Abstract
Vector control is a key intervention against mosquito borne diseases. However, conventional methods have several limitations and alternate strategies are in urgent need. Vector control with endectocides such as ivermectin is emerging as a novel strategy. The short half-life of ivermectin is a limiting factor for its application as a mass therapy tool for vector control. Isoxazoline compounds like fluralaner, a class of veterinary acaricides with long half-life hold promise as an alternative. However, information about their mosquitocidal effect is limited. We explored the efficacy of fluralaner against laboratory reared vector mosquitoes—Aedes aegypti, Anopheles stephensi, and, Culex quinquefasciatus. 24 h post-blood feeding, fluralaner showed a significant mosquitocidal effect with LC50 values in the range of 24.04–49.82 ng/mL for the three different mosquito species tested. Effects on life history characteristics (fecundity, egg hatch success, etc.) were also observed and significant effects were noted at drug concentrations of 20, 25 and 45 ng/mL for Ae. aegypti, An. stephensi, and, Cx. quinquefasciatus respectively. At higher drug concentration of 250 ng/mL, significant mortality was observed within 1–2 h of post blood feeding. Potent mosquitocidal effect coupled with its long half-life makes fluralaner an excellent candidate for drug based vector control strategies.
Similar content being viewed by others
Introduction
Vector borne diseases such as malaria, lymphatic filariasis, dengue and Zika virus pose significant public health threats globally1. Effective vaccines or specific treatment are not available for many of these diseases and vector control is the only option for prevention and control2. However, conventional vector control methods have several limitations and there is an urgent need for alternative control strategies. With the discovery of the potent mosquitocidal effect of the anti-parasitic drug ivermectin, vector control with endectocides is emerging as an alternative strategy3,4. This approach involves mass administration of a systemic insecticidal drug to animal or human population aimed at killing the blood feeding arthropods. Ivermectin is now being explored as a mass drug administration (MDA) tool in humans for malaria control in many settings5,6,7,8. As we progress towards malaria elimination, due to the extensive use of LLIN (Long Lasting Insecticidal Nets) and IRS (Indoor Residual Spraying), mosquitoes’ feeding behavior may change and they may prefer to feed on animals rather than humans9. Hence mass therapy of domestic animals with ivermectin is also suggested10,11,12,13. However, one major drawback for application of ivermectin for vector control is its short half-life (2.07 ± 0.71 days)14. This would necessitate its very frequent administration to human or animal population which may limit its utility in mass therapy. But drugs with shorter half-life tend to be safer and slow release formulation could bypass the need of frequent administration13.
Alternative endectocides with longer half-life which are proved to be safe or having only minimal side effects in animals and humans are highly desirable. Isoxazoline compounds like fluralaner (Bravecto®) and afoxolaner (NexGard®) used as acaricides in veterinary practice have an unusually long half-life (14.27 ± 2.53 days)15,16. After a single oral administration (50 mg/kg) of fluralaner in dogs, maximum plasma concentrations (Cmax) of 5419 ± 2086 ng/mL have been achieved within 24 h and detectable plasma levels were found up to 112 days post administration15,17. The target binding sites of fluralaner are similar to those of ivermectin, although their mechanisms of action differ. Fluralaner acts as a potent inhibitor or antagonist of γ-amino butyric acid (GABA) and l-glutamate-gated chloride channels, inducing spastic paralysis in insects. Notably, it binds to GABA channels more efficiently. In contrast, ivermectin binds more efficiently to glutamate channels and serves as an agonist, resulting in flaccid paralysis in insects18. Isoxazoline compounds may have minimal or no effect on the vertebrate nervous system15. Dogs treated with fluralaner and then exposed to mosquitoes have shown a significant reduction in the survival and fecundity of the fed mosquitoes for up to 13 weeks post-treatment19. Furthermore, fluralaner has demonstrated effectiveness in controlling the transmission of Dirofilaria immitis (heartworm) by killing vector mosquitoes that fed on dogs treated with the drug20.
With its excellent safety profile in animals, long half-life and the potent acaricidal effect, fluralaner can be an alternative choice for drug based vector control strategies. However, information on its mosquitocidal effect is very limited21. A recent study from USA (United States of America) reported its significant lethal effect in different mosquito vector species such as Anopheles stephensi (Liston, 1901), Anopheles gambiae Kisumu, Anopheles gambiae Tiassalé (Gile, 1902), Aedes aegypti New Orleans, Aedes aegypti Cayman (Linnaeus, 1762) and Culex pipiens (Linnaeus, 1758) (Diptera: Culicidae) species21.
In the present study, the adulticidal effect of fluralaner was studied against three vector mosquito species from India: (1) Aedes aegypti, the primary vector of dengue, (2) Anopheles stephensi, the vector of urban malaria, and (3) Culex quinquefasciatus (Say, 1823) (Diptera: Culicidae), the vector of lymphatic filariasis under laboratory conditions. In addition, the effect of fluralaner on fecundity, egg hatch success, immature development and adult emergence success were investigated on mosquitoes fed with different concentrations of the drug.
Results
Estimated LC50 value of fluralaner at different time points
Based on the preliminary results of initial bioassays, experiments were performed with narrow range drug concentrations (with three technical replicates), as described in methodology section for each of the species and the 24 h LC50 (95% CI) value was estimated. The results are summarized in (Table 1). Since the upper limit for 95% CI of 24 h LC50 was exceeding the maximum dosage used for Ae. aegypti and An. stephensi, the mortality data was subjected to cox-regression analysis to compare the survivorship or risk of death between different treatment and control group.
Cox-regression analysis
Ae. aegypti
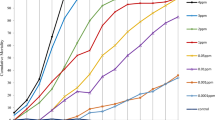
The risk of death was significantly higher in dosage 35 (HR = 150.45 (95% CI: 94.76–238.87), P-value < 0.001) when compared to control i.e., dosage 0 (Table 2) (Fig. 1A).
An. stephensi
The risk of death was significantly higher in dosage 40 (HR = 231.94 (95% CI: 142.68–377.05), P-value < 0.001) when compared to control i.e., dosage 0 (Table 2) (Fig. 1B).
Cx. quinquefasciatus
The risk of death was significantly higher in dosage 60 (HR = 516.41 (95% CI: 292.07–913.07), P-value < 0.001) when compared to control i.e., dosage 0 (Table 2) (Fig. 1C).
Effects on life history characteristics of mosquitoes fed with fluralaner
Fecundity
The overall fecundity (average number of eggs laid per female mosquito) of Ae. aegypti, An. stephensi and Cx. quinquefasciatus females treated with fluralaner at different drug concentrations are given in (Table 3). The mean fecundity differed significantly between treated and control groups at drug concentrations nearing 24 h LC50 value for all the three species tested (P-value < 0.05) (Table 3). At higher drug concentration, dead adults with half of the abdomen filled with eggs were found in the oviposition cups in case of An. stephensi and Cx. quinquefasciatus. In the case of Cx. quinquefasciatus distorted egg rafts were found in the oviposition cups i.e., many eggs were scattered.
Egg hatch success
The egg hatch success of Ae. aegypti, An. stephensi and Cx. quinquefasciatus mosquitoes treated with fluralaner at different drug concentrations are given in Table 4. The mean egg hatch success differed significantly between the treated and control groups (P-value < 0.05) at higher drug concentrations, compared to the effect observed at lower concentrations (Table 4).
Immature development
The overall immature development of Ae. aegypti, An. stephensi and Cx. quinquefasciatus mosquitoes treated with fluralaner at different drug concentrations are given in Table 5. The mean immature development varied significantly between control and treatment groups at higher drug concentrations tested: for Ae. aegypti20 ng/mL; for An. stephensi25 ng/mL; for Cx. quinquefasciatus45 ng/mL (P-value < 0.05 for all species).
Adult emergence success
The overall adult emergence success of Ae. aegypti, An. stephensi and Cx. quinquefasciatus mosquitoes exposed to fluralaner at different drug concentrations are given in Table 6. Certain morphological abnormalities in a few drug exposed mosquitoes at concentration nearing 24 h LC50 (20 ng/mL, 25 ng/mL, 45 ng/mL for Ae. Aegypti, An. stephensi, and Cx. quinquefasciatus respectively) were observed, such as partially emerged adults and emerged adult mosquitoes with their legs attached to the pupal exuviae (Fig. 2A–D).
Estimation of mortality over time
Estimation of mortality at different time points on feeding with different concentrations of the drug for all the three species is depicted in supplementary graphs 1–3. Speed of killing was also studied at a concentration 4–5 folds higher (250 ng/mL) (Fig. 3). When treated with this concentration, An. stephensi showed 100.00% mortality at 3 h of post blood feeding. In the case of Ae. aegypti, 99.00% mortality was recorded at 6 h of post blood feeding. For Cx. quinquefasciatus, it took 7 h to produce 100.00% mortality post blood feeding with fluralaner. The LT50 values for all the three species at given concentration is provided in Table 7.
Discussion
An ideal endectocide for administration as a mass therapy tool for vector control should have potent mosquitocidal effect along with long half-life property so that the drug effect lasts for weeks to months after one-time drug administration. In the present study, oral treatment with fluralaner resulted in significant mosquitocidal effect against the three vector species studied, with the estimated 24 h LC50 values ranging from 24.04 ng/mL to 49.82 ng/mL. The current study is the first attempt that examined the effect of fluralaner against Cx. quinquefasciatus.
Mosquitocidal effect of fluralaner observed in the current study was similar to that of in an earlier study, which reported 24 h LC50 values of 18.48–51.52 ng/mL21. The 24 h LC50 value of fluralaner observed in the earlier study as well as in the current study was lower than that reported for ivermectin (LC50: 44.68–49.40 ng/mL)22. After a single oral administration of fluralaner at 50 mg/kg body weight in dogs, 100 ng/mL concentration of fluralaner was maintained in the blood for a period of 3 months15. Mosquitoes fed on dog treated with fluralaner were reported to have significant reduction in their survival and fecundity for up to 13 weeks post-treatment19. It is noteworthy that the blood level concentration of fluralaner observed in dogs for three months is well above the 24 h LC50 values (24.04–49.82 ng/mL) of fluralaner observed against three species in the current study.
Fluralaner affected the fecundity, egg hatch success, immature development and adult emergence success in all the three species when they were exposed to drug concentrations nearing 24 h LC50 values of 24.04 ng/mL for Ae. aegypti, 33.22 ng/mL for An. stephensi and 49.82 ng/mL for Cx. quinquefasciatus with significant P value. Although these differences were statistically significant, this may not have any importance under field conditions. There was no effect or only minimal effect at lower drug concentrations. In contrast, a significant decrease was observed in the fecundity, egg hatch, larval and pupal development in the groups exposed to a sublethal dose of fluralaner in An. aquasalis 23. Similar finding was reported for ivermectin which showed significant effect on fecundity and egg hatch success at sub lethal concentrations—LC5 value 18.28 ng/mL22. It is curious that fluralaner’s anti-fecundity activity is relatively lesser to that of ivermectin, even though their mortality effects are similar post blood feeding in different mosquito species. One possible reason for this effect could be that ivermectin and isoxazoline compounds may have different mechanism of action regarding the anti-fecundity effect. Further research may be desirable in understanding this differential effect. Though fluralaner did not cause any visible impact on the development of the pupae into adults, certain morphological abnormalities like partially emerged adults and emerged adults with their legs attached to the pupal exuviae were observed. In an earlier study, fluralaner has been shown to affect the adult emergence success in Spodoptera litura Fabricius (Lepidoptera: Noctuidae), even at sub-lethal dose of LC15 morphological abnormalities like notched wings were observed in the adults24.
Studies on susceptible and resistant strains of horn and house flies have shown high level of mortality at lower concentration with fluralaner when compared with imidacloprid25. In a similar study on fipronil and pyrethroid resistant bed bugs, fluralaner outperformed ivermectin with a high mortality rate up to 28 days of post-treatment26.
In the current study, complete mortality was observed within 7 h of post blood feeding at 250 ng/mL drug concentration in all the three species tested. Blood concentration much above this level is expected during the initial weeks in animals treated with fluralaner. This is a notable advantage over ivermectin which takes 72 h to produce 70.00–100.00% morality in mosquitoes at a dose of 93 ng/mL in animal experiments14,27,28 and also this plasma level lasts for less than one day only.
Currently, ivermectin mass therapy is being investigated in peri-domestic animals and humans in field studies for its endectocidal effect and as a complementary tool for malaria vector control10,11,29. Fluralaner, with its comparable mosquitocidal effect to ivermectin coupled with the long half–life may be a good alternative to ivermectin. Additionally, fluralaner was found to be effective in controlling Dirofilaria immitis (heartworm) transmission by killing the vector mosquitoes that fed on drug treated dogs20.
With several emerging and remerging vector borne and zoonotic diseases, there is a great interest in ‘One-Health’ efforts being enforced round the globe. This would be particularly relevant in Africa and Asia where humans and animals live in close proximity and the drug based vector control strategy holds promise in such settings30. Moreover, it would be useful in insecticide resistance management plans due to its distinct binding site from that of the known modulators of ionotropic GABA receptors22,31. Though fluralaner appears to be a promising candidate for vector control, there are some limitations that are to be resolved before proceeding further. High cost of fluralaner is a barrier. Another issue is the safety aspects of fluralaner in human beings. It is not licensed for use in human beings. However, no adverse events were reported when fluralaner and afoxolaner were used for treating scabies in human clinical trials32,33.
There are some limitations of the current study. We used chicken blood, not human blood for carrying out the efficacy trials for convenience. Another limitation is that we have carried out the study only in laboratory maintained colonies and not on field caught mosquitoes. The number of survived mosquitoes assessed for life history characteristics were not comparable between treatment and control groups. The lab obtained results may not be exactly replicable in field conditions. Another limitation is that we did not perform direct blood feeding experiments with mosquitoes fed upon animals treated with fluralaner. This experiment is necessary to understand the effect of the drug on mosquitoes in the context of its metabolism in the treated animals.
In conclusion, our study showed significant oral toxic effect of fluralaner in adult vector mosquitoes with additional effect on life history characteristics (reduced fecundity, egg hatch success, larval development and adult emergence). Fluralaner may be a suitable candidate for future drug based mosquito control strategies. It is required to carry out future safety studies of fluralaner in human beings.
Methods
Extraction of fluralaner from the commercial tablet
Fluralaner was extracted from the commercially available tablets for veterinary use (Bravecto®-purchased from local veterinary drug shop; manufactured by Merck Animal Health, Madison, Vienna, Austria) using the method described by Miglianico et al.21. The tablet was crushed into fine powder using a pestle and mortar and then dissolved in a solvent prepared by mixing dichloromethane and methanol in a 1:1 ratio. The whole mixture was then agitated at room temperature for 1 h with a magnetic stirrer and filtered using Whatman® grade 1 filter paper (Sigma-Aldrich, St. Louis, MO, USA). The filtrate was left overnight for evaporation and the concentrated final product was obtained. Stock solution (5 mg/mL) was prepared in dimethyl sulfoxide (DMSO) (HiMedia, Mumbai, India) and aliquots were frozen at − 20 °C. Prior to each experiment, aliquots were dissolved in phosphate buffer saline (PBS) (Sigma-Aldrich, St. Louis, MO, USA) and added to heparinized chicken blood to achieve the desired final drug concentrations required for the mosquito blood feeding experiments.
Vector mosquitoes and colony maintenance
Laboratory reared mosquito species of Ae. aegypti, An. stephensi and Cx. quinquefasciatus were obtained from the rearing and colonization facility at the Indian Council of Medical Research—Vector Control Research Centre (ICMR-VCRC), Puducherry, India. These strains were originally collected from the Union Territory of Puducherry, India and the colonies have been maintained in the insectary since 1975. Cx. quinquefasciatus were collected in 1975 and Ae. aegypti and An. stephensi were collected in 1976. They were maintained at a constant temperature of 27 ± 2 °C and relative humidity of 80 ± 10% in one-foot Barraud cages (30 cm L × 30 cm W × 30 cm H) and provided with 10% sucrose solution.
Laboratory bioassays with fluralaner
To determine the activity range of fluralaner, initially, bioassays were performed to assess the mortality effect at 24 h using wide range concentrations (20–120 ng/mL). Based on the results of these experiments, a narrow range of four drug concentrations were selected for bioassays to determine LC50 (concentration resulting in 50% mortality after taking a blood meal) value at 24 h of post blood feeding. Drug concentrations of 5 ng/mL, 10 ng/mL, 20 ng/mL and 35 ng/mL were selected for the feeding experiments with Ae aegypti; 5 ng/mL, 15 ng/mL, 25 ng/mL and 40 ng/mL for An. stephensi; and 15 ng/mL, 30 ng/mL, 45 ng/mL and 60 ng/mL for Cx. quinquefasciatus respectively (two drug concentration above and below LC50 value of initial bioassays).
Five days old, non-blood fed female mosquitoes from same batch were used for blood feeding experiments. Prior to blood feeding, cotton pads soaked with 10% sucrose solution were removed and the adult mosquitoes were allowed to starve at least for 12 h to improve the feeding rate. Five mL of heparinized chicken blood containing desired concentration of fluralaner was used for feeding the mosquitoes in the treatment group via artificial blood feeding apparatus made of glass fitted with blood mixing rotor and parafilm for about 60–90 min. Treatment group consists of four drug concentrations and each concentration constituted of three technical replicate cages (each with 50 females). 50 adult females from the same batch of mosquitoes fed on chicken blood with only solvent were used as controls. After blood feeding, mosquitoes (fully engorged) were transferred to clean cages, provided with 10% sucrose solution and the mortality effect was observed at 4, 8, 18, 24, 48, 72 and 96 h of post feeding. 48 h (Ae. aegypti, An. stephensi) and 72 h (Cx. quinquefasciatus) of post blood feeding, survived mosquitoes were observed for fecundity. The experiment was repeated three times on different days using fresh batches of mosquitoes and freshly prepared fluralaner concentration as biological replicates.
In continuation to the bioassay experiments for mortality effect of fluralaner, life history characteristics of survived mosquitoes in each drug treatment groups were assessed in comparison to the control groups. Number of survived mosquitoes in each replicate cage varied with the species and also with the drug concentration.
Effect of fluralaner on fecundity, egg hatch success, immature development and adult emergence success
For fecundity assessment of survived Ae. aegypti and An. stephensi, oviposition cups filled with 75–90 mL water and lined with filter paper were introduced into the cages at 48 h of post blood feeding. For Cx. quinquefasciatus, oviposition cups without filter paper were introduced into the cages at 72 h as it takes 3 days to become fully gravid. All the oviposition cups were placed in the center of the cage. To assess the effect of fluralaner in egg hatch success, eggs laid by survived mosquitoes in treatment group were compared with that of eggs laid by mosquitoes in the control group. For hatching experiment of Ae. aegypti and An. stephensi, five hundred eggs counted under microscope were floated in trays (45 cm L × 30 cm W × 10 cm H) filled with water using a fine tip brush. In case of Cx. quinquefasciatus, as eggs were laid in rafts, numbers were slightly more than five hundred. The rafts were carefully transferred to tray using brush without damaging it. Hundred first instar larvae hatched from eggs laid by survived mosquitoes were observed for development up to pupal stage by providing mosquito larval food [yeast (40%) and dog biscuit (60%)] once in 2 days and were compared with the control group. Rearing water was changed every 2 days. Daily mortality was recorded (if any) in both treatment and control group larvae. Similarly fifty pupae from survived mosquitoes were observed for adult emergence by transferring them into containers filled with 3/4th water and covered with mosquito net having hole in middle plugged with cotton. The results were compared with the control group.
Estimation of speed of killing at high concentration
We performed a set of experiments to assess the speed of killing of the mosquitoes when they were fed with a high concentration of fluralaner. In the initial weeks of drug administration in animals, the blood concentration is expected to be higher which may result in rapid killing of mosquitoes within hours of blood feeding. Consequently, a drug concentration level, specifically five times the highest LC50 value (49.82 ng/mL) obtained in the study, was selected to study the speed of killing. For this, 50 females in triplicates were fed at 250 ng/mL and were observed at hourly interval until complete (100%) mortality was noted in the treatment groups. The experiment was replicated three times on different days with fresh batch of mosquitoes.
Statistical analysis
Mean and standard deviation value from three replicates of different concentration were taken to obtain the data, which was further used for various statistical analysis. Mortality data over time were subjected to Probit regression analysis to estimate LC50 and LT50 values. Mean (SD) and Frequency (%) were used to describe summary values. Mann Whitney U test was used to test the difference in fecundity, egg hatch success, immature survival and adult emergence success between the drug treated and control groups. P-value < 0.05 was considered as statistically significant. SPSS 16.0 was used for carrying out Probit analysis whereas, STATA 14.2 software for other statistical analyses.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on request.
References
WHO. Global vector control response 2017–2030. https://www.who.int/publications-detail-redirect/9789241512978 (2017).
WHO. Vector-borne diseases. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (2020).
Foy, B. D., Kobylinski, K. C., Silva, I. M. D., Rasgon, J. L. & Sylla, M. Endectocides for malaria control. Trends Parasitol. 27, 423–428 (2011).
Imbahale, S. S. et al. Mapping the potential use of endectocide-treated cattle to reduce malaria transmission. Sci. Rep. 9, 5826 (2019).
Foley, D. H., Bryan, J. H. & Lawrence, G. W. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans. R. Soc. Trop. Med. Hygiene 94, 625–628 (2000).
Chaccour, C., Lines, J. & Whitty, C. J. M. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: The potential of oral insecticides in malaria control. J. Infect. Dis. 202, 113–116 (2010).
Foy, B. D. et al. Efficacy and risk of harms of repeat ivermectin mass drug administrations for control of malaria (RIMDAMAL): A cluster-randomised trial. The Lancet 393, 1517–1526 (2019).
Slater, H. C. et al. Ivermectin as a novel complementary malaria control tool to reduce incidence and prevalence: A modelling study. Lancet Infect. Dis. 20, 498–508 (2020).
Kreppel, K. S. et al. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci. Rep. 10, 14527 (2020).
Chaccour, C. J. et al. Ivermectin to reduce malaria transmission: A research agenda for a promising new tool for elimination. Malar. J. 12, 153 (2013).
Pooda, H. S. et al. Administration of ivermectin to peridomestic cattle: A promising approach to target the residual transmission of human malaria. Malar. J. 14, 496 (2015).
Ōmura, S. & Crump, A. Ivermectin and malaria control. Malar. J. 16, 172 (2017).
Chaccour, C. & Rabinovich, N. R. Advancing the repurposing of ivermectin for malaria. The Lancet 393, 1480–1481 (2019).
Kilp, S., Ramirez, D., Allan, M. J., Roepke, R. K. & Nuernberger, M. C. Pharmacokinetics of fluralaner in dogs following a single oral or intravenous administration. Parasit. Vectors 7, 85 (2014).
Comas, W. & Armstrong, R. Bravecto (fluralaner) chewable tablets have been thoroughly evaluated in multiple countries and are approved as a safe and effective flea, tick and mite treatment for dogs. Int. J. Environ. Agric. Res. 4, 36–41 (2018).
Kilp, S., Ramirez, D., Allan, M. J. & Roepke, R. K. Comparative pharmacokinetics of fluralaner in dogs and cats following single topical or intravenous administration. Parasit. Vectors 9, 296 (2016).
Gassel, M., Wolf, C., Noack, S., Williams, H. & Ilg, T. The novel isoxazoline ectoparasiticide fluralaner: Selective inhibition of arthropod γ-aminobutyric acid- and l-glutamate-gated chloride channels and insecticidal/acaricidal activity. Insect Biochem. Mol. Biol. 45, 111–124 (2014).
Jiang, S., Tsikolia, M., Bernier, U. & Bloomquist, J. Mosquitocidal activity and mode of action of the isoxazoline fluralaner. IJERPH 14, 154 (2017).
Evans, C. C. et al. Treatment of dogs with Bravecto® (fluralaner) reduces mosquito survival and fecundity. Parasit. Vectors 16, 147 (2023).
Duncan, K., Barrett, A. W., Little, S. E., Sundstrom, K. D. & Guerino, F. Fluralaner (Bravecto®) treatment kills Aedes aegypti after feeding on Dirofilaria immitis-infected dogs. Parasit. Vectors 16, 208 (2023).
Miglianico, M. et al. Repurposing isoxazoline veterinary drugs for control of vector-borne human diseases. Proc. Natl. Acad. Sci. U.S.A. 115, 29 (2018).
Sampaio, V. S. et al. Filling gaps on ivermectin knowledge: Effects on the survival and reproduction of Anopheles aquasalis, a Latin American malaria vector. Malar J 15, 491 (2016).
Alcântara, J. A. et al. Effect of fluralaner on the biology, survival, and reproductive fitness of the neotropical malaria vector Anopheles aquasalis. Malar. J. 22, 337 (2023).
Liu, D. et al. Toxicity and sublethal effects of fluralaner on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Pest. Biochem. Physiol. 152, 8–16 (2018).
Burgess, E. R. et al. Toxicity of fluralaner, a companion animal insecticide, relative to industry-leading agricultural insecticides against resistant and susceptible strains of filth flies. Sci. Rep. 10, 11166 (2020).
González-Morales, M. A. et al. Systemic veterinary drugs for control of the common bed bug, Cimex lectularius, in poultry farms. Parasit. Vectors 15, 431 (2022).
Bastiaens, G. J. H. et al. Duration of the mosquitocidal effect of ivermectin. Malar. World J. 3, 1–5 (2012).
Walther, F. M., Allan, M. J. & Roepke, R. K. Plasma pharmacokinetic profile of fluralaner (Bravecto™) and ivermectin following concurrent administration to dogs. Parasit. Vectors 8, 508 (2015).
Mekuriaw, W. et al. The effect of ivermectin® on fertility, fecundity and mortality of Anopheles arabiensis fed on treated men in Ethiopia. Malar. J. 18, 357 (2019).
Ruiz-Castillo, P., Rist, C., Rabinovich, R. & Chaccour, C. Insecticide-treated livestock: A potential One Health approach to malaria control in Africa. Trends Parasitol. 38, 112–123 (2022).
Wang, Q. et al. Functional analysis reveals ionotropic gaba receptor subunit rdl is a target site of ivermectin and fluralaner in the yellow fever mosquito, Aedes aegypti. Pest. Manage. Sci. 78, 4173–4182 (2022).
Goldust, M. Oral ivermectin vs. oral fluralaner for the treatment of scabies. Abstract 6533. In American Academy of Dermatology Annual Meeting (2018).
Goldust, M. & Alipour, H. Permethrin 5% cream versus oral afoxolaner for the treatment of scabies a prospective, randomized, controlled, clinical trial study. In Dermcoll, 52nd Annual Scientific Meeting (2019).
Acknowledgements
We gratefully acknowledge the technical assistance of Mr. Shrihari Murmu, Senior Technician-2 and Mr. K. Manimaran, Technical Officer-A, ICMR-VCRC for maintenance of mosquito colonies and blood feeding. We thank Mr. M. Sundharesan, Technical Assistant, ICMR-VCRC for his assistance in extraction of active compound from the commercially available tablet.
Funding
We acknowledge the institutional funding (ICMR-Vector Control Research Centre, Puducherry) provided to carry out the study.
Author information
Authors and Affiliations
Contributions
V.S.K., S.C. and H.K.S. contributed in study design. H.K.S., V.S. and S.V. executed the work, wrote the initial draft of the manuscript. N.M. facilitated extraction of active compound. V.B. worked out statistical analysis for the data. V.S.K. and A.K. critically reviewed the final version of manuscript. All authors read and approved the final version of the manuscript. All authors approved and gave their consent to publish the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shah, H.K., Srinivasan, V., Venkatesan, S. et al. Evaluation of the mosquitocidal efficacy of fluralaner, a potential candidate for drug based vector control. Sci Rep 14, 5628 (2024). https://doi.org/10.1038/s41598-024-56053-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56053-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.