fees from Sidereal Therapeutics, Casma Therapeutics, and MexBrain. RJS has received consulting fees from Vor Biopharma, Smart Immune, Daiichi Sankyo Inc., Neovii, CSL Behring, Bluesphere Bio, Cugene, Jasper, Takeda, Jazz Pharmaceuticals, Precision Biosciences, Alexion, and Rheos Therapeutics. RMB served on advisory boards for Genentech and Merck. SG received research support from BTG International, GE Healthcare, and AstraZeneca, is a member of GlaxoSmithKline’s Global Anemia Council, a consultant for Secretome and Proletariat Therapeutics, and founder of the American Society of Onconephrology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, H., Ali, R., Short, S. et al. AKI treated with kidney replacement therapy in critically Ill allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 59, 178–188 (2024). https://doi.org/10.1038/s41409-023-02136-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02136-8