Abstract
Fungicides or insecticides are popular means of controlling a variety of pathogens and insect pests; however, they can cause harmful effects on both human health and the environment. Different researchers have suggested using plant extracts, which have shown promise in managing fungi and insects. The purpose of this investigation was to explore the antifungal activities of an acetone extract made from the leaves of Indian Hawthorn (HAL) against phytopathogens that are known to harm maize crops, Fusarium verticillioides (OQ820154) and Rhizoctonia solani (OQ820155), and to evaluate the insecticidal property against Aphis gossypii Glover aphid. The HAL extract demonstrated significant antifungal activity against the two fungal pathogens tested, especially at the high dose of 2000 µg/mL. Laboratory tests on the LC20 of HAL extract (61.08 mg/L) versus buprofezin 25% WP (0.0051 mg/L) were achieved on A. gossypii Glover. HAL extract diminished the nymph's production over 72 h and their total reproductive rate. This extract was like buprofezin 25% WP in decreasing the daily reproductive rate, reproductive period, and mean survival percentage. Nevertheless, the newly-born nymphs of treated females with HAL extract attained the highest reduction in survival percentage at 46.00%. Equalized prolongations on the longevity of nymphs to 9.33, 8.33, and 7 days and the total life cycle to 15.00, 14.00, and 12.67 days were realized by HAL extract, buprofezin 25% WP, and the control, respectively. The olfactory choice test on the aphids showed the minimum attraction rate to HAL extract. The HPLC of HAL extract comprised an abundance of phenolic compounds (ferulic acid, gallic acid, 4-hydroxybenzoic acid, salicylic acid, ellagic acid, and pyrogallol), and the concentrations of these compounds vary widely, with salicylic acid being the most concentrated at 25.14 mg/mL. Among the flavonoids, epicatechin has the highest concentration at 11.69 mg/mL. The HAL extract GC–MS consists of various organic compounds, including sesquiterpenes, cyclopropenes, fatty acids, steroids, alcohols, ketones, esters, bufadienolides, opioids, and other organic compounds. The most abundant compounds in the sample are n-hexadecanoic acid (12.17%), followed by 5α, 7αH, 10α-eudesm-11-en-1α-ol (9.43%), and cis-13-octadecenoic acid (5.87%). Based on the findings, it can be inferred that the HAL extract may be a viable option for plants to combat both fungal and insect infestations. This presents an encouraging prospect for utilizing a natural and sustainable approach toward long-term pest management in plants.
Similar content being viewed by others
Introduction
Indian hawthorn, or India hawthorn (Rhaphiolepis indica L.), is a plant species in the family Rosaceae. It is widely distributed throughout tropical and subtropical regions of Asia, including India, China, Japan, and Malaysia. The leaves, stems, and roots of Indian hawthorn have been shown to exhibit a range of pharmacological activities, including anti-inflammatory, antioxidant, hypoglycemic, anticancer, antimicrobial, and hypolipidemic effects, and are a therapeutic agent for treating various diseases1,2.
Regarding the maize crop, according to recent data, Egypt's maize production reached 7.4 million metric tons in 2022, indicating an increase from the 6.4 million metric tons produced in 2020. Throughout the period analyzed, the highest corn production levels were recorded in 2021 and 2022, while the lowest was reported in 2011 at 5.5 million metric tons. Worldwide, the maize crop is devastated by several pathogens, such as fungi of the Fusaria group, which are commonly associated with maize plants and cause diseases in various parts, including seedlings, roots, stalks, and kernels. Fusarium verticillioides, the most prevalent species infecting maize ears in Egypt, leads to ear or kernel rot disease, which can reduce yield and quality and result in mycotoxin accumulation in grain3,4,5. Also, the Rhizoctonia solani fungus is known to infect several other crops besides maize, causing various diseases such as root rot6.
Also, maize crops encounter different pests, including the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae), which is the biggest destroyer of enormous economic crops7,8,9. Its direct sap feeding on the cell contents leads to yield loss. Moreover, it blocks the photosynthesis process caused by the extended superficial growth of black sooty mold resulting from the honeydew exertion of the cotton aphid10.
In recent years, medicinal plants and other natural products used to treat various pathogenic diseases have increased in popularity because of their potential to circumvent pesticide resistance and the unpleasant side effects associated with chemicals11,12,13. In light of these concerns, the search for natural, bio-based alternatives has gained momentum. Botanical extracts have emerged as potential candidates for their cost-effectiveness and inherent bioactive compounds that exhibit antifungal and insect-repellent properties. Studies about the botanical compounds responsible for the biological features of herbal plants have been inspired by the discovery of chemical compounds produced by these plants13,14,15,16. Indian hawthorn is one of these plants according to its phytochemical profile, which contains a variety of bioactive compounds, including flavonoids, triterpenoids, and phenolic acids. The most abundant flavonoids identified in the plant are quercetin, kaempferol, and their glycosides. Triterpenoids such as ursolic and oleanolic acids have also been isolated from the plant1.
There have been several research studies conducted to investigate the efficacy of various extracts against F. verticillioides and R. solani, and the results have been promising, indicating that these extracts possess antifungal activities17,18,19,20. The extracts from different parts of Indian hawthorn species contain unique components compared to their counterparts in other plants that possess distinctive efficiency against many insects, which may qualify them to have an exterminatory role in insecticidal activity1. To compare the extract results, a buprofezin compound was widely used against piercing-sucking hemipteran pests. Buprofezin possesses a particular effect on the biological aspects represented in the diminishing of the chitin contents in successive generations of cotton aphids21,22.
The objectives of this research were to isolate and identify the specific pathogens responsible for causing maize ear rot and root rot, assess the insecticidal and antifungal effects of an acetone extract obtained from Indian hawthorn leaves (HAL) against Aphis gossypii Glover and the isolated fungal pathogens, and identify the primary constituents of the extract using HPLC and GC–MS analysis.
Materials and methods
Fungal strains used in the study
In this study, the fungal strains utilized were obtained from maize and identified morphologically at the genus level using a fungal identification manual. To achieve molecular identification, the ITS region was amplified utilizing universal primers ITS1 and ITS4 with previously reported PCR conditions. Following amplification, the PCR products underwent purification and sequencing through Sanger technology. The resultant sequences were annotated and compared to the GenBank database via alignment with NCBI, and the fungal sequences were subsequently submitted to the GenBank database to obtain accession numbers23,24,25.
HAL extract setting up
Fresh Indian hawthorn (HAL) leaves were collected from the Faculty of Agriculture (Saba Basha) Garden, Alexandria, Egypt, and cleaned thoroughly to ensure that they had not been exposed to any harmful chemicals. The plant material was entirely dried by spreading it on a tray and leaving it in a well-ventilated area. Once the plant material was fully dry, it was ground into a fine powder using a grinder.
The powdered HAL (90 g) was then transferred into a clean glass jar, and acetone (900 mL, 99% ACS reagent purity) was poured into the jar. The jar was sealed tightly and shaken vigorously to ensure that the acetone was thoroughly mixed with the plant material. The mixture was then left to soak for 24 h at room temperature while being shaken occasionally. This allowed the acetone to extract the desired compounds from the plant material. After 24 h, the mixture was filtered through filter paper to remove any solid particles. This step was repeated until the extract was free of any visible particles. The filtered extract was then transferred into a shallow glass dish and allowed to evaporate completely at room temperature in a fume hood with a rotary evaporator at 40 °C (DAIHAN-RVE-05, Singapore). The residue left behind was scraped into a clean, dry container. The HAL extract was stored in a cool, dry place in a dark-colored glass container to prevent light from breaking down the active compounds26. The yield of the extract was calculated as: Yield extract = [HAL dry extract after solvent removal (g)/dry HAL powdered material used in extraction (g)] × 10027.
HAL effect on fungal hyphal growth using food poison technique
To investigate the effect of HAL extract on fungal hyphal growth, a food poisoning technique was employed by adding the extract directly to the culture medium. First, a culture of the test fungus was grown on Potato Dextrose Agar (PDA) plates until it formed a uniform mat of mycelium. The mycelium was then cut into small pieces using a sterile scalpel and transferred onto fresh PDA plates containing different concentrations of HAL extract (500, 1000, and 2000 μg/mL). As a control, PDA plates with no HAL extract were prepared. All plates were incubated at the optimal temperature for the test fungus for 7 days. After this period, the diameter of the fungal colony was measured using a ruler. The hyphal growth (mm) was also measured to determine the effect of the extract on the development of the fungus. This process was repeated three times for each concentration of the extract28,29.
HAL extract insecticidal activity
Tested compounds
-
Acetone extract of Indian hawthorn leaves
-
Buprofezin (Solano 25% WP belongs to the benzoylphenyl urease group, a chitin synthesis inhibitor) was obtained from El-Helb Pesticides and Chemical Co.
Rearing units of cotton aphid
Individuals of Aphis gossypii Glover were collected from the available host plants. The collected aphids were allowed to grow in a rearing unit comprised of cotton seedlings (variety Giza 86) in polypropylene pots (diameter, 10 cm; height, 8 cm), covered up with a muslin jacket. Each rearing unit was placed into a growth incubator, digitally outfitted with a thermostat and a hygrometer, to maintain stable conditions at 25 ± 2 °C, 60% RH, and 16 h of lightning, according to Gaimari and Turner30. Newly ripe seedlings must be replaced by malformed ones resulting from the feeding of aphids. More seedlings must be added to meet the exacerbation of aphid growth. A laboratory strain (LS) of cotton aphids was attained after approximating seven generations31.
Toxicity assay
The toxicity of acetone extract of Indian hawthorn and buprofezin 25% WP against A. gossypii Glover was carried out by using the slide-dipping method invented by Stirbly et al.32. Each tested compound had six gradual concentrations in distilled water. A double-faced piece of Scotch tape was stacked on one surface of a glass slide. A delicate brush was used to load 20 adult females over the surface of the Scotch tape. For each concentration in the tested compound, the slide was dipped for 10 s. Meanwhile, the control slide was dipped in distilled water. Three replicated slides were used for each concentration. The treated slides were incubated under the same laboratory conditions. Every 24 h, the numbers of dead and living aphids were recorded using a stereoscopic microscope. Mortality percentages were corrected according to the formula of Abbott33. The results were subjected to probit analysis34.
Biological aspects
A susceptibility test using the technique of leaf disks on cotton seedlings was accomplished according to Insecticide Resistance Action Committee (IRAC)35. The leaf disks were dipped in the sub-lethal concentration (LC20) of each tested compound and distilled water for control. One leaf disk was attached to the whole cohesive surface of an agar gel 1% filled the bottom of a cup (diameter, 7 cm; height, 2.5 cm).
Adult female
Twenty uniformed adult female cotton aphids (LS) were loaded gently onto the leaf disk. Each cup was capped with micro-hole gauze and tied with a rubber band. Each treatment was replicated with triple cups. The treated cups were incubated under the foregoing laboratory conditions. The affected aphids were monitored every 24 h using a stereoscopic microscope. The daily production of nymphs, total reproduction, survival percentage, and longevity of adult females were recorded after exposure to LC20 of each treatment according to Michelotto et al.36.
The first-born nymphs from treated females
Twenty of the first-born nymphs (produced from the treated females with each tested compound at LC20 in comparison to the control) were monitored for their daily and total survival rate and longevity as performed by Michelotto et al.36.
Choice test of olfactory response
Adult cotton aphids were submitted to an olfactometer set designed by Jaba et al.37. Twenty pre-starved adults were placed in a central cell connected to each of three lateral tubes through a short tube. Each lateral tube contained 3 g of fresh, tender leaf. These leaves were dipped in LC20 of the tested compounds for 10 s versus the control leaf in distilled water. Air was allowed to pass over the hosted leaves in each lateral tube using an air compressor through slime hoses attached. The three-hosted leaves were inset at the same time in the lateral tube. Each treatment had three replicates of olfactometer sets. The number of aphids that responded to each tube was recorded every hour for up to 4h. The retention time of aphids needed to accomplish their movement toward each tube was quantified at the end of the 4th h. The equation of attraction response percentage was expressed by Weeks et al.38 as follows:
where T is the number of attracted aphids to the host tube treated by tested compounds; C is the number of attracted aphids to the control tube; and N is the total number of aphids submitted to the olfactometer set.
High-performance liquid chromatography analysis
High-performance liquid chromatography (HPLC) is a widely used analytical technique for the separation, identification, and quantification of individual components in a mixture. In this study, HPLC Agilent 1260 was used to analyze the target compounds in the samples. Sample preparation involved extracting the target compounds from the sample matrix and filtering it to remove any impurities. The column selected was possessed dimensions of 4.6 mm in diameter and 250 mm in length, featuring a particle size of 5 μm. The mobile phase used consisted of two key components: H2O (A) and 0.05% CF3COOH in CH3CN (B), delivered at a constant flow rate of 0.9 mL/min.
The HPLC system was calibrated using standard compounds of known concentration and purity before running the samples. The samples were then injected into the HPLC system with an injection volume of 5 μL, and a column temperature meticulously maintained at 40 °C. To facilitate the effective separation of sample constituents, we implemented a gradient elution program with distinct phases. Precisely, during the first 5 min, the mobile phase comprised 80% solvent A and 20% solvent B; from 5 to 8 min, it transitioned to 60% solvent A and 40% solvent B; maintaining this composition from 8 to 12 min. Subsequently, from 12 to 16 min, the mobile phase returned to its initial state with 82% solvent A and 18% solvent B, and finally, from 16 to 20 min, it remained consistent with 82% solvent A and 18% solvent B.. The target compounds were separated based on their physicochemical properties and interacted with the stationary phase of the column. The separated compounds were detected by a UV–visible detector, specifically monitored at 280 nm, which generated a signal proportional to the concentration of the compound. The output from the detector was recorded and analyzed using specialized software. The chromatogram showed the retention time and peak area of each compound, which were used to identify and quantify the components in the sample. The HPLC analysis results showed that the samples contained the target compounds at the expected concentrations. The precision and accuracy of the HPLC analysis were validated by the low standard deviation and high recovery rates of the standard compounds. These results demonstrate the suitability of HPLC analysis for the characterization of complex mixtures and the determination of the purity of a compound39.
Gas chromatography–mass spectrometry analysis
Gas chromatography-mass spectrometry (GC–MS) 7000D instrument, manufactured by Agilent Technologies in Santa Clara, CA, USA, was used to analyze the target organic compounds in the sample. Before analysis, the sample was extracted and purified to remove any impurities. The purified sample was injected into the GC column packed with a 5% diphenyl/95% dimethylpolysiloxane stationary phase (HP-5MS), which was coated with a stationary phase that separated the target compounds based on their physical properties. The GC column was heated to separate the target compounds in the sample mixture, and the separated compounds were carried by the carrier gas, helium (purity, 99.99%), flowing at a constant rate of 1 mL/min., through the column and into the mass spectrometer. In the mass spectrometer, the separated compounds were ionized by electron impact ionization,which was set at 70 electronvolts (eV), and the scan time was fixed at 0.2 s, producing charged fragments that were separated based on their mass-to-charge ratio (m/z) range from 40 to 600. The mass spectrometer detected the charged fragments and generated a mass spectrum, which was a unique fingerprint of the compounds in the sample. For each sample injection, a volume of 1 μL was introduced, employing a split ratio of 10:1. The injector temperature was held steady at 250 °C throughout the analysis. The temperature program of the column oven began at 50 °C for an initial 3-min period, followed by a linear ramp of 10 °C per minute until reaching 280 °C. Subsequently, the temperature was raised to 300 °C and maintained for an additional 10 min.
The mass spectra were analyzed using specialized software, which identified the compounds based on their fragmentation patterns and retention times. The output from the mass spectrometer was recorded and analyzed by different liberires, including Wiley Registry 8E and Replib, and the relative abundance and mass-to-charge ratio of each fragment were used to identify and quantify the components in the sample.
Statistical analyses
Statistical analyses were performed using SAS software. An ANOVA (Analysis of Variance) was performed to assess overall treatment effects. A significance level of 0.05 was used, and the LSD (Least Significant Difference) test was employed to determine significant differences among treatment groups40.
Statement of permissions and/or licenses for collection of plant or seed specimens
The authors declare that the maize specimens used in this study are publicly accessible maize cultivars, and we were assigned to NCBI respiratory under accession numbers OQ820154 and OQ820155.
Plant guidelines
Experimental research and field studies on plants, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation—Formal ethical approval is not required.
Results
The procedure for the isolation and characterization of fungal strains
After investigating the maize plant's ear and root, two types of pathogens were isolated and identified as Fusarium and Rhizoctonia species. To further identify and classify these fungi, molecular analysis was performed by sequencing the amplified ITS region, which showed 100% homology with Fusarium verticillioides and Rhizoctonia solani. To make these results widely available, the sequences for the two fungal organisms were deposited in the NCBI database and assigned accession numbers OQ820154 for F. verticillioides and OQ820155 for R. solani.
The impact of HAL extract on fungal strains
The data presented in Table 1 show the effect of Indian hawthorn leaf acetone extract at different concentrations on the hyphal growth of two fungal pathogens, R. solani, and F. verticillioides. The results are shown in terms of mean hyphal growth in millimeters (mm). Based on the results, the HAL extract had a significant inhibitory effect on the hyphal growth of both fungal pathogens, with increasing concentrations leading to greater inhibition. The mean hyphal growth for both pathogens decreased significantly as the concentration of the extract increased from 500 to 1000 μg/mL and 2000 μg/mL, respectively.
For R. solani, the mean hyphal growth decreased from 57.67 to 32.67 mm and 15.33 mm at 500 μg/mL, 1000 μg/mL, and 2000 μg/mL, respectively. Similarly, for Fusarium verticillioides, the mean hyphal growth decreased from 57.33 to 45.00 mm and 12.33 mm at 500 μg/mL, 1000 μg/mL, and 2000 μg/mL, respectively. The results of the statistical analysis revealed that the mean hyphal growth values were significantly different among the concentrations for both fungal pathogens. The mean hyphal growth values for the highest concentration (2000 μg/mL) were significantly different from those of the other concentrations. Furthermore, the mean hyphal growth values for the negative control (Nc) were significantly different from those of all other treatments, indicating that the extract had a significant inhibitory effect on the fungal pathogens. In summary, the HAL extract demonstrated significant antifungal activity against the two fungal pathogens tested. Further studies may be necessary to determine the specific antifungal compounds present in the extract and to evaluate its potential as a natural alternative to synthetic fungicides in plant disease management (Table 1).
Toxicity of the tested HAL extract on the adults of Aphis gossypii
Toxic effect results under laboratory conditions were achieved to determine the LC20 and LC50 values of HAL extract compared to buprofezin 25% WP on the adult stage of A. gossypii Glover after 24 h of exposure (Table 2). Buprofezin 25% WP exhibited toxicity values of 0.022 and 0.0051 mg/L higher than the toxicity of HAL extract at 245.35 and 61.08 mg/L at LC50 and LC20, respectively.
Biological aspects of adults of Aphis gossypii and their newly born nymphs
Adult female
Data on the daily production of the born nymphs per female, reproductive rate, survival percentage, and longevity were evaluated after the exposure of A. gossypii adult female to LC20 of the tested compounds (Table 3).
Indian hawthorn extract had a potent effect on the born nymphs production per female (0.73) compared to buprofezin 25% WP (1.38) and the control (2.07) at 24 h after exposure. Meantime, the results of nymph production per female in HAL extract were comparable to buprofezin 25% WP compared to the control at 48 and 72 h after exposure. All the treatments had no significant differences in the nymph production at 96 h after exposure. The total reproductive rate of HAL extract was significantly decreased (2.08) more than buprofezin 25% WP (2.24) when compared to its counterpart rate in control (15.77). There were no significant differences in the longevity of the adult aphids exposed to all treatments. Significant decreases for HAL extract were comparable to buprofezin 25% WP on the daily reproductive rate, reproductive period, and mean survival percentages compared to the control.
First-day-born nymphs from treated adults
Daily and mean survival percentages and longevity data were accomplished on the first day-born nymphs 7 days after exposure to LC20 of the tested compounds (Table 4).
The daily survival nymphs in HAL extract were vigorously affected with percentages of 90.00, 78.33, and 33.33% compared to buprofezin 25% WP at 100.00, 88.33, and 43.33%, and the control that fulfilled the highest survival percentages at 100.00% along 24, 48, and 72 h after exposure, respectively. Equipollent reductions occurred in the daily survival percentages of nymphs in both treatments of HAL extract and buprofezin 25% WP, which were significantly less than the control at 96, 120, 144, and 168 h of exposure. HAL extract had a significant reduction in the mean survival percentages of nymphs (46.00%) that surpassed buprofezin 25% WP (61.67%) and the control (98.57%). The longevity of nymphs was prolonged in HAL extract to 9.33 days, followed by buprofezin 25% WP at 8.33 days, while in the control it was 7 days.
Total life of Aphis gossypii
The current results of the tested compounds at LC20 exhibited equipollent prolongations on the total life cycle for HAL extract at 15 days and buprofezin 25% WP at 14 days, compared to the highest life duration in control at 12.67 days (Table 5).
Olfactory response of the choice test on the adults of Aphis gossypii
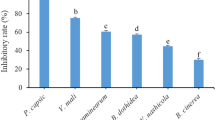
The lateral chamber that hosted the treated leaves with R. indica extract possessed the lowest attraction percentage on aphid individuals (21.67%) compared to buprofezin 25% WP (33.33%) and control treatment (45.00%). There were no significant differences between the treatments for the retention times (Rt) of aphid individuals needed to accomplish their orientations at 4 h of test duration (Fig. 1).
HAL extract yield and polyphenolic content
The yield of HAL extract obtained was found to be 4.33% for every 90 g of initial ground material used. The data in Table 6 and Fig. 2 represent the retention time (in minutes), compounds, and their respective concentrations (mg/mL) for various phenolics and flavonoids. The compounds listed under phenolics are ferulic acid, gallic acid, 4-hydroxybenzoic acid, salicylic acid, ellagic acid, and pyrogallol. The compounds listed under flavonoids are apigenin, rutin, chrysin, epicatechin, quercetin, 7-OH flavone, and acacetin.
The HPLC chromatograms of Indian hawthorn extract show the peaks of the following phenolic compounds identified at a wavelength of 284 nm: 1 = ferulic acid, 2 = gallic acid, 3 = 4-hydroxybenzoic acid, 4 = salicylic acid, 5 = ellagic acid and 6 = pyrogallol; at a wavelength of 273 nm, the peaks represent the following flavonoids: 7 = apigenin, 8 = rutin, 9 = chrysin, 10 = epicatechin, 11 = quercetin and 12 = 7-oh flavone and 13 = acacetin.
The concentrations of these compounds vary widely, with salicylic acid being the most concentrated at 25.14 mg/mL, while ellagic acid has the lowest concentration at 0.55 mg/mL. Among the flavonoids, epicatechin has the highest concentration at 11.69 mg/mL, while apigenin has the lowest concentration at 2.33 mg/mL. Other compounds present in lower concentrations include gallic acid (4.25 mg/mL), pyrogallol (6.18 mg/mL), and chrysin (8.14 mg/mL). The extract also contains various flavonoids, including apigenin (2.33 mg/mL), rutin (3.42 mg/mL), quercetin (7.18 mg/mL), 7-OH flavone (9.15 mg/mL), and acacetin (4.66 mg/mL).
GC–MS content of Indian hawthorn (HAL) extract
From Table 7 and Fig. 3, we concluded that the HAL extract GC–MS consists of various organic compounds, including sesquiterpenes, cyclopropenes, fatty acids, steroids, alcohols, ketones, esters, bufadienolides, opioids, and other organic compounds. The most abundant compounds in the sample are n-hexadecanoic acid (12.17%), followed by 5α,7αH,10α-eudesm-11-en-1α-ol (9.43%), and cis-13-octadecenoic acid (5.87%).
Several compounds appear to be present in relatively low amounts (below 1% relative abundance), including the ketone 2-(4-Nitrobutyryl)cyclooctanone, the steroid Androstan-17-one, 3-ethyl-3-hydroxy-, (5α)-, the fatty acid 11-cis-octadecenoic acid 1tms, and the opioid α-N-Normethadol, among others. Sesquiterpenes and fatty acids are the most common compound classes in the sample, with each class containing 8 compounds. The HAL extract contains several compounds with potential pharmacological activity, such as loperamide, resibufogenin, and α-N-normethadol, which are opioids; and (−)-globulol, caryophyllene oxide, and á-copaene, which have reported anti-inflammatory and analgesic properties.
Discussion
Medicinal plants, which are recognized for their reliability, strength, and environmentally sustainable impact, are being used in the field of environmental management to combat plant diseases and reduce the harmful effects of synthetic antimicrobials. In this study, the acetone extract of Indian hawthorn leaves (HAL) was evaluated for its bioactive substances using various conventional assays. The results revealed the presence of a diverse range of phytochemical components, including salicylic acid, epicatechin, ferulic acid, hydroxybenzoic acid, n-hexadecanoic acid, fatty acid esters, and terpenes, which are known to be biologically active and effective against plant pathogens. These findings are consistent with those of previous studies and suggest that the botanical compound substances in the plant extract play a critical role in its biological activity31,41,42. Also, other researchers43 investigated the relationship between the plant extract's chemical constituents and its therapeutic properties.
After conducting HPLC and GC–MS analyses of HAL extract, we identified several highly potent molecules, including salicylic acid, epicatechin, ferulic acid, and n-hexadecanoic acid. These compounds have been extensively studied for their antimicrobial activities44,45,46. Literature suggests that salicylic acid (SA), a derivative of benzoic acid, is a key component in the resistance of plants against diseases, especially during the process of systemic acquired resistance47. While some studies have suggested that salicylic acid (SA) does not directly exhibit antifungal activity48, other research has indicated that SA can affect various stages of fungal development. For example, SA has been found to hinder conidial germination and hyphae development in Sphaerotheca fuliginea49 and inhibit sclerotia differentiation and growth in Sclerotium rolfsii50. Therefore, SA can have an indirect impact on fungal growth and development.
Our obtained data on the biological aspects, including the daily survival percentage of adult females of A. gossypii Glover and their first day-born nymphs, were potently affected by the LC20 of HAL extract more than buprofezin and the control treatment. Previous studies confirmed the current study on the detected moieties of HAL extract represented in gallic acid, quercetin, apigenin, ellagic acid, and salicylic acid as a primary cause of the lethal activity against A. gossypii Glover51,52,53,54,55. Ellagic acid was found to be the augmented cause of the lethal effect on the larval and pupal stages of Spodoptera litura Fabricius53. In addition, apigenin was detected as the source of the toxic effect on the larval stage of Culex quinquefasciatus due to its capability to inhibit AChE1 and damage the epithelial cells in the mid-gut of the developmental stages55. Moreover, quercetin-3-O-glucoside showed vigorous toxic effects against Aphis craccivora54. Certain concentrations of salicylic acid also could manifest slight reductions in the populations of A. gossypii Glover and Bemisia tabaci Gennadius, mainly attributed to the leverage of the phenolic compounds in the treated leaf contents52. On the other hand, The GC–MS analysis of HAL extract detected specific unsaturated carboxylic fatty acids represented in palmitic acid, n-hexadecanoic acid, 9,12-octadecadienoic acid (Z,Z)-, 11-cis-Octadecenoic acid, known as cis-vaccenic acid, and trans-13-octadecenoic acid that may be causative factors of the insecticidal activity against A. gossypii Glover. This assumption meets previous studies on the insecticidal activity of identical fatty acids on various insect pests56,57,58. It is well known that there is a correlation between the multiplicity of the double bonds in these fatty acid chains and the larvacidal leverages against S. littoralis Boisd59,60. Furthermore, the free carboxyl group in fatty acids could deactivate the systemic stability of cells61 and consequently cause the neat carboxylic fatty acids to attain more toxic effects than their counterparts in the methylated form59.
In our study, HAL extract was comparable to buprofezin at their sub-lethal concentrations (LC20) in prolonging the longevity of the 24h-born nymphs from treated adult females and the total life cycle compared to the control. The presence of the detected moieties of ellagic acid, rutin, and chrysin in HAL extract may be in line with investigations in previous studies53,62,63. Where ellagic acid adversely prolonged the intervals of the developmental stages of S. litura Fabricius53. In addition, the flavonoid rutin was characterized by an obvious prolongation in the developmental duration of larvae and the total life cycle of Spodoptera frugiperda J.E. Smith (Lepidoptera: Noctuidae)62. Chrysin is also thought to possess a prolonged effect on the larval stage and total developmental period of Zeugodacus cucurbitae Coquillett (Diptera: Tephritidae)63.
Regarding the data on the biological aspects of adult females of A. gossypii Glover, potent reductions for R. indica extract at LC20 were exhibited in the daily nymph production per female during the 72 h of exposure. The extract of HAL also had a significant reduction in the total reproductive rate, more than buprofezin, but was comparable to buprofezin on the daily reproductive rate and total reproductive period compared to the control. These results might be correlated to the content of chrysin in the extract of R. indica, as it was previously explored for its role in inhibiting the percentage of adult emergence of Z. cucurbitae Coquillett63. Additionally, flavonoids such as acacetin-7-O-glycoside that were found in extracts of Robinia pseudoacacia L. Fabaceae might have biological effects on Myzus persicae Sulzer (Homoptera: Aphididae)64.
The current study on the olfactory response of adult aphids showed that R. indica extract possessed the lowest attraction percentage on aphids and increased in buprofezin and more in the control at the 4th h of exposure. These findings were tuned to the presence of some phytochemical components in R. indica extract, such as gallic acid and epicatechin65,66. Gallic acid was found to induce epigallocatechin-3-gallate to promote direct defense in the treated plant by stimulating the signals of jasmonic acid66. Flavonoid (+)-catechin also exhibited potent repellency and antifeedant activity against rubber termites, Coptotermes curvignathus Holmgren (Isoptera: Rhinotermitidae)65.
In general, the results of our study indicate that the HAL extract under examination exhibits promising properties as an organic fungicide for combating R. solani and F. verticillioides. Additionally, it shows potential as an insecticide for eradicating cotton aphids. Nevertheless, additional research is necessary to determine the precise mode of action and probable applications of the extract in the agro-fields, thereby warranting further investigation.
Conclusions
To sum up, the current investigation assessed the insecticidal and antifungal characteristics of an acetone extract derived from the leaves of Indian hawthorn (HAL). The extract exhibited some efficacy in impeding the fungal development of Rhizoctonia solani but demonstrated greater effectiveness against Fusarium verticillioides in laboratory settings. HAL extract attained a rational toxic effect on Aphis gossypii Glover. The LC20 of HAL extract vigorously affected the nymph production of adult females of Aphis gossypii Glover over 72 h and their overall reproductive rate. This extract decreased the daily reproductive rate, reproductive period, and mean survival percentage. Nevertheless, the newly born nymphs from treated females with HAL extract faced a decline in the survival percentage and prolongations in the longevity of the nymphs and the overall life cycle of A. gossypii Glover. The olfactory choice of cotton aphids showed a low attraction response to HAL extract. Thence, HAL extract may be sensibly included in control programs of the cotton aphid. HPLC and GCMS analyses revealed that the HAL extract was rich in salicylic acid, ferulic acid, epicatechin, and n-hexadecanoic acid. These findings suggest that the HAL extract may serve as a natural and sustainable solution to manage fungal infestations in plants and combat A. gossypii Glover, offering a preferable alternative to insecticides that can adversely affect human health and the environment in the long run.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Lin, C.-H., Chang, H.-S., Liao, H.-R., Chen, I.-S. & Tsai, I.-L. Triterpenoids from the Roots of Rhaphiolepis indica var. tashiroi and Their Anti-Inflammatory Activity. Int. J. Mol. Sci. 14, 8890–8898 (2013).
Kim, H.-R. et al. Antioxidative effects of Rhaphiolepis indica and Quercus salicina from Jeju. J. Korean Appl. Sci. Technol. 33, 41–50 (2016).
El-Naggar, A. A. A. & Sabry, A. M. Development of Fusarium ear rot disease of maize in three different geographic locations. J. Plant Prot. Pathol. 1, 921–927 (2010).
Kriss, A. B., Paul, P. A. & Madden, L. V. Relationship between yearly fluctuations in Fusarium head blight intensity and environmental variables: A window-pane analysis. Phytopathology 100, 784–797 (2010).
Khan, J., Qi, A. & Khan, M. F. R. Fluctuations in number of Cercospora beticola conidia in relationship to environment and disease severity in sugar beet. Phytopathology 99, 796–801 (2009).
Kluth, C. & Varrelmann, M. Maize genotype susceptibility to Rhizoctonia solani and its effect on sugar beet crop rotations. Crop Prot. 29, 230–238 (2010).
Kaygın, A. T., Görür, G. & Çota, F. Contribution to the aphid (Homoptera: Aphididae) species damaging on woody plants in Bartın, Türkiye. Int. J. Nat. Eng. Sci. 2, 83–86 (2008).
Megahed, H. Annual occurrence and population dynamics, of cotton aphids, Aphis gossypii Glover on specific host plants at Sharkia governorate, Egypt. Egypt. Acad. J. Biol. Sci. A Entomol. 10, 7–18 (2017).
EI-Gindy, M. A., Ibraheem, M. M. A. & Megahed, H. E. Susceptibility of three maize cultivars to aphid infestation and effect of potassium fertilizer levels on aphid. J. Plant Prot. Pathol. 31, 457–463 (2006).
Morando, R. et al. Assessing cotton genotypes for resistance to Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 114, 387–396 (2021).
Abo-Zaid, G., Abdelkhalek, A., Matar, S., Darwish, M. & Abdel-Gayed, M. Application of bio-friendly formulations of chitinase-producing streptomyces cellulosae actino 48 for controlling peanut soil-borne diseases caused by Sclerotium rolfsii. J. Fungi 7, 167 (2021).
Abdelkhalek, A., Al-Askar, A. A., Arishi, A. A. & Behiry, S. I. Trichoderma hamatum strain Th23 promotes tomato growth and induces systemic resistance against tobacco mosaic virus. J. Fungi 8, 228 (2022).
Moawad, H. et al. Retting and degumming of flax using biotechnology eco-friendly approach. Egypt. J. Chem. 62, 2033–2045 (2019).
Duraipandiyan, V., Ayyanar, M. & Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 6, 1–7 (2006).
Jothy, S. L. et al. Cassia spectabilis (DC) Irwin et Barn: A promising traditional herb in health improvement. Molecules 17, 10292–10305 (2012).
Mazumder, P. M., Percha, V., Farswan, M. & Upaganlawar, A. Cassia: A wonder gift to medical sciences. Int. J. Clin. Pharm 1, 16–38 (2008).
Behiry, S. I. et al. Plantago lagopus extract as a green fungicide induces systemic resistance against Rhizoctonia root rot disease in tomato plants. Front. Plant Sci. https://doi.org/10.3389/fpls.2022.966929 (2022).
Behiry, S. I. et al. Chorisia speciosa extract induces systemic resistance against tomato root rot disease caused by Rhizoctonia solani. Agronomy 12, 2309 (2022).
Derbalah, A. et al. Resistance induction and direct antifungal activity of some monoterpenes against Rhizoctonia solani, the causal of root rot in common bean. Life 12, 1040 (2022).
El-Bilawy, E. H. et al. Antiviral and antifungal of Ulva fasciata extract: HPLC analysis of polyphenolic compounds. Sustainability 14, 12799 (2022).
Sohrabi, F., Shishehbor, P., Saber, M. & Mosaddegh, M. S. Lethal and sublethal effects of buprofezin and imidacloprid on Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Prot. 30, 1190–1195 (2011).
Ullah, F. et al. Impact of low lethal concentrations of buprofezin on biological traits and expression profile of chitin synthase 1 gene (CHS1) in melon aphid, Aphis gossypii. Sci. Rep. 9, 12291 (2019).
Dugan, F. M. & Dugan, F. M. The identification of fungi: an illustrated introduction with keys, glossary, and guide to literature. (2006).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guid. Methods Appl. 18, 315–322 (1990).
Sobhy, S. et al. Phytochemical characterization and antifungal efficacy of camphor (Cinnamomum camphora L.) extract against phytopathogenic fungi. Separations 10, 189 (2023).
Abdelkhalek, A., Al-Askar, A. A., Alsubaie, M. M. & Behiry, S. I. First report of protective activity of Paronychia argentea extract against tobacco mosaic virus infection. Plants 10, 2435 (2021).
Al-Askar, A. A. et al. Antimicrobial efficacy and HPLC analysis of polyphenolic compounds in a whole-plant extract of Eryngium campestre. Separations 10, 362 (2023).
Kumar, A., Shukla, R., Singh, P., Prasad, C. S. & Dubey, N. K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innov. Food Sci. Emerg. Technol. 9, 575–580 (2008).
Dissanayake, M. Inhibitory effect of selected medicinal plant extracts on phytopathogenic fungus Fusarium oxysporum (Nectriaceae) Schlecht. Emend. Snyder and Hansen. Annu. Res. Rev. Biol. 4, 133–142 (2014).
Gaimari, S. D. & Turner, W. J. Methods for rearing aphidophagous Leucopis spp. (Diptera: Chamaemyiidae). J. Kansas Entomol. Soc. 363–369 (1996).
Khamis, W. M. et al. Swietenia mahagoni leaves extract: Antifungal, insecticidal, and phytochemical analysis. Separations 10, 301 (2023).
Stribley, M. F., Moores, G. D., Devonshire, A. L. & Sawicki, R. M. Application of the FAO-recommended method for detecting insecticide resistance in Aphis jabae Scopoli, Sitobion avenae (F.), Metopolophium dirhodum (Walker) and Rhopalosiphum padi (L.) (Hemiptera: Aphididae). Bull. Entomol. Res. 73, 107–115 (1983).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 18, 265–267 (1925).
Finney, D. J. Probit Analysis (Cambridge University Press, 1971).
Committee, I. R. A. IRAC susceptibility test methods series method No: 026. (2020).
Michelotto, M. D. & Busoli, A. C. Aspectos biológicos de Aphis gossypii Glover, 1877 (Hemiptera: Aphididae) em três cultivares de algodoeiro e em três espécies de plantas daninhas. Ciência Rural 33, 999–1004 (2003).
Jaba, J., Haseena, B., Tripathy, S., Hosamani, A. C. & Amaresh, Y. S. Olfactory response of cowpea aphid, Aphis craccivora Koch, to host odours and population of conspecifics. J. Biopestic. 3, 405 (2010).
Weeks, E. N. I. et al. A bioassay for studying behavioural responses of the common bed bug, Cimex lectularius (Hemiptera: Cimicidae) to bed bug-derived volatiles. Bull. Entomol. Res. 101, 1–8 (2011).
Abdelkhalek, A. et al. Defense responses and metabolic changes involving phenylpropanoid pathway and PR genes in squash (Cucurbita pepo L.) following Cucumber mosaic virus infection. Plants 11, 1908 (2022).
INC, S. A. S. I. (SAS). PC-SAS user guide, version 8. (2002).
Syame, S. M., Mohamed, S. M., Elgabry, E. A., Darwish, Y. A. A. & Mansour, A. S. Chemical characterization, antimicrobial, antioxidant, and cytotoxic potentials of Swietenia mahagoni. AMB Express 12, 77 (2022).
Youssef, N. H. et al. Antimycotoxigenic activity of beetroot extracts against altenaria alternata mycotoxins on potato crop. Appl. Sci. 11, 4239 (2021).
Chen, J.-J. et al. A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 120, 379–384 (2010).
Behiry, S. I. et al. Urtica dioica and Dodonaea viscosa leaf extracts as eco-friendly bioagents against Alternaria alternata isolate TAA-05 from tomato plant. Sci. Rep. 12, 16468 (2022).
Adamczak, A., Ożarowski, M. & Karpiński, T. M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 9, 109 (2019).
Heflish, A. A. et al. Rhaphiolepis indica fruit extracts for control Fusarium solani and Rhizoctonia solani, the causal agents of bean root rot. Separations 10, 369 (2023).
Ross, A. F. Systemic acquired resistance induced by localized virus infections in plants. Virology 14, 340–358 (1961).
Okuno, T., Nakayama, M., Okajima, N. & Furusawa, I. Systemic resistance to downy mildew and appearance of acid soluble proteins in cucumber leaves treated with biotic and abiotic inducers. Jpn. J. Phytopathol. 57, 203–211 (1991).
Conti, G. G., Pianezzola, A., Violini, G., Maffi, D. & Arnoldi, A. Possible involvement of salicylic acid in systemic acquired resistance of Cucumis sativus against Sphaerotheca fuliginea. Eur. J. Plant Pathol. 102, 537–544 (1996).
Georgiou, C. D., Tairis, N. & Sotiropoulou, A. Hydroxyl radical scavengers inhibit lateral-type sclerotial differentiation and growth in phytopathogenic fungi. Mycologia 92, 825–834 (2000).
Huang, G.-Y. et al. Synthesis and characteristics of (Hydrogenated) ferulic acid derivatives as potential antiviral agents with insecticidal activity. Chem. Cent. J. 7, 1–12 (2013).
El-Sherbeni, A.E.-H.E.-D., Khaleid, M. S., AbdAllah, S. A. E. A. & Ali, O. S. M. Effect of some insecticides alone and in combination with salicylic acid against aphid, Aphis gossypii, and whitefly Bemisia tabaci on the cotton field. Bull. Natl. Res. Cent. 43, 1–7 (2019).
Punia, A., Chauhan, N. S., Kaur, S. & Sohal, S. K. Effect of ellagic acid on the larvae of Spodoptera litura (Lepidoptera: Noctuidae) and its parasitoid Bracon hebetor (Hymenoptera: Braconidae). J. Asia. Pac. Entomol. 23, 660–665 (2020).
Dolma, S. K., Singh, P. P. & Reddy, S. G. E. Insecticidal and enzyme inhibition activities of leaf/bark extracts, fractions, seed oil and isolated compounds from Triadica sebifera (L.) Small against Aphis craccivora Koch. Molecules 27, 1967 (2022).
Samuel, R. et al. Biocontrol efficacy of apigenin isolated from Anisomeles indica (L.) Kuntze against immature stages of Culex quinquefasciatus (Say, 1823) and its in silico studies. Biocatal. Agric. Biotechnol. 48, 102637 (2023).
Moustafa, H. Z., Yousef, H. & El-Lakwah, S. F. Toxicological and biochemical activities of fatty acids against earias insulana (boisd.)(lepidoptera: Noctuidae). Egypt. J. Agric. Res. 96, 503–515 (2018).
Abdullah, R. R. Insecticidal activity of secondary metabolites of locally isolated fungal strains against some cotton insect pests. J. Plant Prot. Pathol. 10, 647–653 (2019).
Farag, S. M., Essa, E. E., Alharbi, S. A., Alfarraj, S. & El-Hassan, G. M. M. A. Agro-waste derived compounds (flax and black seed peels): Toxicological effect against the West Nile virus vector, Culex pipiens L. with special reference to GC–MS analysis. Saudi J. Biol. Sci. 28, 5261–5267 (2021).
Khamis, M. W., El-Desouky, E. S. & Gad, A. A. Toxicity and antifeedant effects of apricot kernel extract and its main components against cotton leaf worm, Spodoptera littoralis (Lepidoptera: Noctuidae) larvae with reference to some physiological effects. Alex. Sci. Exch. J. 37, 637–646 (2016).
Eldesouky, S. E., Khamis, W. M. & Hassan, S. M. Joint action of certain fatty acids with selected insecticides against cotton leafworm, Spodoptera littoralis and their effects on biological aspects. J. Basic Environ. Sci 6, 23–32 (2019).
Maia, M. R. G. et al. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 10, 1–10 (2010).
Silva, T. R. F. B. et al. Effect of the flavonoid rutin on the biology of Spodoptera frugiperda (Lepidoptera: Noctuidae). Acta Sci. Agron. 38, 165–170 (2016).
Puri, S., Singh, S. & Sohal, S. K. Inhibitory effect of chrysin on growth, development and oviposition behaviour of melon fruit fly, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Phytoparasitica 50, 151–162 (2022).
Madanat, H. M., Al Antary, T. M. & Zarga, M. H. A. Identification and isolation of the insecticidal compounds from Robinia pseudoacacia L. (Fabaceae). Fresen Environ. Bull 27, 1838–1849 (2018).
Adfa, M. et al. Insecticidal activity of Toona sinensis against Coptotermes curvignathus Holmgren. Rasayan J. Chem. 10, 153–159 (2017).
Zhang, X. et al. Exogenous application of gallic acid induces the direct defense of tea plant against Ectropis obliqua Caterpillars. Front. Plant Sci. 13, 833489 (2022).
Acknowledgements
The authors express their sincere thanks to the City of Scientific Research and Technological Applications (SRTA-City) and the Faculty of Agriculture (Saba Basha), Alexandria University, Egypt, for providing the necessary research facilities. The authors would like to extend their appreciation to the Researcher Supporting Project number (RSPD2023R752), King Saud University, Riyadh, Saudi Arabia.
Funding
This research was financially supported by the Researcher Supporting Project number (RSPD2023R752), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Methodology and writing the manuscript, W.M.K., S.I.B., A.A.H., G.A., A.A.; Investigation and validation, W.M.K., S.I.B., A.A.H., G.A., A.A., S.A.M.,Y.S. and M.K.G.; Funding, S.A.M. and A.A.Al. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khamis, W.M., Behiry, S.I., Marey, S.A. et al. Phytochemical analysis and insight into insecticidal and antifungal activities of Indian hawthorn leaf extract. Sci Rep 13, 17194 (2023). https://doi.org/10.1038/s41598-023-43749-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43749-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.