Abstract
Sitotroga cerealella is a serious pest of a wide range of stored cereal grains. An essential element of an integrated pest control approach is the application of plant oils as a substitute for chemical insecticides. This study aimed to investigate the fumigant toxicity of Allium sativum and Mentha piperita essential oils against S. cerealella adult moths and the egg parasitoid Trichogramma evanescens. Gas chromatography–mass spectrometry analyses detected that Diallyl trisulfide (37.97%) and dl-Menthol (47.67%) as main compounds in A. sativum and M. piperita, respectively. The results showed that, A. sativum at 10.0, 5.0, and 2.5 µL/L air resulted in 100% insect mortality after 24 h exposure. The concentrations of 10.0 and 5.0 µL/L air of M. piperita oil resulted in 100 and 96% insect mortality, respectively. The parasitoid adult emergence in the F1 reduced when exposed to LC99 of A. sativum and M. piperita oils by 10.89 and 9.67%, respectively. Also, the parasitism of emerged parasitoid decreased by 9.25 and 5.84% (class I-harmless), respectively. Therefore A. sativum and M. piperita have the potential to be used as bio-fumigant for the management of S. cerealella and can be used alongside the T. evanescens in integrated pest management.
Similar content being viewed by others
Introduction
Most of the world’s population depends on cereals as a source of carbs, vitamins, minerals, fiber, oils, fats, and protein1. Therefore, the explosion of the human population in many developing countries led to an increase in the global demand for major production of cereal crops2. Wheat, Triticum sp., is a widespread crop that is grown in various parts of the world and can be attacked by a plethora of pests3. Beetles and moths are among the insect pests that severely harm wheat crops4. The Angoumois grain moth Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) is one of the most significant pests of cereal grains such as wheat, corn, barley, and rice throughout the world. The pest's larvae cause injury to wheat grains by feeding on seed contents, which reduces the weight and nutritional value of the grains and makes them more susceptible to diseases. Synthetic chemicals are commonly used to manage S. cerealella; however, indiscriminate chemical treatment has a number of negative impacts including increased pest resistance and the spread of secondary pests, as well as negative effects on the environment and human health5. As a result, the employment of alternative control techniques has become essential. Finding efficient and eco-friendly alternatives to chemical pesticides presents a significant challenge in limiting the harm wrought by the S. cerealella pest and reducing crop losses. Egg parasitoids have been used in biological control far more frequently than other natural enemies, especially those of the genus Trichogramma (Hymenoptera: Trichogrammatidae). Also, many research studies recently described plant essential oils as eco-friendly agents to control various pests with the least possible effects on the environment and human health. In order to suppress the population of S. cerealella in post-harvest storage, the use of essential oil has been examined as an alternative viable technique for managing this pest. The use of plant essential oils as safe, biodegradable insecticides has gained popularity as a viable substitute for dangerous fumigant pesticides in the fight against stored-product insect pests. Fumigation is a useful technique for controlling pests in stored goods. As a result, numerous researchers investigated the insecticidal activity of essential oils against various stored product pests6,7,8,9,10, the findings of their studies suggest that essential oils have potential for the control of stored-product pests. There have been few attempts to study the effect of the essential oils of garlic (Allium sativum L.) and peppermint (Mentha piperita L.) on the non-target egg parasitoid, T. evanescens. Thus, the objective of this study was to examine the potential fumigant toxicity of A. sativum and M. piperita essential oils against S. cerealella, an economically important pest in stored products, and their effects on the non-target egg parasitoid, T. evanescens.
Materials and methods
Essential oils and their phytochemical screening
Essential oils of A. sativum and M. piperita were obtained from National Research Centre (NRC), Cairo, Egypt. For Gas Chromatography-Mass Spectrometry (GC–MS) analysis, the samples were dissolved in chloroform at the ratio of 50 µL oil:1 mL chloroform and injected into GC. The GC–MS system (Agilent Technologies) was equipped with gas chromatograph (7890B) and mass spectrometer detector (5977A) at NRC. The GC was equipped with HP-5MS column (30 m × 0.25 mm internal diameter and 0.25 μm film thickness). The carrier gas was helium, which was used at a flow rate of 3.0 mL/min at a split ratio (1:10), an injection volume of 1 µL, and the temperature programme as follows: 40 ºC for 1 min; 10 ºC per minute to 200 ºC and held for 1 min; 20 ºC per minute to 220 ºC and held for 1 min; 30 ºC per minute to 320 ºC and held for 3 min. The injector and detector were kept at 250 ºC and 320 ºC, respectively. By using a spectral range of m/z 30–550 and a solvent delay of 2.5 min, mass spectra were produced using electron ionization (EI) at 70 eV. The quad was 150 ºC warmer than the mass temperature of 230 ºC. By comparing the spectrum fragmentation pattern with those found in the Wiley and NIST Mass Spectral Library data, it was possible to identify various constituents of A. sativum and M. piperita oils.
Insects
Sitotroga cerealella
The S. cerealella moths were obtained from naturally infested grains stored in a local warehouse in Cairo governorate, Egypt. The stock culture was maintained for more than 5 generations without exposure to insecticides. The adult moths were reared on intact hard wheat grains inside 1000 mL jars. The resultant moths were collected in special cylinders coated with a wire screen with fine pores that allowed the eggs to pass through and prevent the escape of any moths. In the experiments, the newly emerged adults and freshly deposited eggs were used. Wheat grains were purchased from a local market. In order to kill any insects or parasites present, grains were first stored for about 5 days at − 18 °C before being used. The grains were then adjusted for an additional week before use to the laboratory conditions where the culture was kept. Rearing insects and experiments were carried out under controlled conditions of 28 ± 2 °C, 65 ± 5% RH, and 16:8 (L:D) photoperiod.
Trichogramma evanescens
The egg parasitoid T. evanescens were obtained from the mass rearing of the Laboratory of Biological Control of Insects, Plant Protection Research Institute, Agricultural Research Center, Giza, Egypt. Fresh S. cerealella eggs were glued to paper strips (3 × 8 cm). The strips holding S. cerealella eggs were exposed to T. evanescens adults in glass jars (1000 mL) covered with muslin cloth and tightly tied by a rubber band. Egg strips were renewed daily, and old parasitized eggs were incubated under laboratory conditions. The culture of parasitoid was reared under the previous conditions.
Fumigant toxicity
Sitotroga cerealella
The fumigant toxicity of A. sativum and M. piperita essential oils was examined using S. cerealella moth adult stage. Glass jars of 1000 mL capacity provided with their screw lids were used as exposure chambers. Concentrations were carried out by releasing the required amounts of the tested oil (10.0, 5.0, 2.5, 1.25, 0.625 µL/L air) from an automatic micropipette onto Whatman No.1 filter paper disks (2 cm diameter) and were glued on the underside of the screwcap of the glass jar. Ten adults of S. cerealella (1–3 days old) were released into each jar supplied with 40 g of wheat before the lid was closed tightly and the cover was sealed with parafilm. For control group, the insects were maintained without oil exposure (0 µL/L air). Five replicates were used for each treatment. Insect mortality was checked after 24 h to calculate lethal concentration values of LC99, LC50, and LC25 for the tested oils.
Trichogramma evanescens
Eemergence rate of Trichogramma evanescens
To evaluate the fumigant toxicity of A. sativum and M. piperita essential oils on the emergence rate of the adult parasitoid, an equal number of 1-day-old S. cerealella eggs (110 ± 10) for each treatment were dispersed on self-adhesive strip (1 mm diameter) and exposed to adult parasitoid for 24 h. After 4 days the eggs became black (i.e., clear evidence of parasitism), then the parasitized egg strips were kept in glass jars. Egg strips were exposed to the previously estimated LCs of essential oils vapor on S. cerealella (LC99, LC50, and LC25) for 24 h as described previously. After exposure, the egg strips were placed separately in glass test tubes (Fig. 1), and the number of emergence holes (Fig. 2a) were counted to evaluate the percentage of adult emergence.
Parasitism rate of emerged Trichogramma evanescens (F1)
This experiment was performed to estimate the efficiency of emerged T. evanescens adults (F1) of previous experiment. Freshly mated parasitoid (ten adults/each egg strip) were released in glass jars (1000 mL) containing S. cerealella eggs strips (110 ± 10). After 24 h of exposure to the parasitoids, S. cerealella egg masses were removed from the jars and put into individual transparent test tubes. The number of darkened eggs was counted under binocular to determine the parasitism% (Fig. 2b). As a control, the individuals were maintained in test tubes without any fumes. For each treatment, the studies were repeated ten times.
The International Organization for Biological and Integrated Control (IOBC) proposed four toxic classes: I = harmless, II = slightly harmful, III = moderately harmful, and IV = harmful. These classes are based on the reduction of T. evanescens generation emergence or parasitism by < 30%, 30–79%, 80–99%, and > 99%, respectively11.
The proportion reduced emergence and parasitism rates were computed as12:
Data analysis
The mortality, emergence rate, and parasitism rate results were analyzed using One-Way ANOVA, and Duncan's test was used to determine whether there were significant differences between the treatments (P < 0.05). Average mortality data for each treatment were subjected to probit analysis13 to obtain the lethal concentration (LC) values. LC values were considered significantly different at the P < 0.05 level. Data analyses were performed using SPSS version 18.014.
Results
GC–MS analysis
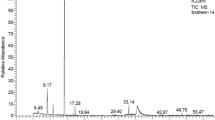
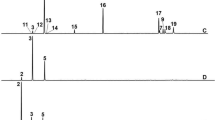
A total of 28 compounds were detected in A. sativum (Table 1, Fig. 3a). The major detected compounds were Trisulfide, di-2-propenyl (Diallyl trisulfide) (37.97%), Trisulfide, methyl 2-propenyl (Allyl methyl trisulfide) (21.65%), and Diallyl disulphide (13.7%). The other sulfur-containing compounds identified in A. sativum were Diallyl tetrasulphide (4.47%), Dimethyl trisulfide (3.33%), Allyl methyl disulfide (2.34%), Diallyl sulfide (1.61%), Dimethyl tetrasulphide (0.38%), Trisulfide, allyl propyl (0.44%), and Trisulfide, methyl propyl (0.32%). Table 2 and Fig. 3b show the chemical composition of M. piperita essential oil, with dl-Menthol (47.67%), Cyclohexanone, 5-methyl-2-(1-methylethyl)- (15.38%), and l-Menthone (10.82%) being the main constituents. Also, Eucalyptol, d-Limonene, and (−)-Carvone were detected in percentages of 9.12, 7.0, and 3.28%, respectively. Whereas, 1R-MENTHYL ACETATE, beta-Pinene, and alpha-Pinene were detected at percentages of 1.22, 1.03, and 0.71%, respectively.
Toxicity study
Fumigant toxicity of A. sativum and M. piperita oils against S. cerealella adult moths is illustrated in Fig. 4. The mortality rate increased as the oil concentration increased after a 24-h exposure. The highest percentage of insect mortality (100%) was observed for A. sativum oil concentrations of 10.0, 5.0, and 2.5 µL/L air. The mortality rate decreased to 80% following 1.25 µL/L air A. sativum fumigation. In contrast, only 6.0% of insects died when A. sativum was applied at the lowest concentration (0.625 µL/L air). Comparably, higher M. piperita oil concentrations (10.0, 5.0 µL/L air) led to a higher mortality rate of 96% and 100%, respectively. Also, 2.5 and 1.25 µL/L air caused mortality percentage > 50% after 24 h exposure (Fig. 4). Toxicity data obtained in Table 3 indicated that LC25, LC50, and LC99 of A. sativum oil against S. cerealella adult moths after 24 h exposure was 0.82, 1.04, and 1.80 µL/L air, respectively. The LC values resulted after fumigation with M. piperita oil were 0.79, 1.88, and 5.65 µL/L air for LC25, LC50, and LC99, respectively.
Effect of A. sativum and M. piperita essential oils on the efficacy of T. evanescens
Results shown in Table 4 demonstrated that the exposure of parasitized eggs of S. cerealella to the fumes of the tested oils affected the emergence rate of the parasitoid. The highest emergence rates of T. evanescens were 92.51% and 93.22% at treatment of LC25 A. sativum and M. piperita, respectively. Furthermore, the adult emergence reduced when parasitized S. cerealella eggs were exposed to the highest tested concentration LC99 of A. sativum and M. piperita oils by 10.89 and 9.67% (class I-harmless). In the same line, the parasitism rate of the emerged female of F1 generation was affected by A. sativum and M. piperita (F = 49.042, P < 0.001and F = 44.54, P < 0.001) respectively. When the fresh S. cerealella eggs were offered to the emerged F1 females after LC99 treatment, the parasitism was decreased by 9.25 and 5.84% (class I), respectively, and these oils were therefore classified as harmless (Table 4).
Discussion
Plant based insecticides such as essential oils are considered as a promising component for pest control and an alternative to synthetic chemicals. The immediate or long-term implications of insecticides on natural enemies should be considered when integrating synthetic or bio-insecticides with biological control agents to control major agricultural pests15. In the current work, the essential oils of A. sativum and M. piperita effectively caused mortality of S. cerealella adult stages. It was observed that the toxicity increased with increasing oil concentration. Earlier studies have mentioned that different essential oils showed an insecticidal impact on stored-product pests10,16,17,18,19. Our results show that A. sativum oil was rich in Diallyl trisulfide (37.97%), Allyl methyl trisulfide (21.65%), and Diallyl disulphide (13.7). Also, M. piperita was found to contain dl-Menthol (47.67%), Cyclohexanone, 5-methyl-2-(1-methylethyl)- (15.38%), and l-Menthone (10.82%), as well as Eucalyptol (9.12%), d-Limonene (7.0%), and (−)-Carvone (3.28%). The primary phytocompounds in essential oils vary in quantity and quality according to location and climate. However, the phytoconstituents of garlic essential oil were relatively similar to the analysis reported by earlier studies20,21, for instance22 who reported that the main volatile compounds of garlic oil were diallyl trisulfide (37.3–45.9%), diallyl disulfide (17.5–35.6%), methyl allyl trisulfide (7.7–10.4%) and the 2-vinyl-1,3-dithiane (3.9–5.9%). Additionally, it was claimed that the three main components of an Egyptian garlic essential oil were diallyl disulfide (25.2%), allyl methyl trisulfide (23.8%), and diallyl trisulfide (21.1%)23. Similar to our findings, the major compounds found in M. piperita were menthol, isomenthone, and limonene24. Previously it was reported that the content of menthol (39.3%) was the highest, followed by menthone (25.2%), menthofuran (6.8%), and menthyl acetate (6.7%)25. Also, menthol (68.0%), menthone (9.5%), isomenthone (8.4%), and menthyl acetate (2.4%) were identified as main compounds of M. piperita essential oil26. However, different production conditions, such as harvest time, location, seasonal factors, and storage time, can cause the components of essential oil in a given plant species to vary27. Insect species, exposure method, and phytochemicals are key indicators of a plant's toxic effect28,29. The current study suggests that the fumigant toxicity of A. sativum and M. piperita oils to S. cerealella might be attributed to their chemical composition. According to our study, 100% of S. cerealella adult mortality was caused by A. sativum oil at concentrations of 10.0, 5.0, and 2.5 μL/L air. Furthermore, M. piperita caused > 90% mortality at 10.0 and 5.0 μL/L air. Over 50% of the insects died, even at the lower concentration of 1.25 μL/L air of both oils. Similarly, it was reported that A. sativum and its two major components, diallyl disulfide and diallyl trisulfide had significant fumigant activity with LC50 values at 1.33, 0.99, and 1.02 μL/L air space, respectively, after 24 h exposure against S. cerealella30. Moreover, A. sativum and bags impregnated with A. sativum and Petroselinum crispum were found to be highly effective against Corcyra cephalonica, S. cerealella, and Trogoderma granarium31,32. Previous study demonstrated that M. piperita had a significant insecticidal effect against stored product insects; Sitophilus oryzae, Rhyzopertha dominica, and Tribolium castaneum33. It was reported that M. piperita and P. nigrum essential oils had a notable toxicity against S. oryzae with LC50 values of 85.0 and 287.7 µL/L air, respectively, after 72 h exposure24. Earlier study indicated that M. piperita oil showed significant fumigation toxicity against T. castaneum, Lasioderma serricorne, and L. bostrychophila (LC50 = 18.1, 68.4, and 0.6 mg/L air, respectively)34. Based on our findings, A. sativum and M. piperita vapors had an effect on the emergence rate of parasitized S. cerealella eggs, the parasitism rate of the adult emerged in the F1 displayed a similar trend. M. piperita and A. sativum reduced the emergence rate by 10.89 and 9.68% in the parental generation T. evanescens when exposed to LC99. Likewise, no treatment affected the parasitism capacity of the T. evanescens F1 generation by more than 9.25%, which classified the oils as harmless based on the IOBC classification. Fewer studies examined the potential toxicity of A. sativum and M. piperita essential oils on non-target egg parasitoid T. evanescens. Alc´antara-de la Cruz et al.35 reported that, the parasitism rate in female T. galloi of the paternal generation was decreased by EOs of Allium sativum, Azadirachta indica, and Carapa guianensis. However, our findings were supported by Ercan et al.36 who reported that, the eggs of Ephestia kuehniella were tolerant to Prangos ferulacea essential oil. The thickness, shape, and permeability of the vitelline membrane, among other chorion features, may play a role in the tolerance of the parasitized S. cerealella eggs. Since essential oils are a source of biologically active vapors, they can possibly act as effective insecticides, as shown by the reported fumigant activity. The findings indicated that the egg parasitoid T. evanescens was relatively unaffected by A. sativum and M. piperita essential oils, and both oils can be used as eco-friendly, effective bio-fumigant for the control of S. cerealella.
Conclusions
In the current study, the fumigant toxicity of A. sativum and M. piperita essential oils against S. cerealella was evaluated. The toxic effect of plant oils is attributed to the presence of phytoconstituents such as Diallyl trisulfide, Allyl methyl trisulfide, and Diallyl disulfide, as well as dl-Menthol, Cyclohexanone, 5-methyl-2-(1-methylethyl)-, l-Menthone, Eucalyptol, d-Limonene, and (−)-Carvone detected in A. sativum and M. piperita essential oils, respectively. Both oils resulted in 100% mortality when S. cerealella moths were fumigated with 10.0% µL/L air after 24 h of exposure. The toxic fumigant effect of both oils on non-target egg parasitoid T. evanescens was also assessed. Three tested concentrations LC99, LC50, and LC25 of A. sativum and M. piperita slightly affected the adult emergence of T. evanescens in F1 and the parasitism%. However, according to the International Organization for Biological and Integrated Control-IOBC's proposed toxic classes, our results indicate that tested oils can be classified as harmless substances to non-target T. evanescens. The results of this study therefore suggest that oils from A. sativum and M. piperita can be used as bio-fumigants to effectively control the S. cerealella storage pest without threatening the egg parasitoid T. evanescens. However, more studies would be needed to ensure their effectiveness under different climatic conditions.
Data availability
All data generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Garutti, M. et al. The impact of cereal grain composition on the health and disease outcomes. Front. Nutr. https://doi.org/10.3389/fnut.2022.888974 (2022).
Sen, D. et al. Potency of UV irradiation in management of rice weevil, Sitophilus oryzae L. JAST-A Revi. Multidiscip. Res. J. 2(1), 61–67 (2016).
Ghodjani, Z., Shakarami, J., Mardani-Talaee, M. & Serrão, J. E. Effect of different wheat cultivars on two sex life table parameters of Sitotroga cerealella (Lepidoptera: Gelechiidae). J. Stored Prod. Res. 101, 102097 (2023).
Atwal, A. S. & Dhaliwal, G. S. Agricultural Pests of South Asia and Their Management (Kalyani Publishers, 2015).
Ileke, K. D. Cheese wood, Alstonia boonei De Wild a botanical entomocides for the management of maize weevil, Sitophilus zeamais (Motschulsky) [Coleoptera: Curculionidae]. Octa. J. Biosci. 2(2), 64–68 (2014).
Sümer Ercan, F., Baş, H., Koc, M., Pandir, D. & Öztemiz, S. Insecticidal activity of essential oil of Prangos ferulacea (Umbelliferae) against Ephestia kuehniella (Lepidoptera: Pyralidae) and Trichogramma embryophagum (Hymenoptera: Trichogrammatidae). Turk. J. Agric. For. 37(6), 719–725 (2013).
Parreira, D. S. et al. Quantifying the harmful potential of ten essential oils on immature Trichogramma pretiosum stages. Chemosphere 199, 670–675 (2018).
Aouadi, G. et al. Screening for insecticidal efficacy of two Algerian essential oils with special concern to their impact on biological parameters of Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae). JPDP. 127, 471–482 (2020).
El-Raheem, A., Sweelam, M., Taka, A., Safaa, M. & Mousa, M. Fumigant toxicity of some plant volatile oils against stored grain weevils. Menoufia J. Plant Prot. 4, 97–106 (2022).
Ghoorchian, M., Rahmani, S. & Weisany, W. Toxicity effects of several medicinal plants essential oils on Angoumois grain moth (Sitotroga cerealella) female adults. J. Plant Dis. Prot. 130, 1263–1271. https://doi.org/10.1007/s41348-023-00792-y (2023).
Sterk, G. et al. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group ‘Pesticides and Beneficial Organisms’. BioControl 44, 99–117 (1999).
Carvalho, G. A. et al. Selectivity of growth regulators and neonicotinoids for adults of Trichogramma pretiosum (Hymenoptera:Trichogrammatidae). Rev. Colomb. Entomol. 36, 195–201 (2010).
Finney, D. J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve Vol. 318 (Cambridge University Press, 1971).
SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. (SPSS Inc).
Poorjavad, N., Goldansaz, S. H., Dadpour, H. & Khajehali, J. Effect of Ferula assafoetida essential oil on some biological and behavioral traits of Trichogramma embryophagum and T. evanescens. BioControl 59, 403–413. https://doi.org/10.1007/s10526-014-9583-x (2014).
Karaborklu, S., Ayvaz, A. N. & Yılmaz, S. Bioactivities of different essential oils against the adults of two stored product insects. Pak. J. Zool. 42, 679–686 (2010).
Karaborklu, S., Ayvaz, A., Yılmaz, S. & Akbulut, M. Chemical composition and fumigant toxicity of some essential oils against Ephestia kuehniella. J. Econ. Entomol. 104, 1212–1219 (2011).
Bounoua-Fraoucene, S., Kellouche, A. N. & Debras, J. F. Toxicity of four essential oils against two insect pests of stored grains, Rhyzopertha dominica (Coleoptera: Bostrychidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Afr. Entomol. 27(2), 344–359. https://doi.org/10.4001/003.027.0344 (2019).
Zimmermann, R. C. et al. Insecticide activity and toxicity of essential oils against two stored-product insects. Crop Prot. 144, 105575. https://doi.org/10.1016/j.cropro.2021.105575 (2021).
Rao, P. P., Nagender, A., Rao, L. J. & Rao, D. G. Studies one the effects of microwave drying and cabinet tray drying on the chemical composition of volatile oils of garlic powder. Eur. Food Res. Technol. 224, 791–795 (2007).
Abu-Lafi, S., Dembicki, J. W., Goldshlag, P., Hanuš, L. O. & Dembitsky, V. M. The use of the ‘cryogenic’ GC/MS and on-column injection for study of organosulfur compounds of the Allium sativum. J. Food Comp. Anal. 17, 235–245 (2004).
Dziri, S., Casabianca, H., Hanchi, B. & Hosni, K. Composition of garlic essential oil (Allium sativum L.) as influenced by drying method. J. Essent. Oil Res. 26(2), 91–96. https://doi.org/10.1080/10412905.2013.868329 (2014).
Romeilah, R. M., Fayed, S. A. & Mahmoud, G. I. Chemical compositions, antiviral and antioxidant activities of seven essential oils. J. Appl. Sci. Res. 6, 50–62 (2010).
Khani, M., Muhamad Awang, R. & Omar, D. Insecticidal effects of peppermint and black pepper essential oils against rice weevil, Sitophilus oryzae L. and rice moth, Corcyra cephalonica (St.). J. Med. Plants 11(43), 97–110 (2012).
NestorBassole, I. H. et al. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 15, 7825–7839 (2010).
Rosato, A. et al. Elucidation of the synergistic action of Mentha piperita essential oil with common antimicrobials. PLoS ONE 13(8), e0200902 (2018).
Galambosi, B. & Peura, P. Agrobotanical features and oil content of wild and cultivated forms of caraway (Carum carvi L.). J. Essent. Oil Res. 8, 389–397 (1996).
Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integr. Pest Manag. Rev. 2, 25–34 (1997).
Park, J. H., Jeon, Y. J., Lee, C. H., Chung, N. & Lee, H. S. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & Lu., newly recorded pest. Sci. Rep. 7, 40902. https://doi.org/10.1038/srep40902 (2017).
Yang, F., Zhu, F. & Lei, C. Insecticidal activities of garlic substances against adults of grain moth, Sitotroga cerealella (Lepidoptera: Gelechiidae). Insect sci. 19(2), 205–212 (2012).
Kumar, R. & Tiwari, S. N. Fumigant toxicity of essential oils against Corcyra cephalonica and Sitotroga cerealella. Environ. Ecol. 36(1), 33–37 (2018).
Elmadawy, A. A., Omar, A. F. & Ismail, T. Bags impregnated with garlic (Allium sativum L.) and parsley (Petroselinum crispum (Mill.) Fuss) essential oils as a new biopesticide tool for Trogoderma granarium Everts, 1898 pest control. Acta agriculturae Slovenica 119(1), 1–13 (2023).
Mackled, M. I., El-Hefny, M., Bin-Jumah, M., Wahba, T. F. & Allam, A. A. Assessment of the toxicity of natural oils from Mentha piperita, Pinus roxburghii, and Rosa spp. against three stored product insects. A. A. Processes 7, 861. https://doi.org/10.3390/pr7110861 (2019).
Pang, X. et al. Toxicity and repellent activity of essential oil from Mentha piperita Linn. leaves and its major monoterpenoids against three stored product insects. Environ. Sci. Pollut. Res. 27, 7618–7627 (2020).
Alcántara-de la Cruz, R. et al. Effects of essential oils on biological attributes of Trichogramma galloi adults. J. Asia Pac. Entomol. 24(2), 64–67 (2021).
Ercan, F., Baş, H., Koç, M., Pandir, D. & Öztemiz, S. Insecticidal activity of essential oil of Prangos ferulacea (Umbelliferae) against Ephestia kuehniella (Lepidoptera: Pyralidae) and Trichogramma embryophagum (Hymenoptera: Trichogrammatidae). Turk. J. Agric. For. 37(6), 719–725 (2013).
Acknowledgements
The financial support for this study was provided by The National Research Centre of Egypt internal research project no. 13050117.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
H.H and S.S.: conceptualization; methodology; investigation; data analysis; writing—original draft, review, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbehery, H.H., Ibrahim, S.S. Potential fumigant toxicity of essential oils against Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) and its egg parasitoid Trichogramma evanescens (Hymenoptera: Trichogrammatidae). Sci Rep 14, 6253 (2024). https://doi.org/10.1038/s41598-024-56611-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56611-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.