Abstract
The Mediterranean fruit fly (medfly) (Ceratitis capitata, Diptera: Tephritidae), is an extremely polyphagous pest that threatens the fruit production and trading industry worldwide. Monitoring C. capitata populations and analysing its dynamics and phenology is considered of outmost importance for designing and implementing sound management approaches. The aim of this study was to investigate the factors regulating the population dynamics of the C. capitata in a coastal and semi-mountainous area. We focused on effects of topography (e.g. elevation), host presence and seasonal patterns of ripening on the phenological patterns considering data collected in 2008. The experimental area is characterized by mixed fruit orchards, and Mediterranean climate with mild winters. Two trap types were used for population monitoring. The female targeted McPhail type and the male targeted Jackson type. Traps were placed in farms located at different elevations and landscape morphology (coastal and semi-mountainous areas). The main crops included citrus, apples, peaches, plums, pears, figs, quinces and apricots. Adult captures were first recorded in May, peaked in mid-summer and mid-autumn and almost ceased at the end of the season (January 2008). Captures in the coastal areas preceded that of highlands by 15 days. Most of the adults detected during the fruit ripening of late stone fruit cultivars (first peak) and citrus (second peak). The probability of capturing the first adults preceded almost three weeks the peak of adult captures either considering the elevation or host focus analyses. The results provide valuable information on the seasonal population trend of C. capitata in mixed fruit Mediterranean orchards and can support the set-up of IPM systems in areas with various landscapes and different hosts throughout the fruit growing season.
Similar content being viewed by others
Introduction
Fruit flies (Diptera: Tephritidae) are considered among the most destructive fruit pests worldwide. The population dynamics of tephritids and phenology patterns are related to several biotic and abiotic factors1,2,3,4. Temperature, humidity and host availability are strongly related with altitude and particularities of landscape in each area generating temporal and spatial gradients that regulate population densities and dispersion of fruit flies5,6,7 and that of other insects8. Despite the wealth of information on seasonal patterns of fruit fly population, the effects of host trees, ripening seasons and elevation have not been elucidated in detail4,9,10,11,12,13,14,15,16.
The Mediterranean fruit fly (medfly), Ceratitis capitata (Wiedemann) (Diptera: Tephritidae), is a tropical multivoltine and extremely polyphagous frugivorous pest that can infest fruits of more than 300 different plant species17,18. Originated in eastern parts of the sub- Saharan Africa19, mostly throughout the intensive international trade of various host fruits and the intense human mobility20 and its plastic and adaptive biological properties21, C. capitata managed to successfully disperse to almost all continents (Africa, Europe, Middle and Near East, several islands of the Indian Ocean, South and Central America, western Australia and Pacific islands) 22,23. In Europe, it has been present throughout the Mediterranean countries for the past couple of centuries. Detections of C. capitata populations have been recently reported in northern parts of the Mediterranean Sea and Central Europe (e.g. Austria, Germany, Switzerland), in black sea (e.g. Romania and Ukraine) and continental Balkan countries such as Romania and Serbia24,25,26. Population modelling studies based on climate change scenarios and ever-increased knowledge on thermal biology limits and response to challenging environments predict a further northward expansion of the geographic range of this species in the near future27,28,29,30.
Insect richness, evenness and population abundance decline with elevation increase due to impoverishment of habitat suitability8,31. Elevation influences temperature, humidity and host occurrence in each area, factors that are strongly correlated with the phenology of insects in general31 and tephritids in particular10,14,15,32,33,34. The adaptation of the insects to higher elevations usually entails reduced number of generations, extending time intervals between the compliance of lifecycles or diapause events and changes on the thermal tolerance, longevity and sexual maturity8,31,35,36. Studies revealed that C. capitata can be found at elevations up to 2000 m from sea level9,10,16,32,35,37. It seems that survival, persistence and thriving of C. capitata populations at such high elevations is accomplished through longer life cycles (extended adult longevity, slower reproduction rates), patchy seasonal occurrence and survival in favorable microclimate habitats11,34,35. The altitudinal effect on C. capitata ecology and behavior may provide valuable information regarding the response of the pest population to climate change and plastic adaptation to challenging environments31.
The small and often mixed fruit orchards that prevail in several European countries constitute favorable environments for the development and persistence of C. capitata populations. The structure of the landscape in such an environment including ripening sequence, dispersion and abundance of key hosts, cultivation practices and slopes may determine epidemic configuration of populations and infestation rates of commercial crops38. Because of its long lifespan and reproduction in the wild39,40 a single C. capitata female can potentially infest both early and late ripening fruit species and/or cultivars, such as apricots or nectarines and apples or citrus, respectively4,7,41. On the other hand, succeeding generations can breed in sequentially ripening hosts. Early ripening hosts are important for the foundation of the first summer generation and late ones may serve as overwintering sources and refugia for females and larvae respectively. Abundance and dispersion of key hosts determine population growth in an area. Infestation rates as well as adult population densities in key hosts provide essential elements to construct predictive models and design sound management interventions. Although plenty of trapping systems used in the past for C. capitata monitoring, the prediction of first adult occurrence during the fruiting season, which can guide farmers’ management strategy, still needs further examination. Likewise, the effect of host dispersion, abundance and ripening season on the population dynamics of C. capitata has received rather limited attention38,42,43,44.
The aim of the current paper was to analyze the population dynamics of the C. capitata in a mixed fruit area that includes pome, prune and citrus fruit. We focused our analysis on effects of key hosts, temperature and the elevation as a major landscape element. Hence, the scope of this study was (a) to appraise the phenology and seasonal biology of C. capitata in mixed fruit orchards, (b) to depict the effect of elevation and host on phenology and (c) to utilize new analytical tools to thoroughly understand population dynamics of C. capitata and present seasonal population trends.
Results
Effect of elevation on the phenological patterns (Study 1)
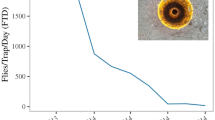
Seasonal patterns of C. capitata captures at four different elevations are given in Fig. 1. The first captures were recorded during May. Captures peaked in September and October and ceased in early January. The total number of captured adults in all plots and traps was 8,194. The highest captures per trap per day were reported at the lowest elevation (Tables S1 and S2). Female captures exceeded those of males in all elevations (Wald x2 = 547.33, df = 1, p < 0.001) (Table S2). Both the active period of trapping as well as the number of captured adults followed an altitudinal gradient. The first captures were reported in the traps deployed at 5 m elevation at the beginning of May while that at 600 m in the middle of July (Fig. 1). A GEE negative binomial model revealed a decrease by 4% on total captures, male and female captures (p < 0.001, p < 0.001 and p < 0.001 for total, male and female captures, respectively) for every 10 m increase in elevation (Table S1).
To depict the seasonal pattern of C. capitata captures in the four different elevations, an “event history” diagram was constructed (Fig. 2). On the lowest elevation (5 m), the first captures were recorded in May, continued throughout the summer, peaked (> 5 captures per trap per day) in both September and October and declined in December, reaching a whole activity period of eight consecutive months. At 200 and 350 m elevation levels, the period of adult captures was shorter (five months), started in June and ended in November, while reached the highest rates in September. The situation was quite different at the highest elevation of 600 m where population activity was reported from the beginning of July until the end of October and reached its peak in September, completing a short flight period of four months.
The effect of elevation on the probability of detecting an adult using the given trapping strategy during the monitoring period is shown in Fig. 3. Both elevation and date were significant predictors of adults’ detection (Wald x2elevation = 23.20, df = 1, p < 0.05) (Wald x2date = 17.44, df = 1, p < 0.05). The detection probability was higher from early July until the end of September in all elevations and increased as elevation decreased. While the predicted probability of detection was more than 0.75 for a period of 5 months (from June until October) at the lowest elevation (5 m), it was lower than 0.4 throughout the season at the higher elevation (600 m) (Fig. 3).
Effect of tree host and temperature on adult captures (Study 2)
Seasonal pattern of C. capitata captures in different hosts are given in Fig. 4. In all hosts the first adults were captured in June. Two picks of captures were recorded, the first in July and the second in September (Fig. 4A, B). The total number of adults captured was 29,564 considering all traps and hosts. We used a separate model for each trap type. Both host (Wald x2 = 91.15, 53.20, df = 3, p < 0.001 for Jackson and Tephri respectively) and temperature (Wald x2 = 80.36, 25.10, df = 1, p < 0.001 for Jackson and Tephri respectively) affected adult captures throughout the season. Captures differed among hosts as the temperature increased (Wald x2 = 79.57, 39.34, df = 3, p < 0.001 for Jackson and Tephri respectively). The highest captures reported in citrus in both Jackson (p < 0.05) and Tephri (p < 0.05) traps (Tables S5 and S6) (Fig. 4A, B). The captures in Jackson traps started in July, peaked in September and declined in November (Fig. 4A). Captures in Tephri traps followed a similar pattern, the only difference being that the capturing period started earlier in June and declined with higher captures in November (Fig. 4B). The highest captures were reported in citrus, while no differences were reported among prunes, pomes and other hosts (Table S5 and S6) (Fig. 4C). Most of the captures reported in citrus, while the captures in prunes, pomes and other hosts were relatively low in the Jackson traps (Fig. 4C) (Table S6).
The effect of host on the probability of detecting an adult using the given trapping system during the monitoring period is shown in Fig. 5. Host, date and temperature were significant factors on adults’ detection in Jackson traps (Wald x2host = 19.37, df = 3, p < 0.001) (Wald x2date = 63.74, df = 1, p < 0.001) (Wald x2temperature = 38.17, df = 1, p < 0.001) (Fig. 5A). Although date and temperature significantly affected the adult detection in Teprhi traps as well, (Wald x2date = 54.30, df = 1, p < 0.05) (Wald x2temperature = 88.04, df = 1, p < 0.05) host was not a significant predictor (Wald x2host = 4.16, df = 3, p = 0.25). Indeed, captures occurred at approximately the same period in all hosts (Fig. 5B). The interaction between the date and the host was not significant as well. The predicted probability of detection was higher from the beginning of August until the mid of September in all host types and traps. In citrus the probability of detecting adults remained higher than 0.5 from early July until the end of November (almost 5 months in a row) in the Jackson traps, while in all the other hosts the same period did not exceed 3 months in a row (Fig. 5A) (Table S5). In the beginning of the capturing period, the probability of detecting an adult using the Tephri trap was higher (> 0.25) than that of Jackson (< 0.25), which was higher than 0.25 only in citrus hosts (Fig. 5).
Discussion
Our results show the activity of adult C. capitata flies, revealed by trap captures, lasted in the area of Lechonia, Volos Greece from the end of May until the end of December. Regardless of elevation and host type following the first detection in May, two peaks in adult captures occurred: the first in the middle of July and the second in September. Both host phenology and elevation regulate population patterns of C. capitata in the area. The probability of detecting adults decreased with an increase in elevation and followed similar seasonal patterns. However, population dynamics followed different patterns in the different elevations. It seems that different factors determine detection efficacy and population density levels. The probability of capturing adults was higher at lower elevations throughout the season. Interestingly, it remained at high levels even at the higher elevation of 600 m from mid-July until mid-September, while in 5 m it exceeded 0.5 from June until November (approximately 5 months). As expected, the hosts considered in the current study (citrus, pome, prunes and other marginal hosts) exerted strong effect on adult captures. Although citrus was reported as the major host for the pest monitoring in the given area, the other two host types, prunes and pomes should also be considered especially at higher elevations where citrus are scarce or absent. The Tephri trap was more efficient early in the season regardless of the host type. However, Jackson traps capture more adults when deployed on citrus trees. Considering the demographic approaches followed to analyze trapping data (e.g. event history diagrams) we gained a better insight on the seasonal and host-related patterns of capture probability, which may be important for managing fruit flies in an area.
The seasonal population fluctuation of C. capitata in the area of Pelion is similar with other areas of southern Europe and western Asia, especially locations with Mediterranean climatic profile. Two populations peaks, in mid-summer and in mid-autumn periods, were reported in Croatia45, Italy15,46 Montenegro47, Spain48, Turkey49, Israel50, and other parts of Greece, particularly in Thessaloniki, northern Greece44, Chios island51 and Crete52. Usually, the peaks of C. capitata population depend on several different factors, such as host ripening period, host abundance and dispersion, temperature, elevation and landscape1,2,3,4,5,6,53.
Across the Mediterranean region, stone fruits and especially apricots and peaches are commonly harvested from early June until the end of August (period of the first peak), followed by the plethora of citrus species that are present from early September until the end of November reaching ripening or harvesting stage (period of the second peak) in the same coastal areas. Pome fruits succeed and overlap in ripening with prunes at the end of summer and citrus at the beginning of autumn. Both peaches and citrus are listed within the most favorable hosts for C. capitata contributing to population increase and are correlated with population peaks15,38,44,45,51,54,55,56.
Evaluating the efficacy of two traps, one targeting males and one capturing most females38,57,58,59 in relation to host, date of capture and temperature, we found out that Tephri performed better than Jackson capturing more flies in all hosts and detecting earlier the first captures regardless of the host. The Jackson performed better in citrus species compared to the other hosts. Our results are in line with those of Papadopoulos et al. (2003) demonstrating that McPhail type traps (e.g. Tephri) baited with food attractants are more suitable for early detection of C. capitata. Tephri trap captures were not affected by the host in our study during the season, in contrast to Papadopoulos and colleagues above, in which the host maturation stage was found to affect detection efficacy especially early in the season. In our case we reported large number of captures and absence of variation between the hosts throughout the season on both traps except of these deployed on citrus that were not present in the area where Papadopoulos and colleagues conducted the above study38,43. Citrus species considered as one of the key hosts for C. capitata population growth and their presence in the orchard may justify high captures in both trap types and increase the probability of detecting the adults51,60. Citrus hosts, especially mandarins, sweet oranges and bitter oranges, are important resources for C. capitata along the Mediterranean region. The fruits remaining on the tree during the winter served as overwintering refugia and also as the first fruit available for infestations at the beginning of the season51. Additionally, the maturation of citrus fruits early in the autumn and the presence of mature fruits until the end of January explains the uninterrupted trapping period of adult medflies. Although Jackson traps baited with trimedlure are by far the most preferred for population monitoring of C. capitata43,44 they capture only males and hence provide no information for detection and population dynamics of females. The inclusion of the labor demanding McPhail type traps increases the detection efficacy and accuracy in depiction of population trends in an area since both sexes are sampled38,58,59. Our results support the use of the two trap types fix to accuracy of population monitoring of C. capitata38,43,58,60.
Ceratitis capitata population abundance and trapping probability are both reduced with an increase in elevation and reduction of host occurrence and dispersion. Elevation has been described as an essential factor affecting the population growth and abundance of fruit flies in general6,16,61 and C. capitata specifically4,5,12,13,34,49. Elevation is strongly connected with host availability and temperature, environmental factors which are contributing to establishing favorable microclimates for C. capitata growth and dispersion5. Increase in elevation usually drives a decrease in temperature which differentiates host availability and ripening period4,5,38. Ceratitis capitata survival and abundance in higher elevations are dramatically affected by the continuous, low temperatures in the beginning of the fruiting season that spans the end of spring 44,48,62. In addition, it is well known that tropical and subtropical fruits including citrus, that are among the most favorable hosts of C. capitata, are not cultivated in higher elevations16,63; hence, C. capitata survival depends mainly on other hosts, such as pome (apples and pears) or stone (apricots and peaches) fruits44,64. Increased winter survival of C. capitata has been reported in coastal Mediterranean areas65,66 where a plethora of favorable hosts such as citrus are cultivated. However, in higher elevations the lack of citrus and the presence of other late ripening hosts, such as pome fruits38,42, in combination with low temperatures during spring delay the population growth and lead to reduced adult captures.
Our data clearly demonstrate that peaks of capture probability precede that of population density by approximately one month adjusting for elevation and host. Trapping probabilities determine the number of traps capturing at least one adult during the observation period. Several studies focus on early detection of first adults since the correlation between population peaks and higher infestation levels seems to be doubtful42,49,51. Early detection is quite relevant in low prevalence or pest free areas and may be related with major management decisions that affect not only control but fruit trading, extirpation, containment and eradication activities21. Bringing new tools to analyze capture data, such as the event history approach followed in the current study, may shed important new light on the detection of invasive fruit flies and the population monitoring under low population densities. In addition, predicting the exponential population growth before reaching the peak67 is extremely important to prevent the destructive infestations. Connecting the dots between trapping probabilities and population densities revealed by flies per trap per day may contribute to develop more accurate population modeling for specific areas and even farms. Apparently, additional factors regulating C. capitata population growth and dispersion such as temperature, humidity and landscape structure and fragmentation should be considered as well21,68.
Conclusion
Our study highlights the effects of elevation and host presence on the population dynamics and dispersion patterns of C. capitata in central Greece. Both elevation and host availability significantly influence the phenology of the fly. Through comprehensive analyses of C. capitata captures at different elevations and in various hosts in mixed European orchards, we lay the groundwork for developing and implementing more effective management strategies to protect fruits. The differences in seasonal shifts of population occurrence at different elevations alter the optimal timing for pest management applications targeting C. capitata in each elevation. The differentiation in population dynamics among hosts refines the seasonal focus on each host, optimizing pest management applications to enhance fruit protection. The results of both studies could be used on modeling approaches focused on predicting population occurrence and dynamics in pest-free areas where similar hosts and topography are present. Similar modeling approaches could utilize these results to evaluate simulations of population dynamics in such landscapes on farm or wider level. The new data analysis tools employed in our study to predict population occurrence before peak events may be adapted for early detection, prevention, and successful pest management applications for C. capitata and other pests.
Materials and methods
Field sites
Two studies were conducted in 2008, in one coastal and three semi-mountainous areas located in mount Pelion, Magnesia, Greece where C. capitata population was monitored. For the first study, four areas located at different elevations were selected (Fig. 6): (a) Kato Lechonia (3–5 m), (b) Paleokastro (198–217 m), (c) Agios Vlasis (331–345 m), (d) Agios Georgios (612–630 m). In the second study, population monitoring, focused on different hosts, was conducted in the coastal plain of Kato Lechonia.
General description of study area
Magnesia county has a rather diverse geography with Pelion Mountain meeting the Aegean Sea to the east and the coastal fertile plain of Kato Lechonia to the west. A total of approximately 320 ha in Pelion are cultivated with stone—pome fruits and citrus69. For the needs of the two studies, an area of approximately 1500 ha in total was selected, stretching from the coast to the slopes of the mount (Fig. 6). Several important C. capitata hosts are cultivated in the area including citrus fruits (bitter oranges, mandarins, sweet oranges), pome fruits (apples, pears, quinces), stone fruits (apricots, peaches, plums) and others (figs and pomegranates). The fruiting period begins in May with apricots, cherries, followed by peaches, pears figs in summer, apples and quinces early in autumn and mandarin, sweet and bitter oranges later in autumn and winter. Conventional, biological and abandoned farms are present in the area as well as scattered summer houses and small towns with numerous backyards. Climatic data were obtained from the Greek national database “Meteo” (http://meteosearch.meteo.gr/) referring to the city of Volos. Detailed description referring to the climatic profile of the area and the climatic data used in this study (Figs. S1 and S2) are provided in the Supplementary materials file (Section S1).
Mediterranean fruit fly monitoring
McPhail type traps (International Pheromone McPhail trap (IPMT) or Tephri McPhail trap) that capture both males and females and Jackson traps capture almost exclusively males, were used to monitor the population dynamics of C. capitata. In the McPhail type traps, the insects were retained in 200 ml of water containing 0.1% of propylenglycol. In the Jackson traps insects were captured on the sticky surface of the trap. Traps were inspected every week, and all captured insects were counted and removed, starting from early May until the end of February 2008. Trimedlure and Biolure dispensers (placed in Jackson and McPhail type traps respectively), were replaced every 3 and 6 weeks respectively. Traps were placed 1.5–1.8 m above the ground in the shaded part of the canopy44,48.
Effect of elevation on adult captures (study 1)
To monitor the adult population at different elevations in the first study, a total of 30 McPhail type traps baited with the three-component lure Biolure (Unipack by Suterra®) containing ammonium acetate, trimethylamine and putrescine, were placed in the above-mentioned four locations (Table 1). Four areas located at different elevations were selected (Fig. 6): (a) Kato Lechonia (3–5 m), (b) Paleokastro (198–217 m, (c) Agios Vlasis (331–345 m), (d) Agios Georgios (612–630 m). Orchards where traps placed were characterized by mixed fruit cultivation type including several important hosts such as citrus fruits (bitter oranges, mandarins, sweet oranges), pome fruits (apples, pears, quinces) and stone fruits (apricots, peaches, plums). Traps placed at a distance further than 30 m between sampling sites, approximately 3–5 traps/ha, except of the higher elevation that the selected orchard was limited to 0.5 ha and we placed 5 traps in 0.5 ha. Detailed description of farms used is provided in Supplementary Information (Table S3).
Effect of host on adult captures (study 2)
For monitoring the seasonal and temporal population trends within the mixed fruit orchards of Kato Lechonia in the second study, we used additional 50 McPhail type traps (Tephri traps) baited with Biolure (Unipack by Suterra®) and 24 Jackson traps baited with trimedlure (Table 2)70. The traps established approximately at a density of ≈1–1.5 traps per hectare throughout the whole coastal area of Kato Lechonia (approximately 100 ha), without focusing on specific orchard types. Conventional, biological and abandoned farms exist in the area were included and sampled. Traps placed on citrus, pome, stone fruits and other hosts (such as figs and walnuts) located at the edges οf the orchards44.
Data analyses
We calculated adult captures per trap per day (Flies/Trap/Day, FTD), the mean and the total number of flies captured during the season among all treatments in both studies. To depict the effect of (a) elevation and date of capture in the first study and (b) host, date of capture and temperature in the second one, on adult detection probability in both experimental approaches, we calculated, for each trap, the probability of capturing at least one adult for each observation date.
For the first study, three different Generalized Estimating Equation models (GEEs) using the negative binomial distribution were used to assess the effect of capture date, and elevation (independent variables) on the a) total number, b) male and c) female fly captures (response variables). GEE models are used for modelling longitudinal data (repeated measurements) as they take into account the within-subject dependencies (here, repeated measurements for each trap)71. Moreover, an event history diagram commonly used in demographic studies was created to depict the seasonal pattern of C. capitata captures in the four different elevations. Each row represents one trap. Squares across rows represent one week and are colored in a gradient from green (0 captures) to yellow (1–20 captures) and red (> 20 captures) according to the number captured adults. Generalized linear models (GLMs) with binomial distribution were applied to portray the effect of elevation (5, 200, 350, 600 m) on the probability of detecting an adult.
For the second study, two different GEE models using the negative binomial distribution were used to investigate the effect temperature and host (prunes, pomes, citrus and others), (independent variables) on the total number of fly captures (response variable) at different trap types, (a) Tephri and (b) Jackson. Generalized Linear Models (GLMs) with binomial distribution were applied to portray the effect of host (prunes, pomes, citrus and others) and trap type on the probability of detecting an adult, followed by post hoc test (Fisher’s Least Significance Difference) to assess the within host differences.
Parameter estimates for the GEE negative binomial models were presented as Incidence Rate Ratios (IRR) with 95% confidence intervals (CI), which is the ratio of the number of captures in a group of interest to the number of captures of the group used as reference. IRRs higher than 1 indicate more trap captures for the group of interest while IRRs lower than 1 indicate more trap captures for the reference group. Parameter estimates for the binomial GLMs were presented as Odds Ratios (OR) with 95% confidence intervals (CI).
Loess curves were used to explore capture change over time. The K-Fold cross validation was employed to identify the best fit with the lowest Root Mean Square Error (RMSE). Package ` caret ` in R was used for the K-Fold cross validation72.
R v4.1.2 (R Core Team 2021, R Foundation of Statistical Computing, Vienna, Austria) in RStudio v2021.09.0 (Rstudio 2021, R Foundation of Statistical Computing, Vienna, Austria) were used for the data analysis and graphical representation (ggplot2)73, of the results. However, in cases where models could not be estimated with R (i.e. GEEs using the negative binomial model), SPSS v29.0 (IBM SPSS Statistics for Windows, IBM Corp, Armonk, NY) was used.
Data availability
Data are available within supplementary materials file.
References
Aluja, M. & Mangan, R. L. Fruit fly (Diptera: Tephritidae) host status determination: Critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol. 53, 473–502 (2008).
Gutierrez, A. P. & Ponti, L. Assessing the invasive potential of the Mediterranean fruit fly in California and Italy. Biol. Invas. 13, 2661–2676 (2011).
Aluja, M., Ordano, M., Guillén, L. & Rull, J. Understanding long-term fruit fly (Diptera: Tephritidae) population dynamics: Implications for areawide management. J. Econ. Entomol. 105, 823–836 (2012).
Flores, S. et al. Population fluctuation of Ceratitis capitata (Diptera: Tephritidae) as a function of altitude in eastern Guatemala. Environ. Entomol. 45, 802–811 (2016).
Puche, H. et al. Effect of elevation and host availability on distribution of sterile and wild Mediterranean fruit flies (Diptera: Tephritidae). Fla. Entomol. 88, 83–90 (2005).
Kounatidis, I. et al. Effect of elevation on spatio-temporal patterns of olive fly (Bactrocera oleae) populations in northern Greece. J. Appl. Entomol. 132, 722–733 (2008).
Sciarretta, A. et al. Analysis of the Mediterranean fruit fly [Ceratitis capitata (Wiedemann)] spatiotemporal distribution in relation to sex and female mating status for precision IPM. PLoS One 13, e0195097 (2018).
McCoy, E. D. The distribution of insects along elevational gradients. Oikos 58, 313–322 (1990).
Vargas, R. L., Harris, E. J. & Nishida, T. Distribution and seasonal occurrence of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) on the Island of Kauai in the Hawaiian Islands. Environ. Entomol. 12, 303–310 (1983).
Harris, E. J., Vargas, R. I. & Gilmore, J. E. Seasonality in occurrence and distribution of Mediterranean fruit fly (Diptera: Tephritidae) in upland and lowland areas on Kauai, Hawaii. Environ. Entomol. 22, 404–410 (1993).
Headrick, D. H. & Goeden, R. D. Issues concerning the eradication or establishment and biological control of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae), in California. Biol. Control. 6, 412–421 (1996).
Israely, N., Ziv, Y. & Oman, S. D. Spatiotemporal distribution patterns of Mediterranean fruit fly (Diptera: Tephritidae) in the central region of Israel. Ann. Entomol. 98, 77–84 (2005).
Israely, N., Ziv, Y. & Galun, R. Metapopulation spatial-temporal distribution patterns of Mediterranean fruit fly (Diptera: Tephritidae) in a patchy environment. Ann. Entomol. 98, 302–308 (2005).
Segura, D. F. et al. Relative abundance of Ceratitis capitata and Anastrepha fraterculus (Diptera: Tephritidae) in diverse host species and localities of Argentina. Ann. Entomol. 99, 70–83 (2006).
Sciarretta, A. & Trematerra, P. Spatio-temporal distribution of Ceratitis capitata population in a heterogeneous landscape in Central Italy. J. Appl. Entomol. 135, 241–251 (2011).
Hassani, I. M., Delatte, H., Ravaomanarivo, L. H., Nouhou, S. & Duyck, P. F. Niche partitioning via host plants and altitude among fruit flies following the invasion of Bactrocera dorsalis. Agric. For. Entomol. 24, 575–585 (2022).
Mitchell, W. C. & Saul, S. H. Current control methods for the Mediterranean fruit fly, Ceratitis capitata, and their application in the USA. Rev. Agric. Entomol. 78, 923–930 (1990).
Liquido, N.J., McQuate, G.T., Hanlin, M.A., & Suiter, K.A. Host plants of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), Version 3.5. USDA Compendium of Fruit Fly Host Information (2017).
White, I. M. & Elson-Harris, M. M. Fruit Flies of Economic Significance: Their Identification and Bionomics (CAB International, 1992).
Szyniszewska, A. M., Leppla, N. C., Huang, Z. & Tatem, A. J. Analysis of seasonal risk for importation of the mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), via air passenger traffic arriving in Florida and California. J. Econ. Entomol. 109, 2317–2328 (2016).
Papadopoulos, N. T., DeMeyer, M., Terblanche, J. S. & Kriticos, D. J. Fruit flies: Challenges and opportunities to stem the tide of global invasions. Annu. Rev. Entomol. 69, 355–373 (2024).
Malacrida, A. R. et al. Globalization and fruitfly invasion and expansion: The medfly paradigm. Genetica. 131, 1–9 (2007).
EPPO (European and Mediterranean Plant Protection Organization). EPPO Global Database. Available online. https://gd.eppo.int [Accessed: 20 October 2023].
Cayol, J. P. & Causse, R. Mediterranean fruit fly Ceratitis capitata Wiedemann (Dipt., Trypetidae) back in Southern France. J. Appl. Entomol. 116, 94–100 (1993).
Egartner, A., Lethmayer, C., Gottsberger, R. A. & Blumel, S. Recent records of the Mediterranean fruit fly, Ceratitis capitata (Tephritidae, Diptera in Austria. IOBC-WPRS Bull. 146, 143–152 (2019).
König, S., Steinmöller, S. & Baufeld, P. Origin and potential for overwintering of Ceratitis capitata (Wiedemann) captured in an official survey in Germany. JPDP 129, 1201–1215 (2022).
Nyamukondiwa, C., Weldon, C. W., Chown, S. L., Le Roux, P. C. & Terblanche, J. S. Thermal biology, population fluctuations and implications of temperature extremes for the management of two globally significant insect pests. J. Insect Physiol. 59, 1199–1211 (2013).
Weldon, C. W., Nyamukondiwa, C., Karsten, M., Chown, S. L. & Terblanche, J. S. Geographic variation and plasticity in climate stress resistance among southern African populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Sci. Rep. 8, 1–13 (2018).
Moraiti, C. A., Verykouki, E. & Papadopoulos, N. T. Chill coma recovery of Ceratitis capitata adults across the Northern Hemisphere. Sci. Rep. 12, 1–12 (2022).
Gilioli, G. et al. Non-linear physiological responses to climate change: The case of Ceratitis capitata distribution and abundance in Europe. Biol. Invas. 24, 261–279 (2022).
Hodkinson, I. D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. Cambridge Philos. Soc. 80(3), 489–513. https://doi.org/10.1017/S1464793105006767 (2005).
Eskafi, F. M. & Kolbe, M. E. Infestation patterns of commonly cultivated., Edible Fruit species by Ceratitis capitata and Anastrepha spp. (Diptera: Tephritidae) in Guatemala and their relationship to environmental factors. Environ. Entomol. 19(6), 1371–1380. https://doi.org/10.1093/ee/19.5.1371 (1990).
Aluja, M. et al. Seasonal population fluctuations and ecological implications for management of Anastrepha fruit flies (Diptera: Tephritidae) in commercial mango orchards in Southern Mexico. J. Econ. Entomol. 89(3), 654–667. https://doi.org/10.1093/jee/89.3.654 (1996).
Duyck, P. F. et al. Lifespan of a Ceratitis fruit fly increases with higher altitude. Biol. J. Linn. Soc. 100(2), 345–350. https://doi.org/10.1111/j.1095-8312.2010.01497.x (2010).
Midgarden D, & Lira E. Ecological relationship of Medfly and coffee in guatemala and Mexico. Fruit flies of economic importance: from basic to applied knowledge. Proc. of the 7th International Symposium on Fruit Flies of Economic Importance, 241–247 (2006).
Karl, I. & Fischer, K. Altitudinal and environmental variation in lifespan in the Copper butterfly Lycaena tityrus. Funct. Ecol. 23(6), 1132–1138. https://doi.org/10.1111/j.1365-2435.2009.01607.x (2009).
Vargas, R. I., Walsh, W. A. & Nishida, T. Colonization of newly planted coffee fields: Dominance of Mediterranean fruit fly over oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 88(3), 620–627. https://doi.org/10.1093/jee/88.3.620 (1995).
Papadopoulos, N. T., Katsoyannos, B. I. & Nestel, D. Spatial autocorrelation analysis of a Ceratitis capitata (Diptera: Tephritidae) adult population in a mixed deciduous fruit orchard in northern Greece. Environ. Entomol. 32(2), 319–326. https://doi.org/10.1603/0046-225X-32.2.319 (2003).
Carey, J. R. et al. Age structure changes and extraordinary lifespan in wild medfly populations. Aging Cell 7(3), 426–437. https://doi.org/10.1111/j.1474-9726.2008.00390.x (2008).
Kouloussis, N. A. et al. Seasonal trends in Ceratitis capitata reproductive potential derived from live-caught females in Greece. Entomol. Exp. Appl. 140(3), 181–188. https://doi.org/10.1111/j.1570-7458.2011.01154.x.Seasonal (2011).
Duarte, F., Calvo, V., Delgado, S., Garcia, F. R. M. & Scatoni, I. Spatio-temporal distribution of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) captures and their relationship with fruit infestation in farms with a diversity of hosts. Florida Entomol. 104(4), 297–306. https://doi.org/10.1653/024.104.0407 (2021).
Israely, N., Yuval, B., Kitron, U. & Nestel, D. Population fluctuations of adult Mediterranean fruit flies (Diptera: Tephritidae) in a Mediterranean heterogeneous agricultural region. Environ. Entomol. 26(6), 1263–1269. https://doi.org/10.1093/ee/26.6.1263 (1997).
Papadopoulos, N. T. et al. Early detection and population monitoring of Ceratitis capitata (Diptera: Tephritidae) in a mixed-fruit orchard in northern Greece. J. Econ. Entomol. 94(4), 971–978. https://doi.org/10.1603/0022-0493-94.4.971 (2001).
Papadopoulos, N. T., Katsoyannos, B. I., Carey, J. R. & Kouloussis, N. A. Seasonal and annual occurrence of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Northern Greece. Ann. Entomol. Soc. Am. 94(1), 41–50 (2001).
Bjeliš M, Radunić D, Masten T, & Kotlar A Spatial distribution and temporal outbreaks of medfly—Ceratitis capitata Wied.(Diptera, Tephritidae) in Republic of Croatia. Proc. of the 8th Slovenian Conference on Plant Protection, 321–325 (2007).
Rigamonti, I. E. Contributions to the knowledge of Ceratitis capitata Wied. (Diptera, Tephritidae) in Northern Italy. II. Overwintering in Lombardy. Bollettino Di Zoologia Agraria e Di Bachicoltura 36(1), 101–116 (2004).
Radonjić, S., Čizmović, M. & Pereira, R. Population dynamics of the Mediterranean fruit fly in Montenegro. Int. J. Insect Sci. 5, 12964. https://doi.org/10.4137/ijis.s12964 (2013).
Escudero-Colomar, L. A., Vilajeliu, M. & Batllori, L. Seasonality in the occurrence of the Mediterranean fruit fly [Ceratitis capitata (Wied.)] in the north-east of Spain. J. Appl. Entomol. 132(9–10), 714–721. https://doi.org/10.1111/j.1439-0418.2008.01372.x (2008).
Tiring, G. & Satar, S. Annual population fluctuations of Mediterranean fruit fly in the Eastern Mediterranean Region of Turkey. Problem of non-marketing fruit. Phytoparasitica 49(5), 807–817. https://doi.org/10.1007/s12600-021-00932-w (2021).
Israely, Ν, Ritte, U. & Oman, S. D. Inability of Ceratitis capitata (Diptera: Tephritidae) to overwinter in the Judean hills. J. Econ. Entomol. 97(1), 33–42. https://doi.org/10.1093/jee/97.1.33 (2004).
Katsoyannos, B. I., Kouloussis, N. A. & Carey, J. R. Seasonal and annual occurrence of Mediterranean fruit flies (Diptera: Tephritidae) on Chios island, Greece: Differences between two neighboring citrus orchards. Ann. the Entomol. Soc. Am. 91(1), 43–51. https://doi.org/10.1093/aesa/91.1.43 (1998).
Michelakis, S. Phenology of the Mediterranean Fruit Fly Ceratitis capitata Wiedemann in Crete. Israel J. Entomol. XXV, 177–180 (1992).
Papadopoulos, N. T., Stavridis, D. G., Zarpas, K. D. & Diamantidis, A. D. The Mediterranean fruit fly in Greece: Current distribution and management. Agrotypos 7, 38–46 (2012).
Schliserman, P., Aluja, M., Rull, J. & Ovruski, S. M. Habitat degradation and introduction of exotic plants favor persistence of invasive species and population growth of native polyphagous fruit fly pests in a Northwestern Argentinean mosaic. Biol. Invas. 16, 2599–2613 (2014).
Ghanim, N. M., Fathy, D. M. & Ramadan, M. M. Occurrence of Mediterranean fruit fly Ceratitis capitata, (Tephritidae: Diptera) in mango orchard and its response to certain ecological factors and different attractants. MEJAS 8, 913–921 (2018).
Dominiak, B. C. & Taylor-Hukins, R. Priority host plants for Ceratitis capitata, Mediterranean fruit fly, based on the host reproduction number for surveillance, trade and eradication programs. Int. J. Trop. Insect Sci. 42, 3721–3727 (2022).
Heath, R. R., Epsky, N. D., Dueben, B. D., Rizzo, J. & Jeronimo, F. Adding methyl-substituted ammonia derivatives to a food-based synthetic attractant on capture of the Mediterranean and Mexican fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 90, 1584–1589 (1997).
Katsoyannos, B. I., Papadopoulos, N. T., Heath, R. R., Hendrichs, J. & Kouloussis, N. A. Evaluation of synthetic food-based attractants for female Mediterranean fruit flies (Dipt., Tephritidae) in McPhail type traps. J. Appl. Entomol. 123(607), 612 (1999).
Katsoyannos, B. I., Heath, R. R., Papadopoulos, N. T., Epsky, N. D. & Hendrichs, J. Field evaluation of Mediterranean fruit fly (Diptera: Tephritidae) female selective attractants for use in monitoring programs. J. Econ. Entomol. 92(583), 589 (1999).
Katsoyannos, B. I. Evaluation of Mediterranean fruit-fly traps for use in sterile-insect-technique programmes. J. Appl. Entomol. 118, 442–452 (1994).
Salazar-Mendoza, P., Peralta-Aragón, I., Romero-Rivas, L., Salamanca, J. & Rodriguez-Saona, C. The abundance and diversity of fruit flies and their parasitoids change with elevation in guava orchards in a tropical Andean forest of Peru, independent of seasonality. PLoS One 16, e0250731 (2021).
Duyck, P. F. & Quilici, S. Survival and development of different life stages of three Ceratitis spp. (Diptera: Tephritidae) reared at five constant temperatures. Bull. Entom. Res. 92, 461–469 (2002).
Hassani, I. Μ et al. Invasion by Bactrocera dorsalis and niche partitioning among tephritid species in Comoros. Bull. Entom. Res. 106, 749–758 (2016).
Krasnov, H. et al. Empirical evidence of the mediterranean fruit fly movement between orchard types. J. Appl. Entomol. 145, 417–426 (2021).
Mavrikakis, P. G., Economopoulos, A. P. & Carey, J. R. Continuous winter reproduction and growth of the Mediterranean Fruit Fly (Diptera: Tephritidae) in Heraklion, Crete, Southern Greece. Environ. Entomol. 29(6), 1180–1187 (2000).
Martínez-Ferrer, M. T. et al. Seasonal and annual trends in field populations of Mediterranean fruit fly, Ceratitis capitata, in Mediterranean citrus groves: comparison of two geographic areas in eastern Spain. SJAR 8, 757 (2010).
Nicácio, J. et al. Spatial distribution and control levels of Anastrepha spp. (Diptera: Tephritidae) in guava orchards. An. Acad. Bras. Cienc. 91(1), 13 (2019).
Lux, S. A. Individual-based modeling approach to assessment of the impacts of landscape complexity and climate on dispersion, detectability and fate of incipient medfly populations. Front. Physiol. 8, 1121 (2018).
Papanastasiou, S. A., Rodovitis, V. G., Bataka, E. P., Verykouki, E. & Papadopoulos, N. T. Population dynamics of Drosophila suzukii in coastal and mainland sweet cherry orchards of Greece. Insects 11, 621 (2020).
Beroza, M., Green, N., Gertler, S. I., Steiner, L. F. & Miyashita, D. H. Insect attractants, new attractants for the Mediterranean fruit fly. J. Agric. Food Chem. 9, 361–365 (1961).
Hardin, J. W. & Hilbe, J. M. Generalized Estimating Equations 2nd edn. (Chapman and Hall/CRC, 2013). https://doi.org/10.1201/b13880S.
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28(5), 26. https://doi.org/10.18637/jss.v028.i05 (2008).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016).
Funding
The European Union's Horizon 2020 research and innovation program under agreement No 818184 (FF-IPM) funded this research. We thank Apostolos Chanias in Magnisia prefecture, as well as other farmers for providing their mixed fruit orchards to execute our field experiments.
Author information
Authors and Affiliations
Contributions
Conceptualization: N.T.P. Data curation: V.G.R., E.V., S.A.P., C.A.M., K.D.Z. Formal Analyses: V.G.R., E.V., N.T.P. Investigation: N.P., C.A.M., S.A.P. Methodology: N.T.P., K. D.Z., Resources: N.T.P. Project administration: N.T.P. Supervision: N.T.P. Writing- original draft: V.G.R., N.T.P., E.V. Writing- reviewing & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodovitis, V.G., Verykouki, E., Zarpas, K.D. et al. Mediterranean fruit fly population phenological patterns are strongly affected by elevation and host presence. Sci Rep 14, 6010 (2024). https://doi.org/10.1038/s41598-024-56714-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56714-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.