Abstract
The management of mosquito resistance to chemical insecticides and the biting behaviour of some species are motivating the search for complementary and/or alternative control methods. The use of plants is increasingly considered as a sustainable biological solution for vector control. The aim of this study was to evaluate the biological effects of the essential oil (EO) of Lippia alba harvested in Abidjan (Côte d’Ivoire) against Anopheles gambiae and Aedes aegypti mosquitoes. Phytochemical compounds were identified by GC–MS. Knockdown and mortality were determined according to the WHO test tube protocol. Contact irritancy was assessed by observing the movement of mosquitoes from a treated WHO tube to a second untreated tube. Non-contact repellency was assessed using a standardised high-throughput screening system (HITSS). Blood meal inhibition was assessed using a membrane feeding assay treated with EO. The EO was identified as the citral chemotype. The EO gave 100% KD60 in both species at a concentration of 1%. Mortalities of 100% were recorded with An. gambiae and Ae. aegypti at concentrations of 1% and 5% respectively. The highest proportions of females escaping during the contact irritancy test were 100% for An. gambiae at 1% concentration and 94% for Ae. aegypti at 2.5% concentration. The 1% concentration produced the highest proportions of repelled mosquitoes in the non-contact repellency tests: 76.8% (An. gambiae) and 68.5% (Ae. aegypti). The blood meal inhibition rate at a dose of 10% was 98.4% in Ae. aegypti but only 15.5% in An. gambiae. The citral chemotype of L. alba EO has promising biological effects in both species that make it a potentially good candidate for its use in mosquito control. The results obtained in this study encourage the further evaluation of L. alba EOs from other localities and of different chemotypes, under laboratory and field conditions.
Similar content being viewed by others
Introduction
Malaria, the most widespread parasitic disease in the world, is the deadliest of the diseases whose parasites are transmitted by mosquitoes1. The morbidity of arboviral diseases such as dengue, chikungunya, yellow fever and Zika virus fever is also a major concern. For example, despite the availability of a vaccine, yellow fever affects 130,000 people and causes 500 deaths each year in the WHO African region alone, where more than 440 million people are at risk2. Control of Anopheles and Aedes mosquitoes, vectors of malaria pathogens and arboviruses respectively, is therefore essential to prevent these vector-borne diseases.
The main methods of vector control adopted by national control programmes and promoted by the WHO are mainly based on chemicals1,2,3. For instance, as part of the fight against malaria vectors, 459 million long-lasting insecticidal nets (LLINs) were distributed worldwide between 2015 and 2017. As for the control of mosquitoes that transmit arboviruses, national programmes use indoor residual spraying (IRS) and spatial spraying of insecticides4,5. Unfortunately, the effectiveness of these tools is getting limited due to the evolution of resistances in most of mosquito vector species to the insecticide molecules recommended for public health use2,4,6,7,8. These resistances are presumed to be due to the use of insecticides in domestic hygiene and public health, and more importantly to the use of the same insecticides types in agricultural production areas9. According to Agossa et al.10, the effectiveness of LLINs decreases considerably in areas of high vector resistance. In addition to the ability of mosquito populations to develop resistance mechanisms, these chemicals can persist in the environment and poison non-target organisms11. Chemical control also changes the behaviour of vectors12. Indoor residual spraying and LLINs are thought to be at the base of the observed switch in Anopheles mosquitoes biting patterns, i.e. from biting majoritarily indoors towards preferentially biting outdoors when human hosts are not protected13,14,15, which may explains the persistence of high residual transmission of malaria in some areas15,16,17. These concerns associated with chemical control are driving research into new and more environmentally friendly vector control alternatives. In this context, several studies have been carried out on the biological activities of natural plant extracts against vector mosquito species18,19,20,21,22,23,24.
Several studies present plant extracts as potential alternatives for mosquito control. For instance, Dua et al.25 showed that a chloroform fraction of fresh Lantana camara (Verbenaceae) flowers prevent 100% of Aedes albopictus bites for 3 h 45 min in the laboratory and 76% for 7 h in the field against mosquitoes of the genus Aedes. Carotol, a major component of the essential oil (hereafter EO) extracted from carrot seeds (Daucus carota sativus L., Apiaceae), has also shown good repellent activity against Aedes aegypti and Anopheles quadrimaculatus26. Artemisia argyi (Asteraceae) EOs collected from seven sites in China have all shown significant repellent activity on Anopheles sinensis27.
Lippia alba (Verbenaceae) is an aromatic medicinal plant native of South America. It is used by local people for its medicinal (e.g. antimalarial, antiviral, digestive, respiratory, sedative and antihypertensive) and culinary properties28,29,30,31. Studies have highlighted its antioxidant, antibacterial, anti-genotoxic, anti-inflammatory and neuro-sedative activities32,33,34,35,36,37. Unlike certain aromatic plants, L. alba has been understudied to evaluate its biological effects on mosquitoes. EOs extracted from leaves and stems harvested in Colombia showed adulticidal activity on Ae. aegypti38. The oil extracted from the whole plant harvested in Bucaramanga (Colombia) showed significant adulticidal activity against Ae. aegypti, in contrast to the EO from leaves harvested in India (Jalukbari, Guwahati, Assam) on adults of Ae. aegypti and Culex quinquefasciatus39,40. L. alba EO could be used as topical repellent40,41,42, although the one evaluated by Castillo et al.39 did not repel Ae. aegypti females. These results prompted the evaluation of this plant species grown in Côte d'Ivoire against Anopheles gambiae and Ae. aegypti, as the phytochemical composition of plants can vary according to their genetic diversity or environmental conditions. This paper presents the phytochemical composition of the EO of L. alba harvested in Abidjan (Côte d'Ivoire) and its adulticidal, repellent and blood meal inhibiting effects on An. gambiae and Ae. aegypti.
Methods
Cultivation of L. alba and extraction of the essential oil
Ornamental L. alba plants were harvested and planted in our experimental field at Nangui Abrogoua University (5° 23ʹ 19ʺ N, 4° 0ʹ 54ʺ W) in February 2021. The species was previously identified at the herbarim of the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire with reference to specimen 009947. Cuttings of 12–17 cm were planted in nursery bags filled with organic substrate from a former poultry farm waste pit. The plants were then watered in the morning and in the evening during one month and then once a day until harvest in May 2021.
The EO was extracted from the leaves by hydro-distillation using a Clevenger43,44,45,46. Five hundred and thirty (530) grams of fresh leaves were placed over 2 L of water in a pressure cooker. 4.6 mL of EO were obtained after two hours of distillation. The EO was stored in an 8 mL shaded bottle at 4 °C.
Mosquitoes
Bioassays were carried out on mosquitoes of the genus Aedes and Anopheles susceptible to chemical insecticides and maintained at the IRD insectarium (27 ± 1 °C and 70 ± 10% relative humidity) in Montpellier: Kisumu strain of An. gambiae from Kenya and SBE strain of Ae. aegypti from Benin. The photoperiod was set at 14 h light and 10 h dark.
GC–MS data acquisition
GC–MS was used to identify the essential oil compounds. 1 µL of a 2% solution of the essential oil diluted in hexane was injected into the Agilent 8860 System chromatograph coupled to the Agilent 5977B GC/MSD mass spectrometer. The oven temperature was initially set at 50 °C for 2 min, before being increased to 110 °C (4 °C/min), 250 °C (8 °C/min) and 310 °C (10 °C/min). The carrier gas was helium at a temperature of 250 °C, a pressure of 7.6522 psi and a total flow rate of 104 mL/min in split mode. The Agilent 122-5032 column (30 m × 250 μm × 0.25 μm) had a constant flow rate of 1 mL/min. The temperature of the mass detector was 300 °C.
Data preprocessing of the GC–MS data
GC–MS data were first analyzed and processed using MzMine 2.53 to visualize the chromatograms and spectra47,48. Then, GC–MS raw data were converted to Analysis Base File (ABF) format using ABF converter (http://www.reifycs.com/AbfConverter/index.html). Next, the converted data were pre-processed using MS-DIAL 4.70 (NSF-JST, Japan), including peak extraction, peak alignment, baseline calibration, deconvolution analysis and peak identification. We selected KovatsRI based on alkanes as a retention index for peak alignment. The deconvoluted spectra in NIST MSP format (GC–MS DB-Public-KovatsRI-VS3) were imported and matched with spectral libraries. Those peaks with an average peak width of 20 scans and minimum peak height above 10,000 amplitudes were selected for peak detection. Peaks with a s-window value of 0.5 and EI spectral cutoff of 5000 amplitudes underwent a deconvolution operation. The identification parameters were set as follows: m/z tolerance of 0.5 Da, retention time tolerance of 0.5 min, EI similarity cutoff value of 70% and identification score cutoff value of 70%. The alignment parameters of retention time tolerance and retention time factor were set to 0.075 min and 0.5, respectively.
Adulticidal tests
The insecticidal activity of the EO of L. alba was evaluated at dilutions of 0.1%, 1%, 2.5% and 5% (v/v). Permethrin (91.8% technical grade) was used as a positive control (0.011 and 0.11 mg/cm2). The EO was diluted in a solution of ethanol plus silicone oil Dow Corning 566 (134 mL ethanol + 66 mL silicone) and permethrin in a solution of acetone plus silicone oil Dow Corning 566 (134 mL acetone + 66 mL silicone). Two milliliters of the different solutions were used to impregnate the filter papers (12 × 15 cm, Whatman®). The tests were conducted with slight modifications to the protocol of Deletre et al.21, at 27 ± 2 °C and 80 ± 10% relative humidity. Two WHO tubes were used, one marked with a red dot for mosquito exposure to impregnated paper and one marked with a green dot containing unimpregnated paper for mosquito holding. A total of 25–30 females aged 3–7 days (post-emergence) were introduced into three holding tubes. After 30 min of acclimatation, the dead mosquitoes, if any, were replaced. The mosquitoes were then transferred to the exposure tubes for 60 min. After exposure time, knocked down mosquitoes (KD60) were counted and all the mosquitoes were transferred back to the holding tubes. Tubes were kept in a climatic chamber (27 ± 2 °C and 80 ± 10% relative humidity) for 24 h. During this time, individuals were provided with a cotton soaked with a 10% honey solution for feeding. The number of dead mosquitoes were checked at 24 h after exposure. For each product and concentration, mosquitoes exposed to papers impregnated with the solvent only served as negative controls. Mortality for treated mosquitoes was corrected by the Abbott formula when mortality in control mosquitoes was between 5 and 20%: (%treatment mortality−%control mortality)/(100−control mortality) × 100.
Contact irritancy tests
The contact irritancy of the EO was evaluated at concentrations of 0.1%, 1%, 2.5% and 5%. The tests were performed in WHO tubes. Two tubes (one marked with a red dot and the other with a green dot) were connected with a slide door unit and placed horizontally on the laboratory bench. Whatman® paper (12 × 15 cm) impregnated with the test solution (2 mL) was placed in the red-dotted tube and unimpregnated paper in the green-dotted tube. The papers were held against the walls of the tubes with metal clips. The device for the negative control consisted of a tube containing a solvent impregnated paper (ethanol and silicone for the essential oil, acetone and silicone for permethrin) and a tube containing unimpregnated paper. Twenty to twenty-four (20–24) females aged 3–7 days after adult emergence were introduced into the tube containing the impregnated paper. After 30 s of acclimatation, the slide unit door was opened and closed after 10 min. Mosquitoes were then killed in a freezer. Mosquitoes present in each tube were then counted and the proportion of passage from treated to untreated tube was determined21,49. Permethrin was evaluated as a positive control. The test was validated when the proportion of escaped mosquitoes in the negative controls was less than 50%. The test was performed three times for each dose. Tests were performed at 27 ± 2 °C and 80 ± 10% relative humidity.
Non-contact repellency tests
The repellent effect of the EO of L. alba was evaluated using a modified High-Throughput Screening System inspired by the methodology proposed in Grieco et al.50 and Deletre et al.21. The system consisted of two cylindrical chambers (treated and untreated) connected by a door. Whatman paper (10 × 30 cm) impregnated with the solution to be tested (3.3 mL) was placed between a rigid transparent film and a mosquito net. The assembly was rolled around a drum so that the transparent film was on the exterior of the armature and the whole was placed in the treated chamber. Unimpregnated paper was placed in the untreated chamber. The treated chamber for the control mosquitoes contained paper impregnated only with solvent (ethanol–silicone oil Dow Corning 566). Twenty to twenty-four fasting females aged 3–7 days (post-emergence) were introduced into the treated chamber. The door was opened after 30 s of acclimatation and closed again after 10 min (Fig. 1). Mosquitoes in each of the chambers were anesthetized with CO2 and then counted. The proportion of passage from the treated to the untreated chamber was then determined. The essential oil was evaluated at 0.1, 1, 2.5 and 5%. The test was performed three times for each dose and was validated when the proportion of escaped mosquitoes in the negative controls was less than 50%. Bioassays were carried out at 27 ± 2 °C and 80 ± 10% relative humidity.
Steps to perform the non-contact repellency test. ① : HITSS metal cylinders, ② : Linking section, ③ : drum, ④ : Rigid transparent film, ⑤ : Insect screen, ⑥ : Whatman paper (30 × 10 cm), ⑦ : Control solution, ⑧ : EO solution, ⑨ : Mosquitoes. (a) Paper impregnation, (b) positioning paper around drum, (c) connecting the cylinders and introducing the mosquitoes into the treated cylinders, (d) opening of the link section doors after 30 s of acclimatation, (e) link section doors closed after 10 min and mosquitoes counted.
Blood feeding inhibition
Solutions of L. alba EO at 1%, 2.5%, 5% and 10% were prepared with absolute ethanol (v/v). The first three solutions were evaluated before the 10% essential oil solution and ethanol was used as a negative control for each test. Glass feeders (Ø = 16.32 mm) were connected to each other by silicon tubing: 12 feeders for the evaluation of the first 3 solutions or 6 feeders for the evaluation of the 10% solution. They were then connected to a water bath equipped with a pump to circulate water through the feeders at 37 °C. The feeders were positioned on the racks at a rate of three feeders per dose. Pre-cut pig gut membranes were placed on the underside of the feeders. Ten (10) µL of the EO solutions were spread on each membrane. The feeders were then turned upside down and 100 µL of rabbit blood was introduced into each feeder. Cups (440 mL) covered with mosquito netting containing 20–29 adult females aged 5–10 days old were placed under each feeder. After one hour, the mosquitoes were removed and stunned in the freezer before counting the blood fed mosquitoes (Fig. 2). The test was validated when the feeding rate in control mosquitoes was at least 75%. The blood meal inhibition rate was defined as: [1−(% fed EO/% fed Ethanol)] × 100. The tests were carried out in 3 replicates at 27 ± 2 °C and 80 ± 10% relative humidity.
Descriptive diagram of the blood meal inhibition test. ①: Water Bath, ② : Feeder ③ : Pipe, ④ : Rack, ⑤ : Rabbit blood, ⑥ : Essential oil solution, ⑦ : Control solution, ⑧ : Mosquitoes, ⑨ : Pig membrane. −: Control line, + : treated line. (a) Setting up the system. (b) Laying of pig membranes on the bottom surfaces of feeders. (c) Impregnation of pig membranes. (d) Introduction of blood into feeders. (e) Place mosquito cups underneath the feeders for 1 h. (f) Count of blood fed and non fed mosquitoes.
Statistical analysis
The data were analysed using R-4.2.1 software51. The proportions of KD60, dead or displaced mosquitoes induced by the essential oil were compared to those induced by the different negative controls using Fisher exact test. The generalized linear mixed model with binomial distribution was used for the analysis of the blood meal inhibition test data. The variables concentrations and cups represented the fixed and random effects respectively. The analysis required the lme4 package52.
Ethics approval and consent to participate
The plant material in this study was used in accordance with the relevant regulations and recommendations.
Results
Identification of EO compounds
The analyses identified 24 compounds, the main ones being geranial (26.79%), neral (19.07%) and geraniol (14.52%) (Fig. 3, Table 1).
Adulticidal tests
The 0.1% EO produced very low KD60 and mortality An. gambiae individuals (7.8% and 4.5% respectively). The mortality recorded at this concentration was not significantly different from the negative control. From the 1% concentration onwards the KD60 and mortality rates were 100% except for the 2.5% which resulted in 97.6% mortality. Permethrin caused 100% KD60 and mortality at 0.011 and 0.11 mg/cm2 (Table 2).
Contrary to the other concentrations, the KD60 and mortality induced by 0.1% EO on Ae. aegypti were not significantly different from the negative control. At 1%, KD60 was 100% but mortality was only 16.4%. The KD60 and mortality rates recorded with the 2.5% solution were 100% and 64.2% respectively. The 5% dose induced 100% KD60 and mortality. Permethrin caused 100% KD60 and mortality at 0.011 and 0.11 mg/cm2 (Table 2).
Contact irritancy tests
The proportions of An. gambiae females escaping from the EO-treated tubes to the untreated tubes were 60.8% and 100% at the 0.1 and 1% doses respectively. These proportions decreased to 79.6% for the 2.5% concentration and 70.4% for the 5% concentration. At concentrations of 0.011 and 0.11 mg/cm2, permethrin caused 23.1 and 48.5% displacement of mosquitoes to untreated tubes, respectively (Table 3).
The 0.1, 1 and 2.5% EO solutions recorded 62.5, 91.5 and 94.1% of Ae. aegypti mosquitoes escaping, respectively. At 5%, the proportion of escaped mosquitoes was 77.4%. Permethrin caused 31.9% displacement of mosquitoes at 0.011 mg/cm2 and 60% displacement at 0.11 mg/cm2 (Table 3).
Non-contact repellency tests
With An. gambiae, the 0.1 and 1% EO solutions repelled 68.1% and 76.8% of mosquitoes respectively, while the 2.5 and 5% solutions produced 43.5% and 26.4% repellency respectively (Table 3).
At 1% EO, the proportion of escaped females was 68.5% for Ae. aegypti mosquitoes. Repellency rates were 33.8% for the 2.5% solution and 11.9% for the 5% solution (Table 3).
Blood meal inhibition
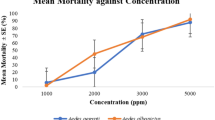
The proportions of blood fed females did not significantly decrease with increasing concentrations in An. gambiae (Tables 4). The EO produced 34.2%, 37.5% and 49% blood feeding inhibition in An. gambiae at concentrations of 1%, 2.5% and 5% respectively. Surprisingly, a significantly lower inhibition (16.7%) was observed with the 10% concentration (Fig. 4).
The mean rates of blood fed Ae. aegypti significantly decreased as concentrations increase (Table 4). The blood feeding inhibition rates increases from 28.4% for the 1% concentration to 98.5% for the 10% concentration (Fig. 4).
Discussion
The management of mosquito resistance to chemical insecticides and the biting behaviour of some species are motivating the search for complementary and/or alternative control methods. We identified the molecules present in the essential oil of L. alba and determined its biological effects (insecticidal, repellent and blood meal inhibition) on An. gambiae and Ae. aegypti. Mainly composed of citral, the EO has been shown to be toxic, irritant and repellent against both species of mosquito. Unexpectedly, the lowest blood meal inhibition rate was recorded with the highest dose of EO with An. gambiae, while this was not observed with Ae. aegypti. These results provide the basis for future work on the impact of L. alba EO formulations for protection against mosquito bites in the field.
The EO evaluated in this study consists mainly of citral (45.86%, geranial and neral being the two isomers of citral) and geraniol (14%). The chemotypes of L. alba EOs, which can be associated with the genetic forms of the plant species, have been characterised according to their main compounds, the variability of which can be related to the morphological characteristics of the leaves and the localities or environmental conditions of production29,53,54,55,56,57,58,59. Hennebelle et al.53 classified the EOs of L. alba into seven chemotypes on the basis of the phytochemical profiles of 4 samples from the French overseas departments and those of 54 other samples reported in 35 publications. This classification can be summarised as follows: (I) citral, linalool, caryophyllene as the major compounds (four subtypes); (II) tagetenone; (III) limonene, carvone in variable amounts, often replaced by related biosynthetic monoterpene ketones (two subtypes); (IV) myrcene; (V) γ-terpinene; (VI) camphor-1,8-cineole and (VII) estragole29.
The insecticidal activity of some EOs may be related to the inhibition of mitochondrial enzymes and acetylcholinesterase (AChE) activities by their compounds60,61. In this study, the EO of L. alba has been shown to be toxic to insecticide susceptible strains of mosquitoes, as it induced 100% KD60 in An. gambiae and Ae. aegypti at doses as low as 1%. Dua et al.18 also obtained 100% KD60 with the EO of L. camara another Verbenaceae mainly composed of caryophyllene, eucalyptol, α-humelene and germacrene-D, at a dose of 4% with Anopheles culicifacies, Anopheles fluviatilis, Anopheles stephensi, Cx. quinquefasciatus and Ae. aegypti. The carvone chemotype of L. alba EO at 0.1% induced 80% mortality in Ae. aegypti adults39, whereas the citral chemotype evaluated in the present study induced 4.5% mortality in An. gambiae and 2.3% in Ae. aegypti at the same dose. This divergence in mortality can be explained either by the higher insecticidal effect of carvone chemotype or by the use of the CDC bottle by Castillo et al.39 and the fact that EO deposited on glass may be more effective than on paper that could adsorb a part of the EO. Significant mortalities were observed in this study from 1% with An. gambiae and 2.5% with Ae. aegypti. Larvicidal activity of several chemotypes of L. alba EO against Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus has also been reported40,62,63,64. Moreover, the carvone and citral chemotypes of L. alba have been shown to be toxic against other insects (Ulomoides dermestoides, Sitophilus zeamais and Tribolium castaneum)65,66 and Rhipicephalus (Boophilus) microplus tick67,68.
The 1% solution of L. alba EO gave 100% KD60 but only 16.4% mortality with Ae. aegypti. These results have also been observed when using intermediate concentrations of EOs as for instance 3–5% solutions of C. nardus, N. cataria and O. americanum which caused 100% KD60 and less than 10% mortality on Ae. aegypti69. The recovery of Ae. aegypti females after knockdown may be explained by natural detoxification mechanisms or by reversible inhibition of receptors in the nervous system as observed with some insecticides, including natural pyrethrins70,71.
In the present study, the 1% concentration induced 76.8 and 68.5% repellency on An. gambiae and Ae. aegypti respectively. The 2.5 and 5% concentrations gave lower repellent rates than those obtained with 1% on both species. As the tests were carried out in hermetically closed cylinders, the 2.5 and 5% concentrations could have started to intoxicate the mosquitoes or impaired their sensory systems. Cutaneous application of the citral chemotype of L. alba EO protects against mosquito bites41. The EO of L. alba, composed mainly of 2, 7 octadione-1-butoxy and 2-isopropenyl-5-methyl hex-4-enol, has a repellent activity, unlike that composed mainly of carvone and limonene39,40. Odour Binding Proteins (OBPs) transport volatile molecules to olfactory receptors which reside in contact with the dendrites of sensory neurons. Citral and limonene showed good docking with OBP1 and OBP22, which have been identified as mediators of olfaction in Ae. aegypti, in addition to acetylcholinesterase72,73. One of the potential mechanisms of action of the repellent compounds in EOs such as Lippia thymoides, Cymbopogon winterianus, Eucalyptus globulus and L. alba is their interaction with OBP141.
The irritant effect observed in the contact repellency test may not only be as a consequence of the intrinsic irritancy of the EO, since a spatial repellency effect was observed for the two mosquito species. In fact, the movement of mosquitoes away from a treated surface without and after contact involves the binding of molecules to specific olfactory receptors on the antennae and specific gustatory receptors on the tarsi, respectively74. These two mechanisms would therefore have resulted in the escape of An. gambiae and Ae. aegypti females from tubes treated with L. alba EO to untreated tubes during contact irritancy tests. This irritancy increased from 60.8 to 100% from 0.1 to 1% concentrations, but fell to 79.6% and 70.4% respectively at 2.5% and 5% on An. gambiae. In Ae. aegypti, the proportion of escaped females was 77.4% with the 5% solution, compared to 94.1% with 2.5%. As the EO showed a neurotoxic effect, the apparent reduction of irritancy are likely to be due to some mosquitoes starting to be intoxicated or being knocked down during the 10 min the slide unit was opened. Reduced irritability caused by a knock down effect has already been observed with DEET and IR3535 against Ae. aegypti75.
The proportions of blood fed An. gambiae ranged from 34 to 49% with 1–5% concentrations of L. alba EO. Wangrawa et al.76 obtained lower proportions of blood fed females using tunnel tests: 20.5% with 1.5% L. camara, 30.3% with 3% Ocimum canum, 35.3% with 2.5% Hyptis spicigera and 19.5% with 2% Hyptis suaveolens. This may be due to the fact that tunnel tests not only measure the direct blood feeding inhibition but also the combined effects of mortality and excito-repellency when mosquitoes are exposed to a treated netting. The highest rate of blood fed females (76.6%) was observed with the 10% concentration of L. alba EO on An. gambiae in the present study. This unexpected observation has already been reported by Hodjati and Curtis77, who obtained higher feeding rates with the highest dose of permethrin on susceptible and resistant populations of An. stephensi. This high dose was also associated with the lowest KD and mortality in their study. L. alba EO had a better effect on blood meal inhibition of Ae. aegypti with an inhibition rate of 98.4% for the 10% concentration. The behavioural differences found in both species as well as the unexpectedly high blood feeding rate of An. gambiae at high concentration will necessitate further investigations.
Conclusion
The investigations carried out showed that the EO of L. alba had very promising knockdown, toxicity, irritant and repellent effects as well as a blood feeding inhibition on An. gambiae and Ae. aegyti. It is clearly a good candidate for the development of sustainable biological products for mosquito control. To this end, further evaluations under field conditions should be carried out to measure the effect of the extract on mosquito bites and/or pathogen transmission. The results obtained have prompted also an evaluation of the biological effects of EOs of L. alba harvested in different localities of west Africa against mosquito vectors of pathogens. Finally, it will be interesting to investigate the impact high doses of L. alba EO on odorant receptors of An. gambiae and their consequences on mosquito behaviour.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Darriet, F. Moustiquaires Imprégnées et Résistance des Moustiques Aux Insecticides (IRD éd, 2007).
Organisation Mondiale de la Santé. Cadre de Mise en Œuvre de L’action Mondiale Pour Lutter Contre les Vecteurs Dans la Région Africaine de l’OMS (Organisation Mondiale de la Santé, 2019).
Organisation Mondiale de la Santé. Entomologie du Paludisme et Lutte Antivectorielle Guide du Participant (Organisation Mondiale de la Santé, 2014).
Organisation Mondiale de la Santé. Action Mondiale Pour Lutter Contre les Vecteurs 2017–2030 (Organisation Mondiale de la Santé, 2017).
World Health Organization. World Malaria Report 2017 (World Health Organization, 2017).
Mouchet, J., Pichon, G., Gayral, P. & Hamon, J. Sensibilité et résistance aux insecticides d’Aedes aegypti en Afrique de l’Ouest et méthodes de contrôle de ce vecteur. Bull. World Health Organ. 45(3), 394–404 (1971).
Etang, J. D. Résistance aux insecticides chez Anopheles gambiae Giles, 1902 au Cameroun : mécanismes et perspectives opérationnelles. Univ. Yaoundé I, PhD thesis (2003).
Organisation Mondiale de la Santé. Procédure Pour Tester la Resistance Aux Insecticides Chez les Moustiques Vecteurs du Paludisme Seconde Edition Genève (Organisation Mondiale de la Santé, 2017).
Tia, E. Situation de la résistance d’Anopheles gambiae (Giles, 1902), vecteur majeur du paludisme, aux pyréthrinoïdes dans cinq écosystèmes agricoles de Côte d’Ivoire. Univ. de Cocody, PhD thesis (2008).
Agossa, F. R. et al. Laboratory and field evaluation of the impact of washings on the effectiveness of LifeNet®, Olyset® and PermaNet® 2.0 in two areas, where there is a high level of resistance of Anopheles gambiae to pyrethroids, Benin, West Africa. Malar. J. 13, 193. https://doi.org/10.1186/1475-2875-13-193 (2014).
Zone humide. Les Moustiques en Zone Humide, un Sujet Piquant (Zone Humide Infos, 2016).
Sangbakembi-Ngounou, C. et al. Diurnal biting of malaria mosquitoes in the Central African Republic indicates residual transmission may be “out of control”. PNAS 119, 21. https://doi.org/10.1073/pnas.2104282119 (2022).
Gatton, M. L. et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution 67(4), 1218–1230. https://doi.org/10.1111/evo.12063 (2013).
Sherrard-Smith, E. et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. PNAS 116, 30. https://doi.org/10.1073/pnas.1820646116 (2019).
Carnevale, P. & Manguin, S. Review of issues on residual malaria transmission. J. Infect. Dis. 223(S2), S61-80. https://doi.org/10.1093/infdis/jiab084 (2021).
Killeen, G. F. et al. Going beyond personal protection against mosquito bites to eliminate malaria transmission: Population suppression of malaria vectors that exploit both human and animal blood. BMJ Glob. Health 2, e000198. https://doi.org/10.1136/bmjgh-2016-000198 (2017).
Organisation Mondiale de la Santé. Cadre pour l’élimination du paludisme. (Organisation Mondiale de la Santé, 2017).
Dua, V. K., Pandey, A. C. & Dash, A. P. Adulticidal activity of essential oil of Lantana camara leaves against mosquitoes. Indian J. Med. Res. 131(3), 434–439 (2010).
Govindarajan, M. & Sivakumar, R. Mosquito adulticidal and repellent activities of botanical extracts against malarial vector, Anopheles stephensi Liston (Diptera:Culicidae). Asian Pac. J. Trop. Med. 4(12), 941–947 (2011).
Bossou, A. D. et al. Chemical composition and insecticidal activity of plant essential oils from Benin against Anopheles gambiae (Giles). Parasit. Vect. 6, 337. https://doi.org/10.1186/1756-3305-6-337 (2013).
Deletre, E. et al. Repellent, irritant and toxic effects of 20 plant extracts on adults of the malaria vector Anopheles gambiae mosquito. PLoS One 8(12), e82103. https://doi.org/10.1371/journal.pone.0082103 (2013).
Ajaegbu, E. E., Danga, S. P. Y., Chijoke, I. U. & Okoye, F. B. C. Mosquito adulticidal activity of the leaf extracts of Spondias mombin L. against Aedes aegypti L. and isolation of active principles. J. Vector Borne Dis. 53(1), 17–22 (2016).
Foko, D. G. A., Bobo, N. M. P., Nyegue, M. A. & Tamesse, J. L. Chemical composition and biocide properties of Clausena anisata (Rutaceae) essential oil against developmental stages of the malaria vector Anopheles coluzzii. Am. J. Essent. Oil Nat. Prod. 6(1), 09–15 (2018).
Baz, M. M. et al. Larvicidal and adulticidal effects of some Egyptian oils against Culex pipiens. Sci. Rep. 12, 4406. https://doi.org/10.1038/s41598-022-08223-y (2022).
Dua, V. K., Pandey, A. C., Singh, R., Sharma, V. P. & Subbarao, S. K. Isolation of repellent ingredients from Lantana camara (Verbenaceae) flowers and their repellency against Aedes mosquitoes. J. Appl. Ent. 127, 509–511. https://doi.org/10.1046/j.1439-0418.2003.00789.x (2003).
Ali, A., Radwan, M. M., Wanas, A. S. & Khan, I. A. Repellent activity of carrot seed essential oil and its pure Compound, carotol, against mosquitoes. J. Am. Mosq. Control Assoc. 34(4), 272–280. https://doi.org/10.2987/18-6751.1 (2018).
Luo, D.-Y., Yan, Z.-T., Che, L.-R., Zhu, J. J. & Chen, B. Repellency and insecticidal activity of seven Mugwort (Artemisia argyi) essential oils against the malaria vector Anopheles sinensis. Sci. Rep. 12, 5337. https://doi.org/10.1038/s41598-022-09190-0 (2022).
Girón, L. M., Freire, V., Alonzo, A. & Cáceres, A. Ethnobotanical survey of the medicinal flora used by the caraibs of Guatemala. J. Ethnopharmacol. 34(2–3), 173–187. https://doi.org/10.1016/0378-8741(91)90035-C (1991).
Hennebelle, T., Sanpaz, S., Joseph, H. & Bauilleul, F. Ethnopharmacologiy of Lippia alba. J. Ethnopharmacol. 116(2), 211–222. https://doi.org/10.1016/j.jep.2007.11.044 (2008).
Tareau, M. A., Palisse, M. & Odonne, G. As vivid as a weed. Medicinal and cosmetic plant uses amongst the urban youth in French Guiana. J. Ethnopharmacol. 116(203), 200–213. https://doi.org/10.1016/j.jep.2017.03.031 (2017).
Agra, M. F., Silva, K. N., Basílio, I. J. L. D., De Freitas, P. F. & Barbosa-Filho, J. M. Survey of medicinal plants used in the region Northeast of Brazil. Rev. Bras. Farmacogn. 18(3), 472–508. https://doi.org/10.1590/S0102-695X2008000300023 (2008).
Hennebelle, T., Sahpaz, S., Gressier, B., Joseph, H. & Bailleul, F. Antioxidant and neurosedative properties of polyphenols and iridoids from Lippia alba. Phytother. Res. 22, 256–258. https://doi.org/10.1002/ptr.2266 (2008).
Chies, C. E. et al. Antioxidant effect of Lippia alba (Miller) N. E. Brown. Antioxidants 2, 194–205. https://doi.org/10.3390/antiox2040194 (2013).
Stashenko, E. E., Jaramillo, B. E. & Martínez, J. R. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) N.E. Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A 1025, 93–103. https://doi.org/10.1016/j.chroma.2003.10.058 (2004).
Oliveira, G. T. et al. Phytochemical characterisation and bioprospection for antibacterial and antioxidant activities of Lippia alba Brown ex Britton & Wilson (Verbenaceae). Nat. Prod. Res. 32(6), 723–731. https://doi.org/10.1080/14786419.2017.1335727 (2018).
López, M. A., Stashenko, E. E. & Fuentes, J. L. Chemical composition and antigenotoxic properties of Lippia alba essential oils. Genet. Mol. Biol. 34(3), 479–488. https://doi.org/10.1590/S1415-47572011005000030 (2011).
Haldar, S. et al. In vivo anti-nociceptive and anti-inflammatory activities of Lippia alba. Asian Pac. J. Trop. Dis. 2(2), S667–S670. https://doi.org/10.1016/S2222-1808(12)60241-2 (2012).
Muñoz, V. J. A., Staschenko, E. & Ocampo, D. C. B. Actividad insecticida de aceites esenciales de plantas nativas contra Aedes aegypti (Diptera: Culicidae). Rev. Colomb. Entomol. 40(2), 198–202 (2014).
Castillo, R. M., Stashenko, E. & Duque, J. E. Insecticidal and repellent activity of several plant-derived essential oils against Aedes aegypti. J. Am. Mosq. Control Assoc. 33(1), 25–35. https://doi.org/10.2987/16-6585.1 (2017).
Mahanta, S., Sarma, R. & Khanikor, B. The essential oil of Lippia alba Mill (Lamiales:Verbenaceae) as mosquitocidal and repellent agent against Culex quinquefasciatus Say (Diptera: Culicidae) and Aedes aegypti Linn (Diptera: Culicidae). JoBAZ 80, 64. https://doi.org/10.1186/s41936-019-0132-0 (2019).
de Brito, G. A. et al. Identification of bioactive compounds against Aedes aegypti (Diptera: Culicidae) by bioassays and in Silico assays. Chem. Biodivers. 18, e2100242. https://doi.org/10.1002/cbdv.202100242 (2021).
Jaramillo-Ramirez, G. I., Logan, J. G., Loza-Reyes, E., Stashenko, E. & Moores, G. D. Repellents inhibit P450 enzymes in Stegomyia (Aedes) aegypti. PLoS One 7(11), e48698. https://doi.org/10.1371/journal.pone.0048698 (2012).
Jantan, I. B., Yalvema, M. F., Ahmad, N. W. & Jamal, J. A. Insecticidal activities of the leaf oils of eight Cinnamomum species against Aedes aegypti and Aedes albopictus. Pharm. Biol. 43(6), 526–532. https://doi.org/10.1080/13880200500220771 (2005).
Akono, N. P., Belong, P., Tchoumbougnang, F., Bakwoils, E.-M. & Fankem, H. Composition chimique et effets insecticides des huiles essentielles des feuilles fraîches d’Ocimum canum Sims et d’Ocimum . L sur les adultes d’Anopheles funestus ss, vecteur du paludisme au Cameroun. J. Appl. Biosci. 59, 4340–4348 (2012).
El-Akhal, F., Guemmouh, R., Greche, H. & El Ouali Lalami, A. Valorisation en tant que bioinsecticide de deux huiles essentielles de Citrus sinensis et Citrus aurantium cultivées au centre du Maroc. J. Mater. Environ. Sci. 5(S1), 2319–2324 (2014).
El-Akhal, F., Greche, H., Ouazzani-Chahdi, F., Guemmouh, R. & El-Ouali Lalami, A. Composition chimique et activité larvicide sur Culex pipiens d’huile essentielle de Thymus vulgaris cultivées au Maroc. J. Mater. Environ. Sci. 6(1), 214–219 (2015).
Pluskal, T., Castillo, S., Villar-Briones, A. & Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 11, 395. https://doi.org/10.1186/1471-2105-11-395 (2010).
Katajamaa, M., Miettinen, J. & Orešič, M. MZmine: Toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics 22, 634–636. https://doi.org/10.1093/bioinformatics/btk039 (2006).
Deletre, E., Martin, T., Duménil, C. & Chandre, F. Insecticide resistance modifies mosquito response to DEET and natural repellents. Parasit. Vect. 12, 89. https://doi.org/10.1186/s13071-019-3343-9 (2019).
Grieco, J. P., Achee, N. L., Sardelis, M. R., Chauhan, K. R. & Roberts, D. R. A novel high-throughput screening system to evaluate the behavioral response of adult mosquitoes to chemicals. J. Am. Mosq. Control Assoc. 21(4), 404–411 (2005).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2022).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
Hennebelle, T., Sahpaz, S., Dermont, C., Joseph, H. & Bailleul, F. The essential oil of Lippia alba: Analysis of samples from french overseas departments and review of previous works. Chem. Biodivers. 3, 1116–1125. https://doi.org/10.1002/cbdv.200690113 (2006).
Yamamoto, P. Y. et al. Performance of ginger grass (Lippia alba) for traits related to the production of essential oil. Sci. Agric. Piracicaba Braz. 65(5), 481–489. https://doi.org/10.1590/S0103-90162008000500006 (2008).
Jannuzzi, H. et al. Avaliação agronômica e identificação de quimiotipos de erva cidreira no Distrito Federal. Hortic. bras. 28(4), 412–417. https://doi.org/10.1590/S0102-05362010000400006 (2010).
Viccini, L. F. et al. Citral and linalool content has been correlated to DNA content in Lippia alba (Mill.) NE. Brown (Verbenaceae). Ind. Crops Prod. 59, 14–19. https://doi.org/10.1016/j.indcrop.2014.04.028 (2014).
Junior, A. Q. S. et al. Seasonal and circadian evaluation of a citral-chemotype from Lippia alba essential oil displaying antibacterial activity. Biochem. Syst. Ecol. 85, 35–42. https://doi.org/10.1016/j.bse.2019.05.002 (2019).
Mendoza, J. D. S. et al. Effect of irrigation depth on biomass production and metabolic profile of Lippia alba (linalool chemotype) essential oil. Agric. Water Manag. 262, 107393. https://doi.org/10.1016/j.agwat.2021.107393 (2022).
de Morais, S. M. et al. Biotechnological potential of essential oils from different chemotypes of Lippia alba (Mill.) N.E.Br. ex Britton & P. Wilson. Bol. Latinoam Caribe Plant Med. Aromat. 21(6), 725–736 (2022).
López, M. D. & Pascual-Villalobos, M. J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 31, 284–288. https://doi.org/10.1016/j.indcrop.2009.11.005 (2010).
Duque, J. E. et al. Insecticidal activity of essential oils from American native plants against Aedes aegypti (Diptera: Culicidae): An introduction to their possible mechanism of action. Sci. Rep. 13, 2989. https://doi.org/10.1038/s41598-023-30046-8 (2023).
Ferreira, R. M. A. et al. A herbal oil in water nano-emulsion prepared through an ecofriendly approach affects two tropical disease vectors. Rev. Bras. Farmacogn. 29, 778–784. https://doi.org/10.1016/j.bjp.2019.05.003 (2019).
Vera, S. S. et al. Essential oils with insecticidal activity against larvae of Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 113, 2647–2654. https://doi.org/10.1007/s00436-014-3917-6 (2014).
Sobrinho, A. C. N., de Morais, S. M., Marinho, M. M., de Souza, N. V. & Lima, D. M. Antiviral activity on the Zika virus and larvicidal activity on the Aedes spp. of Lippia alba essential oil and β-caryophyllene. Ind. Crops Prod. 162, 113281. https://doi.org/10.1016/j.indcrop.2021.113281 (2021).
Caballero-Gallardo, K., Fuentes-Lopez, K., Stashenko, E. E. & Olivero-Verbel, J. Chemical composition, repellent action, and toxicity of essential oils from Lippia origanoide, Lippia alba chemotypes, and Pogostemon cablin on adults of Ulomoides dermestoides (Coleoptera: Tenebrionidae). Insects 14(1), 41. https://doi.org/10.3390/insects14010041 (2023).
Peixoto, M. G. et al. Toxicity and repellency of essential oils of Lippia alba chemotypes and their major monoterpenes against stored grain insects. Ind. Crops Prod. 71, 31–36. https://doi.org/10.1016/j.indcrop.2015.03.084 (2015).
Peixoto, M. G. et al. Acaricidal activity of essential oils from Lippia alba genotypes and its major components carvone, limonene, and citral against Rhipicephalus microplus. Vet. Parasitol. 210, 118–122. https://doi.org/10.1016/j.vetpar.2015.03.010 (2015).
Chagas, A. C. S. et al. Efficacy of 11 Brazilian essential oils on lethality of the cattle tick Rhipicephalus (Boophilus) microplus. Ticks Tick Borne Dis. 7, 427–432. https://doi.org/10.1016/j.ttbdis.2016.01.001 (2016).
Sathantriphop, S., Achee, N. L., Sanguanpong, U. & Chareonviriyaphap, T. The effects of plant essential oils on escape response and mortality rate of Aedes aegypti and Anopheles minimus. J. Vector Ecol. 40(2), 318–326. https://doi.org/10.1111/jvec.12170 (2015).
Georghiou, G. P. Insecticide knockdown and recovery in mosquitoes and its possible significance in control. Mosq. News 22(3), 260–263 (1962).
Duchon, S., Bonnet, J., Marcombe, S., Zaim, M. & Corbel, V. Pyrethrum: A mixture of natural pyrethrins has potential for malaria vector control. J. Med. Entomol. 46(3), 516–522. https://doi.org/10.1603/033.046.0316 (2009).
Leite, N. R. et al. Structure of an odorant-binding protein from the mosquito Aedes aegypti suggests a binding pocket covered by a pH-sensitive ‘“Lid”’. PLoS One 4(11), e8006. https://doi.org/10.1371/journal.pone.0008006 (2009).
Adhikari, K., Sarma, R., Rabha, B. & Khanikor, B. Repellent activity of Citrus essential oils and two constituent compounds against Aedes aegypti. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 92(3), 621–628. https://doi.org/10.1007/s40011-022-01347-1 (2022).
Deletre, E. et al. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 26, 127–142. https://doi.org/10.1007/s00049-016-0214-0 (2016).
Licciardi, S., Herve, J. P., Darriet, F., Hougard, J.-M. & Corbel, V. Lethal and behavioural effects of three synthetic repellents (DEET, IR3535 et KBR 3023) on Aedes aegypti mosquitoes in laboratory assays. Med. Vet. Entomol. 20(3), 288–293. https://doi.org/10.1111/j.1365-2915.2006.00630.x (2006).
Wangrawa, D. W. et al. Larvicidal, oviposition-deterrence, and excito-repellency activities of four essential oils: An eco-friendly tool against malaria vectors Anopheles coluzzii and Anopheles gambiae (Diptera: Culicidae). Int. J Trop. Insect Sci. 41, 1771–1781. https://doi.org/10.1007/s42690-020-00390-7 (2021).
Hodjati, M. H. & Curtis, C. F. Dosage differential effects of permethrin impregnated into bednets on pyrethroid resistant and susceptible genotypes of the mosquito Anopheles stephensi. Med. Vet. Entomol. 11, 368–372. https://doi.org/10.1111/j.1365-2915.1997.tb00424.x (1997).
Acknowledgements
The authors would like to thank the French Embassy in Côte d'Ivoire for the award of a PhD scholarship and Professor Koudou G. Benjamin, Director of Research and Development at CSRS, for his financial and material support for this project. Co-funded by the European Union under the grant No 101086257 (INOVEC Project). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Executive Agency (REA). Neither the European Union nor the European Research Executive Agency (REA) can be held responsible for them. The authors also thank Dr Any Georges Moyabi for his technical assistance in the cultivation of L. alba. We thank the Vectopole platform (IRD, Montpellier) and Bethsabee Scheid for providing technical support. The Vectopole is a platform of the Vectopole Sud Network (http://www.vectopole-sud.fr/) and is part of the LabEx CeMEB (ANR-10-LABX-04-01).
Funding
This work was funded by the strategic support programme for scientific research in Côte d'Ivoire (PASRES_Project N°250), the IRD through MIVEGEC and PHARMADEV Research Units and the European Union under the grant No 101086257 (INOVEC Project).
Author information
Authors and Affiliations
Contributions
F.H.C., A.A. planted the L. alba plants and extracted the EO. C.G. provided the mosquitoes. MR wrote and contributed to the insecticide and repellent test protocols. D.C. designed the blood meal inhibition test system. F.H.C. carried out the bioassays with the help of MR and AV. F.H.C., M.H., D.C. carried out the phytochemical investigations. F.H.C. analysed the bioassay data and drew the diagrams. F.H.C., M.H., D.C., F.C., M.W.K. wrote the manuscript. M.W.K., F.C. provided the scientific supervision of the study. All authors have read and approved the latest version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coulibaly, F.H., Rossignol, M., Haddad, M. et al. Biological effects of Lippia alba essential oil against Anopheles gambiae and Aedes aegypti. Sci Rep 14, 3508 (2024). https://doi.org/10.1038/s41598-024-52801-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52801-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.