Abstract
This study compared muscle strength and foot pressure among patients with metatarsalgia, patients with plantar fasciitis, and healthy controls. A total of 31 patients with foot pain (14 metatarsalgia and 17 plantar fasciitis) and 29 healthy controls participated in the study. The strengths of the plantar flexor and hip muscles were measured using isokinetic and handheld dynamometers, respectively. Foot pressure parameters, including the pressure–time integral (PTI) and foot arch index (AI), were assessed using pedobarography. Compared with the healthy control group, plantar flexor strength was significantly reduced in the affected feet of the metatarsalgia and plantar fasciitis groups (F = 0.083, all p < 0.001); however, hip strength was significantly decreased only in the affected feet of the metatarsalgia group (F = 20.900, p < 0.001). Plantar flexor (p < 0.001) and hip (p = 0.004) strength were significantly lower in the metatarsalgia group than in the plantar fasciitis group. The PTI was lower in the forefeet of the affected feet in the metatarsalgia (p < 0.001) and plantar fasciitis (p = 0.004) groups. Foot AI (p < 0.001) was significantly reduced only in the metatarsalgia group. These results suggest the need to consider the evaluation of muscle strength and foot pressure in both feet for the diagnosis and treatment of foot pain.
Similar content being viewed by others
Introduction
Foot pain is highly prevalent at various ages and is especially common in middle-aged and older women1,2. Foot pain can lead to a variety of musculoskeletal problems and is associated with decreased activities of daily living, impaired balance, and an increased risk of falls1,2,3. Hence, the diagnosis and management of foot pain are important for increasing physical activity and improving quality of life.
In middle-aged and older women, the most common cause of foot pain is plantar fasciitis4, followed by metatarsalgia5. Plantar fasciitis is commonly observed in flat feet and often causes pain in the heel owing to excessive stretching of the plantar fascia6,7. In contrast, metatarsalgia is commonly observed in the cavus foot, and pain often occurs in the forefoot in the region of the metatarsal heads7 owing to restricted joint mobility8. Foot pain is often determined based on pain location and foot posture. Indeed, foot posture affects foot kinematics during walking8,9, which may affect lower-extremity muscle function10,11,12,13,14 and foot pressure15. A recent study12 reported differences in lower-extremity muscle strength and foot pressure between patients with plantar fasciitis and flatfoot and those with a normal foot. Another study9 reported differences in foot pressure among normal, flat, and cavus feet and the association of foot posture with foot pressure during gait. However, the reasons for the differences in muscle strength, foot pressure, and foot posture among patients with metatarsalgia and those with plantar fasciitis, especially compared with healthy controls, are not clear. To the best of our knowledge, no studies have directly compared muscle strength, foot pressure, and foot posture among patients with metatarsalgia, patients with plantar fasciitis, and healthy controls. Moreover, study data on metatarsalgia are limited.
Therefore, the present study compared muscle strength, foot pressure, and foot posture among patients with metatarsalgia, patients with plantar fasciitis, and healthy controls. We hypothesized that patients with metatarsalgia and plantar fasciitis would show decreased muscle strength, increased foot pressure, and different foot postures compared with healthy controls.
Methods
Study design and setting
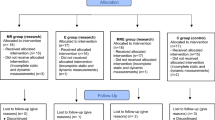
This prospective comparative case–control study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for non-pharmacological treatments and obtained informed consent from all patients. This study was conducted from July 2018 to August 2022 and was approved by our Institutional Review Board (2018AN0168). Outpatients presenting for physiotherapy with a primary report of foot pain were screened according to the eligibility criteria. Among a total of 109 enrolled patients, only those with forefoot or rearfoot pain were selected by an orthopedic surgeon. Metatarsalgia and plantar fasciitis were evaluated by physical examination, plain radiography, and ultrasonography. The inclusion criteria were pathological effusion in the metatarsophalangeal joints for metatarsalgia16 and fascia thickening of > 4 mm for plantar fasciitis17. A total of 78 patients with affected muscle strength, foot pressure, and walking were excluded for the following reasons: (1) bilateral foot pain; (2) other concomitant foot pain (i.e., hallux valgus and Achilles tendonitis); (3) knee osteoarthritis with Kellgren–Lawrence grade > 2; 4) previous foot and ankle surgeries; (5) inability to perform the tests owing to pain; (6) neurogenic deformities; (7) administration of analgesics and anti-inflammatory drugs within 4 weeks or injections in the past 6 months; (8) gastrocnemius and hamstring muscle tightness; and (9) anatomical problems of the feet such as plantar plate tears, plantarflexed metatarsal bone, and metatarsal length discrepancy. Finally, 31 patients with foot pain (14 metatarsalgia and 17 plantar fasciitis) participated in this study. We also included 29 healthy controls with no history of ankle or foot trauma, or foot pain symptoms were selected from our database and agreed to participate in this study (Table 1).
Measurements and outcome variables
Muscle strength
At our institution, the strengths of the plantar flexor and hip muscles were measured in patients with foot pain using an isokinetic dynamometer and a handheld dynamometer, respectively. Plantar flexor strength was evaluated for five repetitions of plantar flexion at 30°/s using an isokinetic device (Biodex Multi-Joint System 4, Biodex Medical Systems, Inc.) for each leg with each participant in a semi-seated position and 20° knee flexion12.
Isometric hip muscle strength was measured using a handheld dynamometer (microFET2, Hoggan Scientific, LLC, Salt Lake City, UT, USA, 2016) with 45° flexion, 20° abduction of the hip joint, and 90° flexion of the knee joint with the participants lying on their side. The evaluation was performed twice and the average value was used12.
Muscle strength was calculated in this study by normalizing the peak torque to body weight. Plantar flexor and hip muscles strength were recorded as Nm kg−1 × 100 and kgf/kg, respectively. The intraclass correlation coefficients (ICCs) for plantar flexor and hip muscle strength were 0.84 and 0.87, respectively.
Foot pressure and foot posture
Foot pressure was measured using pedobarography (Tekscan, MA, USA) and recorded at 50 Hz while walking for 2 m. All participants were evaluated after stepping on the pressure platform for three steps on the affected foot while walking the 2 m distance. The foot pressure parameters were evaluated using the pressure–time integral (PTI).
PTI was defined as the time integral of the mean pressure (N/cm2 s) in each of the three areas (forefoot, midfoot, and rearfoot) during walking18, and is a better indicator than peak pressure19.
Foot posture was assessed using the foot arch index (AI)9,20. The foot AI was calculated as the entire footprint area divided by the area of the middle third of the footprint (AI = B/A + B + C), and has demonstrated acceptable reliability and validity.20 Based on a previous study20, the cut-off scores for foot AI were defined as follows: high-arched (< 0.21), normal (0.21–0.28), and low-arched (> 0.28). The high- and low-foot AI scores were defined as low-arched and high-arched foot postures, respectively, in the present study.
Statistical analysis
The sample size calculation was based on a > 10% difference in plantar flexor muscle strength between groups, a significance α level of 0.05, and a power (1–β) of 0.812, which was considered clinically significant. To determine the sample size, we conducted an a priori power analysis using one-way analysis of variance (ANOVA), which revealed that a minimum sample size of 36 participants was sufficient to detect a 10% difference in plantar flexor strength between the groups (effect size f(V): 0.567). The power required to detect the differences in muscle strength was 0.837.
We used the Shapiro–Wilk test to verify normal data distributions. The Kruskal–Wallis test was performed to compare demographic data between the groups. One-way ANOVA was performed to determine significant differences in all outcome variables between the groups. When between-group comparisons were made, Tukey’s correction was applied as a post hoc test (p < 0.017). Correlations among pain visual analog scale (VAS), muscle strength, foot pressure, and foot AI were assessed using Pearson’s correlation coefficients. Data were analyzed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA). The significance level was set at α = 0.05.
Ethics approval and consent to participate
Korea University Anam Hospital approved this study (2018AN0168). The study was performed in accordance with the ethical standards as laid out in the 1964 Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Results
Participant age, weight, height, or body mass index (p > 0.05) did not differ significantly between groups.
Comparisons of muscle strength between the groups
In the affected feet (Table 2 and Fig. 1), plantar flexor (F = 44.175, p < 0.001) and hip (F = 20.900, p < 0.001) strengths differed significantly between the groups. Following the post hoc test, compared with the healthy control group, the plantar flexor strength was significantly lower in the metatarsalgia (58.8 ± 9.9 vs. 31.1 ± 9.0; 95% confidence interval [CI], 20.3 to 35.1; p < 0.001) and plantar fasciitis (58.8 ± 9.9 vs. 44.7 ± 8.0; 95% CI 7.1–21.0; p < 0.001) groups, whereas hip strength was significantly decreased only in the metatarsalgia (57.1 ± 10.4 vs. 36.1 ± 9.8; 95% CI 12.9 to 29.1; p < 0.001) group. Plantar flexor (44.7 ± 8.0 vs. 31.1 ± 9.0; 95% CI 5.4–21.9; p < 0.001) and hip (48.4 ± 9.5 vs. 36.1 ± 9.8; 95% CI 3.4–21.3; p = 0.004) strength were significantly lower in the metatarsalgia group compared with that in the plantar fasciitis group.
Comparisons of foot pressure and AI between groups
In affected feet (Table 3 and Fig. 1), the PTI differed significantly in the forefoot (F = 24.211, p < 0.001) and rearfoot (F = 31.105, p < 0.001) between groups. Following the post hoc test, compared with the healthy control group, PTI was low in the forefoot in the metatarsalgia (25.0 ± 2.9 vs. 18.7 ± 2.4; 95% CI 4.0–8.5; p < 0.001) and plantar fasciitis (25.0 ± 2.9 vs. 22.1 ± 2.9; 95% CI 0.6–5.2; p = 0.004) groups, but significantly higher in the rearfoot (metatarsalgia: 22.9 ± 3.4 vs. 28.7 ± 5.1; 95% CI −9.2 to −2.5; plantar fasciitis: 22.9 ± 3.4 vs. 32.7 ± 4.6; 95% CI −13.0 to −6.7; all p < 0.001). In particular, PTI in the forefoot (18.7 ± 2.4 vs. 22.1 ± 2.9; 95% CI 0.2–7.7; p = 0.004) was significantly lower in the metatarsalgia group compared with that in the plantar fasciitis group.
In the unaffected feet, PTI of the rearfoot differed significantly among the groups (F = 47.317, p < 0.001). Following the post hoc test, compared with that of the healthy controls, PTI in the rearfoot was significantly higher in the metatarsalgia (26.4 ± 3.2 vs. 35.1 ± 5.5; 95% CI −11.8 to −5.8; p < 0.001) and plantar fasciitis (26.4 ± 3.2 vs. 36.2 ± 2.8; 95% CI −12.7 to −7.0; p < 0.001) groups. The PTI did not differ significantly between the metatarsalgia and plantar fasciitis groups.
Foot AI (F = 28.019, p < 0.001) was significantly reduced in the metatarsalgia group compared with that in the plantar fasciitis (0.19 vs. 0.27; 95% CI −0.1 to 0; p < 0.001) and healthy control (0.19 vs. 0.24; 95% CI −0.1 to 0; p < 0.001) groups, but did not differ significantly between the plantar fasciitis and healthy control groups (Fig. 1, p > 0.017).
Correlations between pain VAS, muscle strength, foot pressure, and foot AI
Pain VAS in the metatarsalgia and plantar fasciitis groups showed a significant negative correlation with plantar flexor strength in the affected feet (r = −0.423, p = 0.018) and a positive correlation with PTI in the rearfoot of the unaffected feet (r = 0.529, p = 0.002, Table 4).
Discussion
This study compared muscle strength, foot pressure, and foot posture among patients with metatarsalgia, patients with plantar fasciitis, and healthy controls. Our findings showed the weakest plantar flexor and hip muscle strength in patients with metatarsalgia. Metatarsalgia was also associated with low forefoot pressure. Furthermore, patients with metatarsalgia and plantar fasciitis had high pressures in the rearfoot of both feet. Finally, foot posture differed only in the metatarsalgia group.
The plantar fasciitis group in this study showed significantly reduced plantar flexor strength compared with that in the healthy control group. This result is consistent with those previously reported21,22. However, the reasons why the plantar flexor and hip muscle strength decreased more in the metatarsalgia group than in the plantar fasciitis and control groups are not known. One possible explanation may involve compensatory strategies to reduce forefoot pain. The forefoot should be used to increase plantar flexor strength. However, patients with metatarsalgia may compensate for increased pain when using their forefeet during the muscle strength test. Similarly, the plantar flexor23,24,25 and hip24,26 muscles are mainly used for single-leg stance and forward propulsion during walking. However, patients with metatarsalgia may use compensatory strategies to reduce the activity of the plantar flexors and hip muscles to reduce pain during these movements. Therefore, our findings also showed decreased forefoot-PTI in patients with metatarsalgia compared with the plantar fasciitis and healthy control groups, indicating a lack of sufficient foot adaptation for forward propulsion during walking. However, further studies are needed to clarify whether plantar flexor and hip muscle weaknesses are the cause or result of metatarsalgia.
In this study, rearfoot-PTI was significantly higher in the metatarsalgia and plantar fasciitis groups than in the healthy control group. One possible explanation for this finding is foot posture. Previous studies19,27 have reported increased rearfoot-PTI in patients with foot pain and an association between foot posture and foot pain and plantar pressure. Our results showed that metatarsalgia was associated with cavus foot posture (foot AI < 0.21, high-arched foot) and differed from heathy controls. Burns et al.19 and Buldt et al.9 reported higher rearfoot-PTI in the cavus foot than in healthy controls owing to a lack of load bearing in the midfoot, and that this foot posture was related to foot pain14. Another possible explanation may be weakness of the plantar flexor muscles. Such weakness may lead to a lack of ankle moment, which may alter walking patterns. Ong et al.25 and Bruel et al.28 suggested that plantar flexor muscle weakness may cause heel walking, indicating a higher load on the heel. Furthermore, in this study, compared with the healthy controls, the PTI was higher in the rearfoot of feet unaffected by metatarsalgia and plantar fasciitis. Although the reason for this finding is unclear, it may be explained by the overuse of the contralateral foot owing to an altered walking pattern owing to foot pain. Sullivan et al.29 reported that patients with foot pain change their walking patterns as a strategy to reduce pain. Both feet are used synchronously while walking; therefore, biomechanical stress on one side can naturally increase the use of the other. Therefore, patients with unilateral foot pain may have bilateral foot problems30. Arie et al.31 reported that biomechanical alterations accounted for > 90% of all etiological factors. Taken together, the results of this study and those of previous studies may explain to the higher plantar pressure on the rearfoot in patients with metatarsalgia and plantar fasciitis.
Clinical implication
The results of this study revealed decreased plantar flexor and hip muscle strength in the following order: patients with metatarsalgia < patients with plantar fasciitis < healthy controls. Metatarsalgia may be a compensatory strategy for reducing pressure on the forefoot. Additionally, the metatarsalgia and plantar fasciitis groups showed increased rearfoot pressure. Moreover, the metatarsalgia group showed a high-arched foot posture, whereas the plantar fasciitis group showed a posture similar to that in the healthy control group. Hence, caution is needed when diagnosing these diseases based solely on foot posture. Previous studies reported no difference in foot posture between individuals with and without foot pain32,33. Therefore, whether the results in this study are the cause or result of metatarsalgia or plantar fasciitis is unknown; however, our results suggest that muscle strength and foot pressure should be evaluated in both feet for the diagnosis and treatment of foot pain.
Limitations
This study has some limitations. First, the intrinsic foot muscles were not measured. These muscles play important roles in foot posture and function. Previous studies10,34,35,36 reported that alterations in the function of the intrinsic foot muscles owing to changes in foot posture may lead to variation in foot posture and function. Therefore, further studies on intrinsic foot muscles are needed to clarify the results of the present study. Second, although walking speed can affect foot pressure results37, we did not evaluate this factor. Third, we did not assess neural subsystems. McKeon et al.10 reported that foot core systems such as the passive (ligament and plantar fascia), active (foot muscles), and neural (neuromuscular control) subsystems, play significant roles in normal foot function. Therefore, assessment of neuromuscular control abilities such as dynamic postural stability34 should be considered in patients with foot pain. Finally, randomized controlled trials with larger sample sizes are required to validate our results.
Conclusion
Compared with the healthy controls, patients with foot pain, such as metatarsalgia and plantar fasciitis, showed decreased plantar flexor and hip muscle strength. Foot pressure was low in the forefoot of the affected foot, but high in the rearfoot of the affected and unaffected feet. In particular, compared with patients with plantar fasciitis and the healthy controls, patients with metatarsalgia demonstrated weaker plantar flexor and hip muscles, low forefoot pressure, and high foot posture.
Data availability
The data that support the findings of this study are available from author, Jin Hyuck Lee but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Furthermore, all data generated or analyzed during the current study will not be disclosed due to policy of the Korea University Anam Hospital Research Ethics Board.
References
Thomas, M. J. et al. The population prevalence of foot and ankle pain in middle and old age: A systematic review. Pain 152, 2870–2880. https://doi.org/10.1016/j.pain.2011.09.019 (2011).
Hendry, G. J., Fenocchi, L., Woodburn, J. & Steultjens, M. Foot pain and foot health in an educated population of adults: Results from the Glasgow Caledonian University Alumni Foot Health Survey. J Foot Ankle Res 11, 48. https://doi.org/10.1186/s13047-018-0290-1 (2018).
Hill, C. L., Gill, T. K., Menz, H. B. & Taylor, A. W. Prevalence and correlates of foot pain in a population-based study: the North West Adelaide health study. J Foot Ankle Res 1, 2. https://doi.org/10.1186/1757-1146-1-2 (2008).
Hawke, F. & Burns, J. Understanding the nature and mechanism of foot pain. J Foot Ankle Res 2, 1. https://doi.org/10.1186/1757-1146-2-1 (2009).
Naranjo-Ruiz, C. et al. Influence of foot type on the clinical outcome of minimally invasive surgery for metatarsalgia. A prospective pilot study. Front Surg 8, 748330. https://doi.org/10.3389/fsurg.2021.748330 (2021).
Buchbinder, R. Clinical practice. Plantar fasciitis. N. Engl. J. Med. 350, 2159–2166. https://doi.org/10.1056/NEJMcp032745 (2004).
Burns, J., Landorf, K. B., Ryan, M. M., Crosbie, J. & Ouvrier, R. A. Interventions for the prevention and treatment of pes cavus. Cochrane Database Syst. Rev. 2007, Cd006154, https://doi.org/10.1002/14651858.CD006154.pub2 (2007).
Buldt, A. K. et al. Centre of pressure characteristics in normal, planus and cavus feet. J Foot Ankle Res 11, 3. https://doi.org/10.1186/s13047-018-0245-6 (2018).
Buldt, A. K. et al. Foot posture is associated with kinematics of the foot during gait: A comparison of normal, planus and cavus feet. Gait Posture 42, 42–48. https://doi.org/10.1016/j.gaitpost.2015.03.004 (2015).
McKeon, P. O., Hertel, J., Bramble, D. & Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 49, 290. https://doi.org/10.1136/bjsports-2013-092690 (2015).
Angin, S., Mickle, K. J. & Nester, C. J. Contributions of foot muscles and plantar fascia morphology to foot posture. Gait Posture 61, 238–242. https://doi.org/10.1016/j.gaitpost.2018.01.022 (2018).
Lee, J. H., Shin, K. H., Jung, T. S. & Jang, W. Y. Lower extremity muscle performance and foot pressure in patients who have plantar fasciitis with and without flat foot posture. Int. J. Environ. Res. Public Health 20. https://doi.org/10.3390/ijerph20010087 (2022).
Lee, J. H., Jung, H. W. & Jang, W. Y. A prospective study of the muscle strength and reaction time of the quadriceps, hamstring, and gastrocnemius muscles in patients with plantar fasciitis. BMC Musculoskelet. Disord. 21, 722. https://doi.org/10.1186/s12891-020-03740-1 (2020).
Tong, J. W. & Kong, P. W. Association between foot type and lower extremity injuries: systematic literature review with meta-analysis. J. Orthop. Sports Phys. Ther. 43, 700–714. https://doi.org/10.2519/jospt.2013.4225 (2013).
Buldt, A. K., Allan, J. J., Landorf, K. B. & Menz, H. B. The relationship between foot posture and plantar pressure during walking in adults: A systematic review. Gait Posture 62, 56–67. https://doi.org/10.1016/j.gaitpost.2018.02.026 (2018).
Albano, D. et al. Plantar forefoot pain: Ultrasound findings before and after treatment with custom-made foot orthoses. Radiol. Med. 126, 963–970. https://doi.org/10.1007/s11547-021-01354-8 (2021).
Draghi, F., Gitto, S., Bortolotto, C., Draghi, A. G. & Ori Belometti, G. Imaging of plantar fascia disorders: findings on plain radiography, ultrasound and magnetic resonance imaging. Insights Imaging 8, 69–78, https://doi.org/10.1007/s13244-016-0533-2 (2017).
Waaijman, R. & Bus, S. A. The interdependency of peak pressure and pressure-time integral in pressure studies on diabetic footwear: no need to report both parameters. Gait Posture 35, 1–5. https://doi.org/10.1016/j.gaitpost.2011.07.006 (2012).
Burns, J., Crosbie, J., Hunt, A. & Ouvrier, R. The effect of pes cavus on foot pain and plantar pressure. Clin. Biomech. (Bristol, Avon) 20, 877–882, https://doi.org/10.1016/j.clinbiomech.2005.03.006 (2005).
Menz, H. B., Fotoohabadi, M. R., Wee, E. & Spink, M. J. Visual categorisation of the arch index: A simplified measure of foot posture in older people. J Foot Ankle Res 5, 10. https://doi.org/10.1186/1757-1146-5-10 (2012).
McClinton, S., Collazo, C., Vincent, E. & Vardaxis, V. Impaired foot plantar flexor muscle performance in individuals with plantar heel pain and association with foot orthosis Use. J. Orthop. Sports Phys. Ther. 46, 681–688. https://doi.org/10.2519/jospt.2016.6482 (2016).
Kibler, W. B., Goldberg, C. & Chandler, T. J. Functional biomechanical deficits in running athletes with plantar fasciitis. Am. J. Sports Med. 19, 66–71. https://doi.org/10.1177/036354659101900111 (1991).
Sutherland, D. H., Cooper, L. & Daniel, D. The role of the ankle plantar flexors in normal walking. J. Bone Joint Surg. Am. 62, 354–363 (1980).
Liu, M. Q., Anderson, F. C., Schwartz, M. H. & Delp, S. L. Muscle contributions to support and progression over a range of walking speeds. J. Biomech. 41, 3243–3252. https://doi.org/10.1016/j.jbiomech.2008.07.031 (2008).
Ong, C. F., Geijtenbeek, T., Hicks, J. L. & Delp, S. L. Predicting gait adaptations due to ankle plantarflexor muscle weakness and contracture using physics-based musculoskeletal simulations. PLoS Comput. Biol. 15, e1006993. https://doi.org/10.1371/journal.pcbi.1006993 (2019).
Neptune, R. R., Sasaki, K. & Kautz, S. A. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 28, 135–143. https://doi.org/10.1016/j.gaitpost.2007.11.004 (2008).
Ribeiro, A. P., Sacco, I. C., Dinato, R. C. & João, S. M. Relationships between static foot alignment and dynamic plantar loads in runners with acute and chronic stages of plantar fasciitis: a cross-sectional study. Braz J Phys Ther 20, 87–95. https://doi.org/10.1590/bjpt-rbf.2014.0136 (2016).
Bruel, A. et al. Investigation of neural and biomechanical impairments leading to pathological toe and heel gaits using neuromusculoskeletal modelling. J. Physiol. 600, 2691–2712. https://doi.org/10.1113/jp282609 (2022).
Sullivan, J., Burns, J., Adams, R., Pappas, E. & Crosbie, J. Plantar heel pain and foot loading during normal walking. Gait Posture 41, 688–693. https://doi.org/10.1016/j.gaitpost.2015.01.025 (2015).
Ríos-León, M., Ortega-Santiago, R., Madeleine, P., Fernández-de-Las-Peñas, C. & Plaza-Manzano, G. Topographical pressure pain sensitivity maps of the feet reveal bilateral pain sensitivity in patients with unilateral plantar heel pain. J. Orthop. Sports Phys. Ther. 49, 640–646. https://doi.org/10.2519/jospt.2019.8813 (2019).
Arie, E. K., Moreira, N. S., Freire, G. S., Dos Santos, B. S. & Yi, L. C. Study of the metatarsal formula in patient with primary metatarsalgia. Rev Bras Ortop 50, 438–444. https://doi.org/10.1016/j.rboe.2015.06.018 (2015).
Menz, H. B. & Morris, M. E. Determinants of disabling foot pain in retirement village residents. J. Am. Podiatr. Med. Assoc. 95, 573–579. https://doi.org/10.7547/0950573 (2005).
Paiva de Castro, A., Rebelatto, J. R. & Aurichio, T. R. The relationship between foot pain, anthropometric variables and footwear among older people. Appl. Ergon. 41, 93–97. https://doi.org/10.1016/j.apergo.2009.05.002 (2010).
Wei, Z., Zeng, Z., Liu, M. & Wang, L. Effect of intrinsic foot muscles training on foot function and dynamic postural balance: A systematic review and meta-analysis. PLoS One 17, e0266525. https://doi.org/10.1371/journal.pone.0266525 (2022).
Angin, S., Crofts, G., Mickle, K. J. & Nester, C. J. Ultrasound evaluation of foot muscles and plantar fascia in pes planus. Gait Posture 40, 48–52. https://doi.org/10.1016/j.gaitpost.2014.02.008 (2014).
Amaha, K., Arimoto, T. & Kitamura, N. Effect of toe exercises and toe grip strength on the treatment of primary metatarsalgia. J. Orthop. Surg. Res. 15, 580. https://doi.org/10.1186/s13018-020-02113-7 (2020).
Lung, C. W. et al. Effects of walking speeds and durations on the plantar pressure gradient and pressure gradient angle. BMC Musculoskelet. Disord. 23, 823. https://doi.org/10.1186/s12891-022-05771-2 (2022).
Acknowledgements
This project is supported by LG Electronics Inc (Yeoui-daero, Yeongdeungpo-gu, Seoul, Korea) and we thank Taegu Kang, Jina Lee, Hyung Yoon, Homin Lee, Sunghoon Jung, Taejung Kwon, Taehoon Kim, and Suin Seo for their contributions to data acquisition and preparation in this study.
Funding
This work was supported by the Korea Medical Device Development Fund granted by the Korean government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, and Ministry of Food and Drug Safety) (KMDF_PR_20200901_0039, KMDF_PR_20200901_0293, RS_2023_00243310). This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2022R1A2C2092726), Korea University Anam Hospital, Seoul, Republic of Korea (Grant No. K2305161, K2313001, K2312991, I2300231, I2203971).This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant number: HI22C1463 and HR22C1302), and technically by 4P Lab, Co., Ltd. for data analysis.
Author information
Authors and Affiliations
Contributions
J.H.L., J.H., and H.P. collected and analyzed the patient clinical data. H.K., W.S., D.A.C., and C.H.S. interpreted the data. J.H.L. and W.Y.J. were major contributors to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J.H., Hwang, J., Park, H. et al. Muscle strength and foot pressure vary depending on the type of foot pain. Sci Rep 14, 5857 (2024). https://doi.org/10.1038/s41598-024-56490-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56490-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.