Abstract
Limited information is available regarding the association between preoperative lung function and postoperative pulmonary complications (PPCs) in patients with esophageal cancer who undergo esophagectomy. This is a retrospective cohort study. Patients were classified into low and high lung function groups by the cutoff of the lowest fifth quintile of forced expiratory volume in 1 s (FEV1) %predicted (%pred) and diffusing capacity of the carbon monoxide (DLco) %pred. The PPCs compromised of atelectasis requiring bronchoscopic intervention, pneumonia, and acute lung injury/acute respiratory distress syndrome. Modified multivariable-adjusted Poisson regression model using robust error variances and inverse probability treatment weighting (IPTW) were used to assess the relative risk (RR) for the PPCs. A joint effect model considered FEV1%pred and DLco %pred together for the estimation of RR for the PPCs. Of 810 patients with esophageal cancer who underwent esophagectomy, 159 (19.6%) developed PPCs. The adjusted RR for PPCs in the low FEV1 group relative to high FEV1 group was 1.48 (95% confidence interval [CI] = 1.09–2.00) and 1.98 (95% CI = 1.46–2.68) in the low DLco group relative to the high DLco group. A joint effect model showed adjusted RR of PPCs was highest in patients with low DLco and low FEV1 followed by low DLco and high FEV1, high DLco and low FEV1, and high DLco and high FEV1 (Reference). Results were consistent with the IPTW. Reduced preoperative lung function (FEV1 and DLco) is associated with post-esophagectomy PPCs. The risk was further strengthened when both values decreased together.
Similar content being viewed by others
Introduction
Postoperative pulmonary complications (PPCs) occur in 16–67% of patients after esophagectomy, which accounts for two-thirds of the deaths associated with esophagectomy and affects the long-term survival rate in patients with esophageal cancer1,2,3,4,5,6,7. Therefore, to improve surgical treatment outcomes as well as the long-term survival rate, it is important to identify risk factors of PPCs in patients with esophageal cancer who are expected to undergo esophagectomy.
Lung function measurement is one of the important determinants for the risk stratification of patients who undergo thoracic surgery. Previous studies showed that forced expiratory volume in 1 s (FEV1) or diffusing capacity of carbon monoxide (DLco) could predict PPCs in lung cancer patients following lung resection surgery8,9,10. In addition, these measurements were found to be useful to identify a high-risk group in patients who undergo extra-pulmonary surgery11,12. However, in terms of PPCs after esophagectomy, studies mainly have focused on the types of surgery for predicting the occurrence of PPCs5,13,14,15. Although some studies have examined the relationship between lung function and post-esophagectomy PPCs5,16,17,18, these are limited by their study design (single center with a single surgeon), patient enrollments in the past, small numbers of participants, reliance on multiple imputation due to missing data, lack of consideration for individual components of PPCs. Moreover, it would be of value to consider lung function parameters together for the estimation of PPCs.
In this regard, this study aimed to evaluate the association between several preoperative lung function and the occurrence of PPCs and its components (atelectasis requiring bronchoscopic intervention, pneumonia, and acute lung injury [ALI]/acute respiratory distress syndrome [ARDS]) in patients with esophageal cancer who underwent esophagectomy.
Results
Patients’ characteristics
The baseline characteristics of 810 patients who underwent esophagectomy for esophageal cancer were summarized in Table 1. PPCs occurred in 19.6% (n = 159) of patients with esophageal cancer. Compared who did not develop PPCs, those who developed PPCs were more likely to be older, had more cardiovascular diseases and lower albumin, and underwent more thoracotomy. Preoperative lung function measurements, including FVC %pred, FEV1%pred, and DLco %pred were lower in patients who developed PPCs than those who did not develop PPCs.
The incidence of PPCs by FEV1%pred
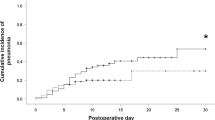
As shown in Fig. 1a and Table 2, the rate of overall PPCs tended to increase as FEV1%pred decreased (Q1 group, 29.7%; Q2 group, 23.0%; Q3 group, 18.1%; Q4 group, 14.2%; and Q5 group, 12.3%; P for trend < 0.01). The increasing trend in the incidence of pneumonia and ALI/ARDS was significant according to FEV1%pred.
Post-esophagectomy pulmonary complications. (a) PPC by the quintiles of FEV1%pred, (b) PPC by the quintiles of DLco %pred. PPCs, postoperative pulmonary complications; FEV1, forced expiratory volume in 1 s; %pred, %predicted; DLco, diffusing capacity of the lung for carbon monoxide, Q1, the lowest quintile; Q5, the top quintile.
The relative risk (RR) of overall PPCs was highest in Q1 group of FEV1%pred followed by Q2, Q3, Q4, and Q5 (Table 3). This significance of trend remained after adjustment for covariables. Table 4 shows the RR of overall and individual PPCs in the low FEV1 group versus high FEV1 group. The RR of overall PPCs in the low FEV1 group was significantly higher than that of the high FEV1 group. For individual PPCs, the low FEV1 group had a significantly higher risk of pneumonia compared to the high FEV1 group. The results using the inverse probability treatment weighting (IPTW) were similar to the multivariable-adjusted model.
The incidence of PPCs by DLco %pred
A trend of gradual increase in PPCs was observed as DLco %pred is decreased from Q5 to Q1 (P for trend < 0.01, Fig. 1b and Table 2). The increasing trend in the incidence of atelectasis requiring bronchoscopic toileting, pneumonia, and ALI/ARDS was observed according to FEV1%pred.
The RR of overall PPCs in Q2, Q3, Q4, and Q5 groups versus Q1 group of DLco %pred is summarized in Table 3. The RR of overall PPCs was highest in Q1 group of FEV1%pred followed by Q2, Q3, Q4, and Q5, and this trend remained significant after adjustment for covariables. Compared to high DLco group, the RR of overall PPCs and individual components of PPCs (atelectasis requiring bronchoscopic toileting, pneumonia, and ALI/ARDS) significantly higher in low DLco group. The results using the IPTW were similar to the multivariable-adjusted model.
Joint effect of FEV1%pred DLco %pred for the occurrence of overall PPCs
The adjusted RR of overall PPCs was highest in patients with low DLco %pred and low FEV1%pred followed by low DLco %pred and high FEV1%pred, high DLco %pred and low FEV1%pred, and high DLco %pred and high FEV1%pred (Reference, Table 5). The results using the IPTW were similar to the multivariable-adjusted model.
Discussion
In this retrospective cohort study in patients with esophageal cancer who underwent esophageal resection, we observed significant association between low levels of preoperative lung functions (DLco and FEV1) and the occurrence of PPCs: low FEV1 group had an approximately 1.5-fold increased risk of PPCs than the high FEV1 group and the risk of PPCs was approximately 2.0-fold higher in the low DLco group compared to the high DLco group. Importantly, when both lung function parameters were considered together, patients with both low DLco and low FEV1 showed 2.3-fold increased risk of developing PPCs compared to patients with both high DLco and high FEV1.
Our study expanded previous findings examining the predictive ability of preoperative lung function testing for PPCs in patients with esophageal cancer. Pulmonary function testing is commonly performed before not only for lung resection surgeries but also for extra-pulmonary surgeries to assess the risk of morbidity and mortality related to the surgery. Previous studies have shown that reduced lung function is an important contributor in predicting the occurrence of PPCs8,9,10,11,12. However, in the case of esophageal cancer, despite the higher risk of PPCs occurrence than in other surgeries1,2,3,4,5,6,7, only few studies have examined the association between preoperative lung function and PPCs after esophagectomy. For example, one previous study revealed that low FEV1 was associated with delayed weaning of mechanical ventilation: but this study was limited by an analysis of a small number of patients (n = 60) performed by a single surgeon, and this study did not evaluate PPCs other than delayed weaning of mechanical ventilation5. In 2018, Dutch group reported low DLco as an independent predictor of the major PPCs (Clavien-Dindo classification IIIb or higher: intervention requiring general anesthesia, life-threatening complications requiring intensive care, organ dysfunction, or death) after esophagectomy for esophageal cancer17. They suggested 85% as an ideal cutoff for DLco %pred. They also found preoperative FEV1%pred was significantly lower in patients presenting major PPCs (P = 0.011), but the significance did not remain in multivariable-adjusted model. Another study from the United States in patients with esophageal cancer treated with surgical resection after chemoradiation similarly showed a close relationship between PPCs and pre-treatment DLco, while pre-treatment FEV1 was related to the development of gastrointestinal complications18. Therefore, in agreement with and expanding upon previous findings, our results concerning the potential role of the preoperative values of DLco and FEV1 in the development of PPCs warrant further studies on constructing a predictive model for preventing PPCs.
One of notable approach in our study might be a joint effect analysis for PPCs. This approach incorporated a previous study that showed FEV1 and DLco were independently associated with PPCs after esophagectomy16. Our study has much larger numbers of study participants (n = 810 versus n = 516), and that study multiply imputed data because of large volume of missing data, which is not recommended method for handling missing values currently19. In addition, our study found that DLco plays a slightly more significant role than FEV1 in predicting PPCs after esophagectomy. While both FEV1 and DLco exhibited associations with post-esophagectomy PPCs in patients with esophageal cancer during individual analyses, statistical significance was achieved solely for DLco across all components of PPCs (atelectasis requiring bronchoscopic toileting, pneumonia, and ALI/ARDS). Moreover, in a fully adjusted model, DLco showed a larger effect size compared to FEV1. Several explanations could exist. First, DLco may show better performance over FEV1 by its ability to reflect general conditions of body as well as lung function itself. DLco can be influenced by body mass index, anemia, and nutritional conditions, whereas FEV1 is mainly influenced by the mechanics of the chest system20,21,22. Indeed, preoperative nutrition status, albumin level, as well as hand grip strength were closely associated with the development of post-esophagectomy PPCs23,24,25. Second, in terms of lung physiology, DLco could assess physiologic function of the lung more comprehensively than testing airflow (FEV1). For example, a reduced DLco could be related to obstructive lung diseases, as restrictive lung diseases, pulmonary vascular disorders, and other systemic diseases26. However, regarding this, since not much has been revealed, further studies are necessary. Our study findings may indicate that the strategies to prevent PPCs should consider preoperative measurement of DLco in patients with esophageal cancer who are planned to undergo esophagectomy.
Our study has several limitations. First, this study was performed in a single study with a retrospective design. Temporal causality might not be guaranteed. Second, we used the lowest quintile as a cutoff of FEV1 and DLco, and the cutoff values of FEV1%pred and DLco %pred were different. However, it was found that a sensitivity analysis using 80%pred as a cutoff of FEV1 and DLco showed similar results (Supplementary Table 1). Third, the patients in our study had relatively persevered pulmonary function. The mean values of FVC %pred, FEV1%pred, and DLco %pred were all > 80. Thus, our results might not be generalizable across all patients with esophageal cancer who underwent esophagectomy, and this warrants further study especially in patients with low lung function. Finally, we used smoking status as a binary variable. However, it should be noted that other confounders, such as pack-years, could affect the observed findings, which were not collected in our study.
In conclusion, reduced preoperative lung function, FEV1 and DLco, was significantly associated with an increased probability of PPCs after esophagectomy in patients with esophageal cancer. Decreased value of preoperative DLco seems to play a slightly more negative role for the development of PPCs than FEV1. In addition, there was more intensified association with PPCs when FEV1 and DLco were decreased together. Our study suggests that preoperative lung function could be useful for the stratification of patients at risk for PPCs who underwent esophagectomy for esophageal cancer.
Methods
Patients
This study enrolled 848 patients with clinical stage I–III esophageal cancer who underwent curative R0 esophagectomy at Samsung Medical Center between January 2013 and December 2017. Patients who received neoadjuvant treatment were not included in this study. After excluding 25 patients who did not have preoperative lung function measurements, 10 patients with pathologic types other than squamous cell carcinoma ad adenocarcinoma, and 3 patients who were diagnosed with pathologically stage IV after esophagectomy, a total of 810 patients were analyzed (Fig. 2).
Institutional Review Board of Samsung Medical Center (IRB no. 2020-06-056) approved the study protocol and waived the informed consent from the participants since the nature of this study was retrospective and patient data were anonymized. This study was conducted in accordance with the Declaration of Helsinki. All procedures were performed in accordance with the relevant guidelines and regulations.
Lung function measurements
Spirometry and DLco measurements were performed by using Vmax 22 (SensorMedics, CA, USA) according to the American Thoracic Society/European Respiratory Society criteria27,28. Absolute values of FEV1, forced vital capacity (FVC), and DLco were obtained, and the percentage of predicted values (% pred) for FEV1, FVC, and DLco was calculated by using a reference equation obtained on analysis of a representative South Korean sample29,30.
Since the optimal cutoff values of FEV1%pred and DLco %pred for PPCs after esophagectomy are not established, patients were classified into high and low pulmonary function groups based on the quintiles of pulmonary measurements. The high FEV1 group was defined as those with quintiles 2–5 (Q2–5) of FEV1%pred and the low FEV1 group as those with quintile 1 (Q1) of FEV1%pred. Similarly, high DLco group was defined as those with Q2–5 of DLco %pred and low DLco group as those with Q1 of DLco %pred.
Other variables
Baseline demographics and behavioral information, including patient age, sex, body mass index, smoking status, comorbidities, and laboratory findings were collected through retrospective review. Information, including postoperative pathological stage, histological types, and surgical methods were also collected.
PPCs
PPCs were defined as the occurrence of one or more of the followings after esophagectomy: (1) atelectasis requiring bronchoscopic toileting; (2) pneumonia (at least three among leukocytosis, pulmonary infiltrate or consolidation, fever [> 38 °C], culture-positive, or use of antibiotics); or (3) ALI/ARDS (PaO2/FiO2 < 300 and bilateral infiltrate seen on chest radiograph with no evidence of congestive heart failure or volume overload). All PPCs in this study were assessed by using the Clavien-Dindo classification31.
Statistical analyses
Categorical variables were described as frequency and percentage, and continuous variables were described as median and interquartile range or mean and standard deviation. Categorical variables were compared using Pearson’s chi-squared test or Fisher’s test, as appropriate. Continuous variables were compared with the t-test or Mann–Whitney U test depending on the normality of the data.
We used a modified multivariable-adjusted Poisson regressive model to estimate the RR and confidence interval by using the robust error variances32. We adjusted for age, sex, smoking history (never and ever smoker), body mass index (kg/m2), the presence of pulmonary comorbidities (yes and no), the presence of cardiovascular comorbidities (yes and no), albumin (g/dL), pathologic stage (I, II, and III), tumor location (cervical/upper esophagus, mid esophagus, and low esophagus/esophagogastric junction), type of surgery (open thoracoscopic surgery, video-assisted thoracoscopic surgery, and robotic surgery), and extent of lymph node dissection (two or fewer locations and three locations), and surgical time (hours).
Subgroup analyses were performed to identify the association between preoperative lung function and specific types of PPCs; atelectasis requiring bronchoscopic toileting, pneumonia, and ALI/ARDS. Sensitivity analyses were conducted by 80%pred, a well-known practical cutoff value of FEV1 and DLco.
In addition, to investigate whether there is a joint effect with FEV1 and DLco on the relationship with PPCs, we further classified patients into four groups as follows: high FEV1/high DLco group, low FEV1/high DLco group, high FEV1/low DLco, and low FEV1/low DLco.
Besides of multivariable-adjusted Poisson model, an additional IPTW model was used to adjust for any potential group imbalances. To compute IPTW for multiple groups, a multinomial logit model was used to generate propensity score, and weights were assigned as the inverse of the probability of the groups (1/probability [treatment 0], 1/probability [treatment 1], 1/probability [treatment 2], etc.).
All tests were two-sided and a P < 0.05 was considered to be statistically significant. All analyses were performed using STATA version 15 (StataCorp, LP, USA).
Ethical approval
Institutional Review Board of Samsung Medical Center (IRB no. 2020-06-056) approved the study protocol and waived the informed consent from the participants since the nature of this study was retrospective and patient data were anonymized. This study was conducted in accordance with the Declaration of Helsinki. All procedures were performed in accordance with the relevant guidelines and regulations.
Data availability
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Change history
02 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-58398-9
References
Bailey, S. H. et al. Outcomes after esophagectomy: A ten-year prospective cohort. Ann. Thorac. Surg. 75, 217–222. https://doi.org/10.1016/s0003-4975(02)04368-0 (2003).
Biere, S. S., Maas, K. W., Cuesta, M. A. & van der Peet, D. L. Cervical or thoracic anastomosis after esophagectomy for cancer: A systematic review and meta-analysis. Dig. Surg. 28, 29–35. https://doi.org/10.1159/000322014 (2011).
Inoue, J. et al. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dis. Esophagus 26, 68–74. https://doi.org/10.1111/j.1442-2050.2012.01336.x (2013).
Schieman, C. et al. Patterns of operative mortality following esophagectomy. Dis. Esophagus 25, 645–651. https://doi.org/10.1111/j.1442-2050.2011.01304.x (2012).
Avendano, C. E., Flume, P. A., Silvestri, G. A., King, L. B. & Reed, C. E. Pulmonary complications after esophagectomy. Ann. Thorac. Surg. 73, 922–926. https://doi.org/10.1016/s0003-4975(01)03584-6 (2002).
Baba, Y. et al. Prognostic impact of postoperative complications in 502 patients with surgically resected esophageal squamous cell carcinoma: A retrospective single-institution study. Ann. Surg. 264, 305–311. https://doi.org/10.1097/sla.0000000000001510 (2016).
Rutegård, M., Lagergren, P., Rouvelas, I., Mason, R. & Lagergren, J. Surgical complications and long-term survival after esophagectomy for cancer in a nationwide Swedish cohort study. Eur. J. Surg. Oncol. 38, 555–561. https://doi.org/10.1016/j.ejso.2012.02.177 (2012).
Ha, D., Mazzone, P. J., Ries, A. L., Malhotra, A. & Fuster, M. The utility of exercise testing in patients with lung cancer. J. Thorac. Oncol. 11, 1397–1410. https://doi.org/10.1016/j.jtho.2016.04.021 (2016).
Brunelli, A., Kim, A. W., Berger, K. I. & Addrizzo-Harris, D. J. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of chest physicians evidence-based clinical practice guidelines. CHEST 143, e166S-e190S. https://doi.org/10.1378/chest.12-2395 (2013).
Lee, H. et al. Prognostic value of 6-min walk test to predict postoperative cardiopulmonary complications in patients with non-small cell lung cancer. CHEST 157, 1665–1673. https://doi.org/10.1016/j.chest.2019.12.039 (2020).
Jeong, B.-H. et al. Development of a prediction rule for estimating postoperative pulmonary complications. PLOS ONE 9, e113656. https://doi.org/10.1371/journal.pone.0113656 (2014).
Shin, B. et al. Airflow limitation severity and post-operative pulmonary complications following extra-pulmonary surgery in COPD patients. Respirology 22, 935–941. https://doi.org/10.1111/resp.12988 (2017).
Hulscher, J. B. et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N. Engl. J. Med. 347, 1662–1669. https://doi.org/10.1056/NEJMoa022343 (2002).
Biere, S. S. et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 379, 1887–1892. https://doi.org/10.1016/s0140-6736(12)60516-9 (2012).
Briez, N. et al. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br. J. Surg. 99, 1547–1553. https://doi.org/10.1002/bjs.8931 (2012).
Ferguson, M. K., Celauro, A. D. & Prachand, V. Prediction of major pulmonary complications after esophagectomy. Ann. Thorac. Surg. 91, 1494–1500. https://doi.org/10.1016/j.athoracsur.2010.12.036 (2011).
Goense, L. et al. Pulmonary diffusion capacity predicts major complications after esophagectomy for patients with esophageal cancer. Dis. Esophagus 32, 1456. https://doi.org/10.1093/dote/doy082 (2019).
Wang, J. et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 86, 885–891. https://doi.org/10.1016/j.ijrobp.2013.04.006 (2013).
Jakobsen, J. C., Gluud, C., Wetterslev, J. & Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med. Res. Methodol. 17, 162. https://doi.org/10.1186/s12874-017-0442-1 (2017).
Sahebjami, H., Doers, J. T., Render, M. L. & Bond, T. L. Anthropometric and pulmonary function test profiles of outpatients with stable chronic obstructive pulmonary disease. Am. J. Med. 94, 469–474. https://doi.org/10.1016/0002-9343(93)90080-9 (1993).
Dinakara, P., Blumenthal, W. S., Johnston, R. F., Kauffman, L. A. & Solnick, P. B. The effect of anemia on pulmonary diffusing capacity with derivation of a correction equation. Am. Rev. Respir. Dis. 102, 965–969. https://doi.org/10.1164/arrd.1970.102.6.965 (1970).
Magnussen, H. et al. What can we learn from pulmonary function testing in heart failure?. Eur. J. Heart Fail. 19, 1222–1229. https://doi.org/10.1002/ejhf.946 (2017).
Sato, S. et al. Hand grip strength as a predictor of postoperative complications in esophageal cancer patients undergoing esophagectomy. Esophagus 15, 10–18. https://doi.org/10.1007/s10388-017-0587-3 (2018).
Qi, Q., Song, Q., Cheng, Y. & Wang, N. Prognostic significance of preoperative prognostic nutritional index for overall survival and postoperative complications in esophageal cancer patients. Cancer Manag. Res. 13, 8585–8597. https://doi.org/10.2147/cmar.S333190 (2021).
Saito, T. et al. Novel prognostic score of postoperative complications after transthoracic minimally invasive esophagectomy for esophageal cancer: A retrospective cohort study of 90 consecutive patients. Esophagus 16, 155–161. https://doi.org/10.1007/s10388-018-0645-5 (2019).
Hughes, J. M. & Pride, N. B. Examination of the carbon monoxide diffusing capacity (DL(CO)) in relation to its KCO and VA components. Am. J. Respir. Crit. Care Med. 186, 132–139. https://doi.org/10.1164/rccm.201112-2160CI (2012).
Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J. 26, 319. https://doi.org/10.1183/09031936.05.00034805 (2005).
American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique—1995 update. Am. J. Respir. Crit. Care Med. 152, 2185–2198. https://doi.org/10.1164/ajrccm.152.6.8520796 (1995).
Choi, J. K., Paek, D. & Lee, J. O. Normal predictive values of spirometry in Korean population. Tuberc. Respir. Dis. 58, 230–242 (2005).
Park, J. O., Choi, I. S. & Park, K. O. Normal predicted values of single-breath diffusing capacity of the lung in healthy nonsmoking adults. Korean J. Intern. Med. 1, 178–185. https://doi.org/10.3904/kjim.1986.1.2.178 (1986).
Dindo, D., Demartines, N. & Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae (2004).
Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706. https://doi.org/10.1093/aje/kwh090 (2004).
Funding
This research was supported by a grant of the Korean Cancer Survivors Healthcare R&D Project through the National Cancer Center, funded by the Ministry of Health & Welfare, Republic of Korea (HA23C0515).
Author information
Authors and Affiliations
Contributions
T Kim and YJ Jeon: Writing - original draft; H Lee: investigation; D Kang: methodology and formal analysis; HK Kim and HY Park: Writing - review and editing, supervision, and project administration; TH Kim, SY Park, YS Hong, G Lee, J Lee, S Shin, JH Cho, YS Choi, J Kim, J Cho, JI Zo, and YM Shim: Validation. All the authors discussed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Funding section in the original version of this Article was omitted. Full information regarding the correction made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, T., Jeon, Y.J., Lee, H. et al. Preoperative DLco and FEV1 are correlated with postoperative pulmonary complications in patients after esophagectomy. Sci Rep 14, 6117 (2024). https://doi.org/10.1038/s41598-024-56593-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56593-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.