Abstract
We investigated whether pulmonary function tests (PFTs) can predict pulmonary complications and if they are, to find new cutoff values in current open lung resection surgery. In this observational study, patients underwent open lung resection surgery at a tertiary hospital were analyzed (n = 1544). Various PFTs were tested by area under the receiver-operating characteristic curve (AUCROC) to predict pulmonary complications until 30 days postoperatively. In results, PFTs were generally not effective to predict pulmonary complications (AUCROC: 0.58–0.66). Therefore, we could not determine new cutoff values, and used previously reported cutoffs for post-hoc analysis [predicted postoperative forced expiratory volume in one second (ppoFEV1) < 40%, predicted postoperative diffusing capacity for carbon monoxide (ppoDLCO) < 40%]. In multivariable analysis, old age, male sex, current smoker, intraoperative transfusion and use of inotropes were independent risk factors for pulmonary complications (model 1: AUCROC 0.737). Addition of ppoFEV1 or ppoDLCO < 40% to model 1 did not significantly increase predictive capability (model 2: AUCROC 0.751, P = 0.065). In propensity score-matched subgroups, patients with ppoFEV1 or ppoDLCO < 40% showed higher rates of pulmonary complications [13% (21/160) vs. 24% (38/160), P = 0.014], but no difference in in-hospital mortality [3% (8/241) vs. 6% (14/241), P = 0.210] or mean survival duration [61 (95% CI 57–66) vs. 65 (95% CI 60–70) months, P = 0.830] compared to patients with both > 40%. In conclusion, PFTs themselves were not effective predictors of pulmonary complications. Decision to proceed with surgical resection of lung cancer should be made on an individual basis considering other risk factors and the patient's goals.
Similar content being viewed by others
Introduction
Accurate assessment of lung function has been regarded as vital for prevention of pulmonary complications after lung resection1. Predicted postoperative forced expiratory volume in 1 s (ppoFEV1) and predicted postoperative diffusing capacity for carbon monoxide (ppoDLCO) are commonly used to predict pulmonary complications in thoracic surgery1. Many previous studies from the 1980s through the 2000s observed that morbidity and mortality significantly increased in patients whose ppoFEV1 or ppoDLCO were < 40% of predicted normal values2,3,4,5, and patients with limited pulmonary function are often denied curative resection due to the possibility of postoperative respiratory failure.
However, the 40% cutoff points were not determined by objective analysis and were generally empirical and based on expert opinion2,3,4,5. Currently, many patients with ppoFEV1 or ppoDLCO < 40% undergo surgery with acceptable morbidity and mortality, especially when minimally invasive techniques are used6. Notably, one study suggested that FEV1 and DLCO are not associated with pulmonary complications in patients who had lobectomy using video-assisted thoracoscopic surgery (VATS)7. Accordingly, lower cutoff such as 30% ppoFEV1 and ppoDLCO is being increasingly used in many institutions8. However, the previous cutoff points for ppoFEV1 and ppoDLCO remain widely accepted for open lung resection surgery. With modern advances in anesthetic, surgical, and postoperative care, the predictive capability of pulmonary function tests (PFTs) needs to be re-evaluated for open lung resection surgery, especially when we consider that two-year survival was higher (62% vs. 18%) in patients who had surgery compared to those who were denied an operation in this high-risk group9.
Therefore, we performed an observational cohort study in patients who underwent open lung resection surgery for non-small cell lung cancer. Our primary objectives were to determine whether various PFTs can predict pulmonary complications within 30 postoperative days, and if they are, to find new cutoff values in open lung resection surgery. Whether PFTs are related to in-hospital mortality and long-term survival were also analyzed.
Methods
Study design
This was a single-center, observational cohort study to find the new cutoff values of PFTs which are related to pulmonary complications for open lung resection surgery. The Samsung Medical Center Institutional Review Board approved this study and waived the requirement for informed patient consent (Approval No. SMC 2018-12-056-001). The study proposal and statistical plan were registered at the Institutional Review Board before accessing patient data. The study was conducted in accordance to the original protocol. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology checklist for reporting observational studies. All methods were performed in accordance with the ethical principles of the 1964 Declaration of Helsinki and its later amendments.
Patient records
We collected study data from electronic medical records at our institution from patients who had an open lobectomy or more extensive open thoracotomy for non-small cell lung cancer between January 2009 and December 2013 (n = 1544). Patients were followed up for mortality until December 31, 2015. Metastasectomy cases and those featuring concomitant surgery with other departments were excluded. The primary objective was to identify pulmonary complications occurring until 30 days postoperatively.
The following information was collected from electronic medical records: preoperative data included PFTs, age, sex, body mass index (BMI), comorbidities, American Society of Anesthesiologists (ASA) physical status, smoking, alcohol consumption, previous lung diseases, pulmonary tuberculosis, neoadjuvant therapy, cancer cell types and clinical tumor node metastasis (TNM) stages, and plan for surgery and postoperative analgesia. Comorbidities included hypertension, diabetes mellitus, renal dysfunction (estimated glomerular filtration rate < 60 ml/min/1.73 m2), cerebrovascular disease, cardiac disease, and pulmonary disease. Cerebrovascular disease included cerebral infarction, cerebral hemorrhage, dementia, Parkinson’s disease, and Alzheimer’s disease. Cardiac disease included coronary artery disease and heart failure. Underlying pulmonary disease included chronic obstructive pulmonary disease, bronchiectasis, asthma, and interstitial lung disease. Current smokers were defined as patients who were presently smoking or had stopped smoking within 1 month prior to surgery. Heavy drinking was defined as the consumption of ≥ 15 drinks per week for men and ≥ 8 drinks per week for women, in accordance with Centers for Disease Control and Prevention guidelines (https://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm).
Intraoperative data collected included the duration of surgery, requirement for transfusion, use of hydroxyethyl starch, and use of continuous inotropes (dopamine, dobutamine, or epinephrine continuous infusion) or vasopressors (phenylephrine or norepinephrine continuous infusion) during operation.
Postoperative data were collected from the institutional thoracic surgery registry and included the incidence rates of pulmonary and other complications, duration of intensive care unit (ICU) and hospital stays, and occurrence of in-hospital mortality and long-term survival. Other complications within the first 30 days included new-onset arrhythmia, myocardial infarction, renal complication, cerebral infarction, seizure, pulmonary thromboembolism, and surgical complications. Renal complication was defined as an Acute Kidney Injury Network classification ≥ 2. Surgical complications included prolonged air leak (≥ 5 days), prolonged effusion (≥ 5 days), chylothorax, vocal cord palsy, empyema, wound infection, wound dehiscence, and bronchopleural fistula.
Pulmonary function tests
FEV1, DLCO, forced vital capacity (FVC), FEV1/FVC, and reduction in forced expiratory flow at 25–75% of the pulmonary volume (FEF25-75) were analyzed in this study. The values of FEV1 and DLCO were expressed as a percentage of the values predicted by age, sex, and height10. Calculations of ppoFEV1 and ppoDLCO were performed by multiplying FEV1 and DLCO values by the percentage of functional lung tissue remaining after resection11. ppoFEV1 and ppoDLCO were calculated based on anatomical resection. Blood gas analysis, six-minute walk tests, tests of expired gas analysis during exercise, and lung quantitative perfusion scans were not routinely performed; thus, these parameters were not analyzed in the current study.
Primary endpoint: pulmonary complications
Pulmonary complications included pneumonia, acute respiratory distress syndrome (ARDS), and atelectasis requiring bronchoscopy within 30 days of surgery. ARDS was defined in accordance with the 2012 Berlin criteria, as acute (within 1 week of a known clinical insult) hypoxemic respiratory failure [(ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (< 300 mm Hg)], with bilateral opacities on chest imaging not fully explained by cardiac failure or fluid overload12. Pneumonia was defined as meeting three of five characteristics: fever, leukocytosis, chest x-ray with infiltrate, positive culture from sputum, or treatment with antibiotics. Atelectasis was diagnosed only when bronchoscopy toileting was performed.
Institutional protocol for anesthesia, surgery, and postoperative management
Most patients received balanced anesthesia, which was a combination of volatile anesthetic agents, non-depolarizing neuromuscular blocking agents, and continuous intravenous infusion of remifentanil. Lactated Ringer’s solution was used as maintenance fluid and was infused at a rate of 3–5 ml/kg/h. If intraoperative bleeding occurred, 5% human albumin (Green Cross, Gyeonggi, Korea) or 6% hydroxyethyl starch (Fresenius Kabi, Bad Homburg, Germany) was administered. Transfusion was performed for resuscitation if the transfusion cutoff was reached (hemoglobin < 8 g/dl). A protective ventilation protocol was implemented for all patients. Pulmonary resection was performed using standard posterolateral thoracotomy with mediastinal lymph node dissection. All patients were routinely extubated at the end of surgery unless the attending anesthesiologists or surgeons decided not to.
Patients remained in the ICU for one day for postoperative management. Analgesic methods were determined in accordance with each surgeon’s preference, as well as the existence of contraindications for regional analgesia. Maintenance fluid was administered at a rate of 1–2 ml/kg/h in the ICU and ward. Patients were encouraged to ambulate beginning on postoperative day one and also participated in a daily physiotherapy program, which included deep-breathing exercises, incentive spirometry, and chest physiotherapy delivered by physiotherapists and attending nurses during the ICU and ward stays.
Statistical analysis
This observational cohort study did not conduct an a priori statistical power calculation and the sample size was based on the available data. Our primary objective was to determine whether various PFTs can predict pulmonary complications. We constructed receiver operating characteristic curves (ROC) using logistic regression model and assessed the predictive capacity of each PFT. Co-variates were not adjusted for primary ROC analysis. We defined tests showing an area under the ROC (AUCROC) > 0.7 as optimal13. Pairwise comparisons of ROC curves for each PFT were conducted by DeLong’s method (hypothesis: true difference in AUC is not equal to 0)14.
For post hoc test, multivariable logistic regression was used to assess the risk factors for pulmonary complications. All preoperative and intraoperative variables were entered into analysis. The multivariable analysis was based on a backward stepwise method, with P < 0.05 for the inclusion of variables and P > 0.10 for the removal of variables. The goodness of fit was examined by the Hosmer–Lemeshow test. AUCROC was compared between models with and without PFT data.
Propensity score matching was also performed as a post hoc study. Groups were divided into below (ppoFEV1 < 40% or ppoDLCO < 40%) or above (both ≥ 40%) according to the previous cutoff points for ppoFEV1 and ppoDLCO. Matched variables included age, sex, BMI, ASA physical status, current smoking, heavy drinking, underlying comorbidities, cell types, clinical TNM stage, neoadjuvant therapy, and plan for surgery and postoperative analgesia. One-to-one matching was performed using the nearest-neighbor method with a caliper width of 0.3 (pulmonary complications), 0.2 (in-hospital mortality), and 0.4 (long-term survival) in a pairwise manner. We analyzed pulmonary complications and in-hospital mortality between below and above groups using conditional logistic regression. Long-term survival between below and above groups was analyzed using the Kaplan–Meier method and stratified Cox regression. Stratum variable was a group (ppoFEV1 or ppoDLCO < 40% vs. both > 40%). The proportional hazards assumption was tested by Schoenfeld residual graph. Participants who were not available due to follow-up loss or non-occurrence of outcome event were right censored for long-term survival analysis. Analyses of clinical end points were reported using available data with no imputation for missing data given the low observed rate of missingness (< 5%). An independent t-test or the Wilcoxon rank-sum test were used to determine significant differences in continuous variables. The chi-squared test or Fisher’s exact test was used to compare categorical variables. Patient demographic and clinical data are summarized as frequency (percentage) for categorical variables and mean ± standard deviation or median (interquartile range) for continuous variables. The normality of the data distribution was evaluated with the Shapiro–Wilk test. All reported P values were two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were performed using MedCalc for Windows (version 7.3; MedCalc Software, Mariakerke, Belgium) or SAS (version 9.2; SAS Institute, Cary, NC, USA).
Results
Characteristics of patients, surgery, and complications
The analysis cohort comprised all 1544 patients who underwent an open lobectomy or more extensive open thoracotomy for lung cancer between January 2009 and December 2013 at a single institution. The surgeries included lobectomy (n = 1187), sleeve lobectomy (n = 180), and pneumonectomy (n = 177). Patients were followed for mortality until December 31, 2015. The median follow-up time was 40 months for long-term prognosis [range: 0 months (in-hospital mortality) to 83 months]. Baseline patient and operative characteristics compared between pulmonary complications (+) and (−) patients are shown in supplementary Table S1.
Outcome data including pulmonary and other complications are shown in supplementary Table S2. Pulmonary complications developed in 12% of patients (178/1544) [ARDS: 6% (96/1544), pneumonia: 6% (100/1544), and atelectasis requiring bronchoscopy: 2% (25/1544)]. The ICU and hospital stays were longer in pulmonary complications (+) patients than in (−) patients. In-hospital mortality rates were 0.7% (9/1366) and 17% (30/178) in pulmonary complications (−) and (+) patients, respectively.
PFTs and pulmonary complications
Figure 1 shows scatter plots of ppoFEV1 and ppoDLCO between pulmonary complications (+) and (−) patients; the distribution pattern of ppoFEV1 and ppoDLCO did not differ between pulmonary complications (+) and (−) patients (P = 0.605). Patients with pulmonary complications were scattered widely rather than being concentrated in the left lower corner of the scatter plots (i.e., ppoFEV1 and ppoDLCO < 40%).
Scatter diagrams of ppoFEV1 and ppoDLCO in the PPC (+) and (−) patients. Each dot indicates the ppoFEV1 and ppoDLCO of one patient. Open circles indicate a PPC (−) patient. Filled stars indicate a PPC (+) patient. There is no difference between the scatter plots of PPC (+) and PPC (−) patients (P = 0.605). The diagonal line and accompanying dotted lines represent the regression line with 95 percent confidence limits, respectively. ppoFEV1 predicted postoperative expiratory volume in 1 s, ppoDLCO predicted diffusing capacity for carbon monoxide, PPCs postoperative pulmonary complications.
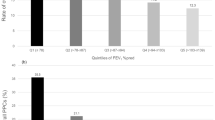
The ROC curves of each PFT are shown in Fig. 2. PFTs performed poorly when predicting pulmonary complications (AUCROC: 0.58–0.66). ppoDLCO showed the highest AUCROC [0.66; 95% confidence interval (CI) 0.64, 0.69] among PFTs. The AUCROC of ppoFEV1 was 0.64 (95% CI 0.62, 0.66). For FVC, FEV1/FVC, and FEF25–75, the AUCROC was 0.58 (95% CI 0.55, 0.60), 0.62 (95% CI 0.60, 0.64), and 0.62 (95% CI 0.59, 0.64), respectively. AUCROC values did not statistically differ from each other, except between ppoDLCO and FVC (P = 0.010), and between ppoFEV1 and FVC (P = 0.005). Combining ppoFEV1 and ppoDLCO (AUCROC 0.67; 95% CI 0.63, 0.71) did not improve discrimination of pulmonary complications compared with ppoDLCO alone (AUCROC 0.66; P = 0.297).
AUCROC of various pulmonary function tests. Pulmonary function tests performed poorly in predicting postoperative pulmonary complications (AUCROC: 0.58–0.66). AUCROC values did not statistically differ from each other, except between ppoDLCO and FVC (P = 0.010), and between ppoFEV1 and FVC (P = 0.005). AUCROC area under the receiver operating characteristic curve, CI confidence interval, ppoFEV1 postoperative expiratory volume in 1 s, ppoDLCO diffusing capacity for carbon monoxide, FEF25–75 a reduction in forced expiratory flow at 25–75% of the pulmonary volume, FEV1/FVC forced expiratory volume in 1 s/forced vital capacity.
Because PFTs were not effective predictors of pulmonary complications, we could not present new cutoff points for preoperative PFTs. We used previous cutoffs for post-hoc analysis.
Post-Hoc analysis: multivariable analysis for pulmonary complications
Multivariable logistic regression was performed to identify the risk factors for pulmonary complications using the previous cutoffs (dependent variable: presence of pulmonary complications, supplementary Table S3). Independent co-variates for pulmonary complications were age ≥ 66 years, male sex, current smoker, intraoperative transfusion and use of inotropes. The AUCROC was 0.737 (95% CI 0.695, 0.778) without PFT data (Model 1). ppoFEV1 or ppoDLCO < 40% was an independent risk factor in multivariable analysis (Fig. 3) but tis adddition to other co-variates did not significantly increase the predictive capability [Model 2, AUCROC 0.751 (95% CI 0.712, 0.791); Model 1 vs. model 2, P = 0.065; supplementary Fig. S2, supplementary Table S3].
Post-Hoc analysis: propensity score matching
Patients were divided into two groups, Above (ppoDLCO ≥ 40% and ppoFEV1 ≥ 40%) and Below (ppoDLCO < 40% or ppoFEV1 < 40%), and propensity score matching was performed to balance confounding factors between the two groups (Supplementary Table S4, Supplementary Fig. S1). After propensity score matching, pulmonary complications were higher in Below group compared to Above group [24% (38/160) vs. 13% (21/160); OR 2.4 (95% CI 1.2, 4.7); P = 0.014]. However, there was no difference in in-hospital mortality [Above vs. Below groups: 3% (8/241) vs. 6% (14/241); OR 1.75 (95% CI 0.74, 4.17); P = 0.210]. For long-term survival, the Kaplan–Meier survival analysis showed no difference between Above and Below groups; the mean survival duration was 61 (95% CI 57, 66) months and 65 (95% CI 60, 70) months (hazard ratio 0.95, 95% CI 0.62, 1.47; P = 0.830) in Above and Below groups, respectively (Fig. 4).
Kaplan–Meier survival analysis of the above (ppoDLCO ≥ 40% and ppoFEV1 ≥ 40%) and below (ppoDLCO < 40% or ppoFEV1 < 40%) groups in the propensity score-matched data. There was no difference between the two groups (P = 0.830, hazard ratio 0.95, 95% confidence interval: 0.62, 1.47). ppoFEV1 postoperative expiratory volume in 1 s, ppoDLCO diffusing capacity for carbon monoxide.
Discussion
In the current study, PFTs themselves were not effective predictors of pulmonary complications in open lung resection surgery; therefore, we could not present new cutoff points for preoperative PFTs.
In this study, we used ROC analysis, which has not been employed previously in relation to PFTs. Based on the ROC curves (Fig. 2) and scatter plots of ppoFEV1 and ppoDLCO (Fig. 1), we conclude that the performance of various PFTs for the prediction of pulmonary complications is unsatisfactory. Considering that we place great emphasis on ppoFEV1 and ppoDLCO for surgical decision-making, this is quite disappointing; however, these results may explain why patients with low ppoFEV1 and ppoDLCO often have good postoperative outcomes6,9,15,16.
The reason for poor predictability of PFTs seems to be the existence of other risk factors of equal or greater importance such as operative factors, and patient's other comorbidities. Age ≥ 66 years (OR 1.86), male sex (OR 3.37), current smoker (OR 1.59), all had a significant impact on pulmonary complications in multivariable analysis. Intraoperative events such as transfusion (OR 2.32) and use of inotropes (OR 1.90) also had a great influence on pulmonary complications (Fig. 3, supplementary Table S3). Another reason is lack of information on cardiopulmonary interaction in the current PFTs. To evaluate pulmonary function in relation to cardiac reserve, physiologic tests, such as the six-minute walk tests or expired gas analysis during exercise test are required, but these physiologic tests were not included in the present studies and are only selectively recommended for patents with poor PFTs in the current guidelines3,4,9. These physiologic pulmonary tests, however, are increasingly regarded as a more important predictor of morbidities in thoracic surgery patients17,18.
Previously, the influence of PFTs on patient outcomes has been controversial. Decades ago, several studies showed higher complication rates or mortality after lobectomy in patients with low ppoDLCO or ppoFEV11,19,20,21. One study even reported that the patients with ppoFEV1 or ppoDLCO < 60% had significantly poorer short-and long-term postoperative outcomes in early-stage non-small cell lung cancer (retrospective, n = 391)22. However, in a more recent retrospective study, marginal PFT status was not associated with the development of a major complication following lobectomy and was not an independent predictor of mortality when other variables were controlled (n = 1259)23. All these previous reports included both VATS and open surgery.
When VATS and open thoracotomy were compared, FEV1 and DLCO (< 60%) were predictors of pulmonary complications when lobectomy was performed through thoracotomy but not through VATS (n = 340)7, and VATS patients had a longer survival duration than those with open thoracotomy among patients with low ppoDLCO or ppoFEV1 (< 40%) (n = 84, 54 vs. 20 months)16. However, these two retrospective studies were conducted between 1997 and 2009.
Our study is one of the largest observational studies (n = 1544) comprised of open lung resection surgery and includes a significant number of patients with ppoDLCO or ppoFEV1 < 40% (n = 238). We observed that ppoFEV1 and ppoDLCO themselves were not effective predictors of pulmonary complications, and addition of previous cutoff to other risk factors did not significantly increase the risk predictive capability of the model. In propensity score-matched subgroups, patients with ppoFEV1 or ppoDLCO < 40% showed higher rates of pulmonary complications. However, we regard that the incidence (13% vs. 24%; P = 0.014) is acceptable considering poor PFTs in these patients. In addition, the higher incidence of pulmonary complications in these patients did not result in difference in in-hospital mortality. And in patients who survived the early postoperative period, the long-term survival was similar at the mean survival duration (61 months vs. 65 months, Above vs. Below groups, respectively). These findings support a surgical option in this high-risk group and is considered meaningful because two-year survival was higher (62% vs. 18%) in patients who had surgery compared to those who were denied an operation9.
An additional finding of the current study was that combining ppoFEV1 and ppoDLCO did not result in superior prediction of pulmonary complications compared to ppoDLCO alone. ppoFEV1 and ppoDLCO are regarded as two separate measures of pulmonary function (ppoFEV1 for airway flow; ppoDLCO for alveolar-vascular gas exchange); thus, the absence of an additive effect was unexpected. For possible explanations, some patients may not have performed the spirometric test properly; FEV1 is heavily dependent on patient cooperation24. In addition, in patients with moderate to severe chronic obstructive pulmonary disease, or with tumor-generated endobronchial obstruction, resection of the most affected parenchyma or relief of obstruction may actually improve FEV1 postoperatively19,25,26,27. In contrast, DLCO is related to the total functioning surface area of the alveolar-capillary interface and is generally independent of patient effort. Therefore, it is a more sensitive measure of parenchymal damage than spirometric measurements28. This may be the reason that there was no additive effect between ppoFEV1 and ppoDLCO.
Our study has some limitations. First, this was a single-center study. Other hospitals with different patient care protocols may thus produce different results. Second, our study involved patients who had open lung resection surgery. Thus, more favorable results may be possible with less invasive thoracic surgeries, such as VATS. Third, we did not test 30% cutoff in our post-hoc analysis because only small number of patients belonged to this category (31 patients with a ppoFEV1 < 30%, 33 patients with a ppoDLco < 30%, and 12 patients with both values < 30%). Forth, there is a possibility of selection bias in our cohort. The surgeons were not blinded to the ppoFEV1 and ppoDLCO values; thus, if surgery was offered to the patients whose ppoDLCO and ppoFEV1 < 40%, it may be that those patients were relatively healthy with respect to comorbidities, or may have been offered less extensive surgery. We attempted to adjust for this possible bias by using propensity score matching.
In conclusion, PFTs themselves were not effective in predicting pulmonary complications, and the evidence supporting the decision to operate based on PFTs is limited. Previous cutoff 40% still has an influence in pulmonary complications after open lung resection surgery but no impact on mortality when other variables are controlled. Therefore, decision to proceed with surgical resection should be made on an individual basis with consideration of the patient's goals and physiologic state.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kearney, D. J., Lee, T. H., Reilly, J. J., DeCamp, M. M. & Sugarbaker, D. J. Assessment of operative risk in patients undergoing lung resection: Importance of predicted pulmonary function. Chest 105, 753–759. https://doi.org/10.1378/chest.105.3.753 (1994).
Markos, J. et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am. Rev. Respir. Dis. 139, 902–910. https://doi.org/10.1164/ajrccm/139.4.902 (1989).
British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 56, 89–108. https://doi.org/10.1136/thorax.56.2.89 (2001).
Colice, G. L., Shafazand, S., Griffin, J. P., Keenan, R. & Bolliger, C. T. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 132, 161s–177s. https://doi.org/10.1378/chest.07-1359 (2007).
Liptay, M. J. et al. Diffusion lung capacity for carbon monoxide (DLCO) is an independent prognostic factor for long-term survival after curative lung resection for cancer. J. Surg. Oncol. 100, 703–707. https://doi.org/10.1002/jso.21407 (2009).
Burt, B. M., Kosinski, A. S., Shrager, J. B., Onaitis, M. W. & Weigel, T. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J. Thorac. Cardiovasc. Surg. 148, 19–28. https://doi.org/10.1016/j.jtcvs.2014.03.007 (2014).
Berry, M. F. et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann. Thorac. Surg. 89, 1044–1051. https://doi.org/10.1016/j.athoracsur.2009.12.065 (2010).
Brunelli, A. et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 34, 17–41. https://doi.org/10.1183/09031936.00184308 (2009).
Puente-Maestú, L. et al. Early and long-term validation of an algorithm assessing fitness for surgery in patients with postoperative FEV1 and diffusing capacity of the lung for carbon monoxide < 40%. Chest 139, 1430–1438. https://doi.org/10.1378/chest.10-1069 (2011).
Philip, H. et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur. Respir. J. 40, 1324–1343. https://doi.org/10.1183/09031936.00080312 (2012).
Slinger, P. & Campos, J. Anesthesia for Thoracic surgery in Miller's anesthesia (ed. Gropper, M) 1648–1716 (Elsevier, 2020).
Ranieri, V. M. et al. Acute respiratory distress syndrome: The Berlin definition. JAMA 307, 2526–2533. https://doi.org/10.1001/jama.2012.5669 (2012).
Obuchowski, N. A., Lieber, M. L. & Wians, F. H. Jr. ROC curves in clinical chemistry: Uses, misuses, and possible solutions. Clin. Chem. 50, 1118–1125. https://doi.org/10.1373/clinchem.2004.031823 (2004).
DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845. https://doi.org/10.2307/2531595 (1988).
Paul, S. et al. Outcomes of lobectomy in patients with severely compromised lung function (predicted postoperative diffusing capacity of the lung for carbon monoxide % ≤ 40%). Ann. Am. Thorac. Soc. 10, 616–621. https://doi.org/10.1513/AnnalsATS.201305-117OC (2013).
Lau, K. K., Martin-Ucar, A. E., Nakas, A. & Waller, D. A. Lung cancer surgery in the breathless patient–the benefits of avoiding the gold standard. Eur. J. Cardio Thorac. Surg. 38, 6–13. https://doi.org/10.1016/j.ejcts.2010.01.043 (2010).
Bolliger, C. T., Wyser, C., Roser, H., Solèr, M. & Perruchoud, A. P. Lung scanning and exercise testing for the prediction of postoperative performance in lung resection candidates at increased risk for complications. Chest 108, 341–348. https://doi.org/10.1378/chest.108.2.341 (1995).
Lee, H. et al. Prognostic value of 6-min walk test to predict postoperative cardiopulmonary complications in patients with non-small cell lung cancer. Chest 157, 1665–1673. https://doi.org/10.1016/j.chest.2019.12.039 (2020).
Brunelli, A. & Rocco, G. Spirometry: Predicting risk and outcome. Thorac. Cardiovasc. Surg. 18, 1–8. https://doi.org/10.1016/j.thorsurg.2007.10.007 (2008).
Santini, M., Fiorello, A., Vicidomini, G., Di Crescenzo, V. G. & Laperuta, P. Role of diffusing capacity in predicting complications after lung resection for cancer. Thorac. Cardiovasc. Surg. 55, 391–394. https://doi.org/10.1055/s-2007-965326 (2007).
Ferguson, M. K. & Vigneswaran, W. T. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann. Thorac. Surg. 85, 1158–1164. https://doi.org/10.1016/j.athoracsur.2007.12.071 (2008).
Ozeki, N. et al. Marginal pulmonary function is associated with poor short- and long-term outcomes in lung cancer surgery. Nagoya J. Med. Sci. 79, 37–42. https://doi.org/10.18999/nagjms.79.1.37 (2017).
Taylor, M. D. et al. Marginal pulmonary function should not preclude lobectomy in selected patients with non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 147, 738–744. https://doi.org/10.1016/j.jtcvs.2013.09.064 (2014).
Brunelli, A. et al. Predictors of early morbidity after major lung resection in patients with and without airflow limitation. Ann. Thorac. Surg. 74, 999–1003. https://doi.org/10.1016/s0003-4975(02)03852-3 (2002).
Linden, P. A. et al. Lung resection in patients with preoperative FEV1 < 35% predicted. Chest 127, 1984–1990. https://doi.org/10.1378/chest.127.6.1984 (2005).
Brunelli, A. et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: A prospective follow-up analysis. Chest 131, 141–147. https://doi.org/10.1378/chest.06-1345 (2007).
Baldi, S. et al. Does lobectomy for lung cancer in patients with chronic obstructive pulmonary disease affect lung function? A multicenter national study. J. Thorac. Cardiovasc. Surg. 130, 1616–1622. https://doi.org/10.1016/j.jtcvs.2005.06.049 (2005).
Hegewald, M. J. Diffusing capacity. Clin. Rev. Allergy Immunol. 37, 159–166. https://doi.org/10.1007/s12016-009-8125-2 (2009).
Author information
Authors and Affiliations
Contributions
J.W.C., H.J., and H.J.A. designed the research; J.W.C., H.J.A., M.Y., and J.A.K. performed the research; D.K.K., S.H.L., K.K., and J.C. analyzed the data; and J.W.C., H.J., and H.J.A. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, J.W., Jeong, H., Ahn, H.J. et al. The impact of pulmonary function tests on early postoperative complications in open lung resection surgery: an observational cohort study. Sci Rep 12, 1277 (2022). https://doi.org/10.1038/s41598-022-05279-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05279-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.