Abstract
Much evidence has accumulated to show that inflammation and nutritional status are associated with the prognosis of patients with various cancers. The present study was designed to explore the prognostic role of the LANR in NPC patients receiving definitive radiotherapy and to construct a nomogram for predicting patient survival. This study retrospectively reviewed 805 NPC patients (604 in the training cohort and 201 in the validation cohort) who received definitive radiotherapy between January 2013 and December 2019. The clinical data and pretreatment laboratory test data, including lymphocyte count, neutrophil count, and serum ALB concentration, were collected for all patients. The LANR was calculated as the albumin × lymphocyte/neutrophil ratio. Patients in the training cohort and validation cohort were categorized into high-LANR and low-LANR groups according to the corresponding cutoff values. The independent prognostic factors for overall survival (OS), progression-free survival (PFS), relapse-free survival (RFS), and metastasis-free survival (MFS) were evaluated by univariate and multivariate Cox regression analyses, and a nomogram was subsequently constructed. The performance of the nomogram was evaluated by the concordance index (C-index) and calibration curve. A low LANR (< 14.3) was independently associated with worse OS, PFS and MFS in NPC patients. A prognostic prediction nomogram was established based on T stage, N stage, Eastern Cooperative Oncology Group (ECOG) score, treatment modality, and LANR and was validated. The C-indices of the nomograms for OS and PFS in the training cohort were 0.729 and 0.72, respectively. The C-indices of the nomograms for OS and PFS in the validation cohort were 0.694 and 0.695, respectively. The calibration curve revealed good consistency between the actual survival and the nomogram prediction. Patients with NPC with low pretreatment LANR had a poor prognosis. The nomogram established on the basis of the LANR was efficient and clinically useful for predicting survival in NPC patients who underwent definitive radiotherapy.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is a common head and neck cancer in southern China and southeast Asia1. Radiotherapy is the pillar of treatment. With the development of radiotherapy and medical treatments, the prognosis of NPC patients, especially locally advanced NPC patients, has significantly improved2. However, approximately 10% of patients will suffer from relapse, and 20% will suffer from metastasis3. The TNM staging system is the most prevalent prognostic system used in clinical practice. However, the TNM staging system is not sufficient for accurate differentiation due to the heterogeneity of patients. Thus, the exploration of additional biomarkers for make personalized and accurate predictions is challenging and serves an unmet clinical need.
Promoting inflammation and deregulating metabolism are widely accepted hallmarks of cancer4. Numerous studies have demonstrated a significant correlation between inflammatory and nutritional biomarkers and patient prognosis in various types of tumors. Blood immune cells, including neutrophils, lymphocytes, monocytes and platelets, are major mediators of the host inflammatory response and could be used as biomarkers of host immune-inflammation status. Several previous studies have demonstrated that a series of indices, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII), are based on blood cell counts and can predict patient prognosis in various cancers, including NPC5,6,7. The serum ALB concentration is the classical biomarker for nutritional status, and previous studies have shown that it is a prognostic factor in various malignancies8,9,10. The LANR is a novel biomarker composed of the serum ALB concentration and the NLR. The LANR is an independent prognostic indicator for patients with resectable colorectal cancer11. However, the prognostic role of the LANR in NPC patients has not yet been reported.
In the present study, we retrospectively analyzed the prognostic value of the LANR and other nutritional and inflammation indices in a cohort of NPC patients. A nomogram was constructed to establish a prediction model for the prognosis of NPC patients.
Methods and materials
Patients
The data from patients diagnosed with NPC who underwent definitive radiotherapy with or without chemotherapy at the Second Xiangya Hospital, Central South University, from January 2013 to December 2019 were retrospectively analyzed. The inclusion criteria were as follows: (1) had histologically diagnosed NPC, (2) had complete clinicopathological data and laboratory test results, and (3) lacked distant metastasis. The exclusion criteria were as follows: (1) incomplete follow-up data; (2) received anticancer therapy before admission; (3) had a history of chronic inflammatory diseases or other malignancies; and (4) had a recent acute infectious disease. All patients recruited were randomly allocated to a training cohort (n = 604) and a validation cohort (n = 201) at a ratio of 3:1. This research was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. The requirement for written informed consent was waived by the ethical committee of the Second Xiangya Hospital, Central South University.
Data collection
The demographic and clinical pathological data and laboratory results were obtained from the hospital medical records system. Data regarding patient age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS) score, T stage, N stage, clinical stage, treatment modality, height, body weight, neutrophils, lymphocytes, and serum ALB were collected. The primary biomarker was calculated as follows: LANR = albumin × lymphocyte/neutrophil. All patients were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system based on enhanced MRI.
Treatment
The treatment of NPC patients was based on international guidelines. All patients were treated with intensity-modulated radiation therapy (IMRT). The gross tumor volume and clinical tumor volume were defined according to previous guidelines12. The prescribed dose was 70 Gy for PGTV, 60 Gy for PTV1 and 54 Gy for PTV2. Radiotherapy was delivered at a dose of 1.82–2.12 Gy daily for 5 days per week. Concurrent chemotherapy (cisplatin or nedaplatin) and targeted therapy (nimotuzumab) were administered according to the stage and tolerability of the patients. Induction chemotherapy (IC) and/or adjuvant chemotherapy (AC) were also given to a proportion of patients according to guideline recommendations and tolerability.
Follow-up
Patients were followed up by telephone review or inpatient and outpatient medical records. The patients were followed up every 3 months in the first year after the end of treatment, every 3 to 6 months in the second to fifth years after the completion of treatment, and annually after 5 years after the completion of treatment. The primary site was evaluated by enhanced MRI, and distant metastasis was evaluated by enhanced CT and SPECT. PET–CT was used in a proportion of patients when enhanced CT and MRI were not sufficient to make a diagnosis. The last follow-up date was April 30, 2023. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or to the last follow-up date. PFS was defined as the time between the date of diagnosis and the date of disease progression or death. Metastasis-free survival (MFS) was defined as the time between the date of diagnosis and the date of distant metastasis. Relapse-free survival (RFS) was defined as the time between the date of diagnosis and the date of locoregional relapse. The major endpoint of the study was OS.
Statistical analysis
SPSS statistical software (version 22.0; SPSS, Inc., Chicago, IL, USA) and R software version 4.1.3 (The R Foundation, Vienna, Austria) were used for the data analysis. Receiver operating characteristic (ROC) curves were used to calculate the cutoff value for the LANR. The Chi-square test was used to analyze the associations between LANR and clinicopathological features. The Kaplan–Meier method was used to calculate survival curves. Multivariate analysis was based on the Cox regression model. A two-sided P value < 0.05 was considered indicative of statistical significance. A nomogram was used to visualize the prognostic strength of the indices for OS and PFS. The C-index was defined as the difference between the predicted values of the Cox model in survival analysis and the true values and was used to evaluate the predictive ability of the model, and calibration curves were generated to evaluate the degree of calibration of the clinical prediction models.
Results
Patient characteristics
A total of 805 NPC patients were enrolled in this retrospective study; 72.1% were male, and 27.9% were female. The median age was 49 years (range 14–83 years). In the whole cohort, 260 (32.3%) patients received concurrent chemoradiation therapy (CCRT), 545 (67.7%) patients received non-CCRT, 109 patients received radiation therapy concurrent with nimotuzumab, and 436 patients received radiation therapy alone. The median OS was 61 months (IQR 44.4–80.5), and the median PFS was 57.7 months (IQR 40.1–78.9). The 3- and 5-year OS rates of the whole cohort were 87.2% (95% CI 85.4–88.8%) and 78.9% (95% CI 76.7–80.4%), respectively. Among all the patients, 83 (10.3%) had local regional recurrence (primary site recurrence and/or cervical lymph node metastasis), 143 (17.8%) had experienced metastasis, and 195 (24.2%) died. The baseline data of the training cohort (n = 604) and validation cohort (n = 201) are presented in Table 1. The distributions of these baseline characteristics did not significantly differ between the two cohorts (all P > 0.05).
Identification of independent prognostic factors
By setting overall survival status as the endpoint, we used ROC analysis to determine the cutoff value of the LANR. The optimal cutoff value of the LANR was 14.3, with a sensitivity of 73.7% and specificity of 65.1% (Fig. S1). The associations between LANR and the clinicopathological characteristics of the patients are shown in Table 2. LANR status was not associated with any clinicopathological characteristic in the training cohort (all P > 0.05). A low LANR was associated with a higher T stage in the validation cohort (P = 0.028).
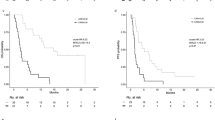
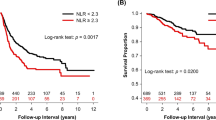
K‒M analysis and the log-rank test showed that the LANR was significantly associated with OS, PFS, and MFS (Fig. 1). In addition to the LANR, age, T stage, N stage, and clinical stage were associated with patient OS in the training cohort (Fig. 2A). T stage, N stage, and clinical stage were associated with patient PFS (Fig. 2B). T stage, N stage, and treatment modality were associated with patient MFS. (Fig. 2C).
The variables that were significantly correlated with patient prognosis according to univariate analysis were incorporated into multivariate analysis. Cox regression analysis revealed that LANR (HR 0.650, 95% CI 0.460–0.919, P = 0.015), age (HR 1.700, 95% CI 1.122–2.575, P = 0.012), T stage (HR 1.828, 95% CI 1.265–2.642, P = 0.001), and N stage (HR 2.228, 95% CI 1.239–4.009, P = 0.008) were independent prognostic factors for OS (Fig. 2A). The LANR (HR 0.613, 95% CI 0.446–0.843, P = 0.003), T stage (HR 1.846, 95% CI 1.314–2.595, P < 0.001), and N stage (HR 1.999, 95% CI 1.183–3.380, P = 0.010) were independent prognostic factors of PFS (Fig. 2B). LANR (HR 0.515, 95% CI 0.348–0.763, P < B0.001), T stage (HR 1.780, 95% CI 1.169–2.711, P = 0.007), N stage (HR 2.633, 95% CI 1.270–5.462, P = 0.009), and treatment modality (HR 0.503, 95% CI 0.308–0.823, P = 0.006) were independent prognostic factors for MFS (Fig. 2C). The results found in the training cohort were further proven in the validation cohort (Fig. 3).
Nomogram construction and validation
The nomogram models of the training cohort were established based on the variables that were significantly correlated with OS and PFS (Fig. 4A,B). The area under the curve (AUC) values for 3-, 5- and 7-year OS were 0.698, 0.658, and 0.671, respectively (Fig. 4C). The area under the curve (AUC) values for 3-, 5- and 7-year PFS were 0.718, 0.658, and 0.687, respectively (Fig. 4D). The C-indices of the nomogram models for OS and PFS were 0.729 and 0.72, respectively. The calibration plots and DCA curves for the probability of OS (Fig. 5A) and PFS (Fig. 5B) showed excellent consistency between the nomogram model predictions and actual observations. The nomogram models were further validated in the validation cohort. The models also exhibited excellent predictive ability, with C-indices of 0.694 and 0.695 (Fig. 6).
Nomograms for predicting 3-, 5-, and 7-year survival probabilities in the training cohort of NPC patients. (A) Nomogram incorporating the LANR and other clinicopathological characteristics for OS prediction in the training cohort; (B) nomogram incorporating the LANR and other clinicopathological characteristics for PFS prediction in the training cohort; (C) ROC curves for the nomogram for predicting OS in the training cohort; (D) ROC curves for the nomogram for predicting PFS in the training cohort. OS overall survival, PFS progression-free survival.
Nomograms, calibration curves and DCA curves in the validation cohort of NPC patients. (A) Nomogram incorporating the LANR and other clinicopathological characteristics for OS prediction in the validation cohort; (B) nomogram incorporating the LANR and other clinicopathological characteristics for PFS prediction in the validation cohort; (C) ROC curves, calibration curves and DCA curves for the nomogram for predicting OS in the validation cohort; (D) ROC curves, calibration curves and DCA curves for the nomogram for predicting PFS in the validation cohort. DCA decision curve analysis, ROC receiver operating characteristic.
Discussion
In the present study, we retrospectively analyzed the prognostic value of a novel immune-nutritional index (LANR) and other clinical parameters in a cohort of 805 NPC patients and demonstrated that the LANR is an independent prognostic factor for OS, PFS and MFS. Furthermore, a nomogram combining the LANR and other independent prognostic factors (T stage, N stage, Eastern Cooperative Oncology Group [ECOG] score and treatment modality) was established, and the C-index and calibration curve showed robust predictive performance and good prognostic accuracy in both the training and validation cohorts.
The TNM staging system is the most widely used prognostic system for NPC patients. However, the mere use of TNM staging is not sufficient for stratifying patients due to the heterogeneity of cancer and the host. Therefore, identifying biomarkers to facilitate more accurate patient stratification is an unmet clinical need. Systemic inflammation is widely accepted as a hallmark of cancer4 and plays important roles in the development and progression of cancer cells as well as the prognosis of cancer patients. Lymphocytes are the main mediators of host antitumor immunity. The level of CD8+ T cells infiltrating the microenvironment is considered to represent host antitumor immunity and is correlated with increased sensitivity to immune therapy and improved prognosis13,14. Past reports have shown that lymphopenia induced by treatment, including radiation therapy, chemotherapy and surgery, is associated with a worse prognosis in patients with various cancers15,16,17. Neutrophils are another immune cell involved in antitumor immunity. Neutrophils can inactivate T cells by secreting various cytokines, resulting in tumor immune evasion and progression18,19,20. Moreover, tumor-infiltrated neutrophils can promote tumor angiogenesis21, and tumor-neutrophil crosstalk sustains prolonged tumor activation22. Hence, various blood-based immunity-related biomarkers, including the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and systemic immune-inflammation index (SII), have been reported to be independent prognostic factors in cancer patients23,24,25. An elevated NLR is associated with worse survival in patients with various cancers, including NPC26,27. The underlying mechanism between the NLR and survival is partly mediated by CD3+ T-cell infiltration28.
Malnutrition is a common complication of tumors and is specifically prevalent in head and neck cancers29. The mechanism underlying cancer-related malnutrition includes reduced intake caused by cancer-related symptoms, treatment toxicity, excess catabolism and systemic inflammation. The serum ALB concentration is the most classical index of nutritional status. A decreased level of ALB is correlated with excess systemic inflammation that affects cancer progression30. The role of malnutrition as a prognostic factor for cancer patients has been widely explored. Several clinical studies have demonstrated that the serum ALB concentration is an independent prognostic factor in several malignant tumors31,32,33.
In the present study, we established a novel immune and nutritional index, the LANR, that is more comprehensive than a single immune or nutritional index. The prognostic importance of the LANR has been reported in several clinical studies. Liang et al.11 reported that a low preoperative LANR was a predictor of worse overall and progression-free survival in resectable colorectal cancer patients. Moreover, Takashi Oshima et al. reported that the LANR was an independent prognostic factor for gastric cancer34 and gastroesophageal junction cancer35. In our study, we also demonstrated that the LANR was an independent prognostic factor for OS and PFS in NPC patients. Moreover, we found that the influence of the LANR on NPC patient prognosis mainly relies on distant metastasis, not on local relapse, which indicates that immune and nutritional status mainly affect cancer metastasis and that the underlying mechanism is worth further exploration.
There are several limitations in our study. First, this was a single-center retrospective study, which will inevitably introduce bias to the results. Although we established a validation cohort for internal validation, external validation is lacking, which impairs the robustness and generalizability of the study. Multicenter research is planned for further validation of the results of the present study. Second, several other prognostic factors were not included in the analysis. For example, the EBV-DNA copy number is generally accepted as a prognostic factor in NPC patients. However, a standard examination procedure has not yet been established, which makes the results from different centers incomparable. Due to the different testing methods used at our center and the missing data for a proportion of patients, these patients were not included in our analysis. Therefore, we could not compare EBV-DNA and LANR, but such an investigation would be a worthwhile aspect in future research.
Conclusions
In summary, a low pretreatment LANR is related to a worse prognosis and is an independent factor for OS, PFS and DMFS in NPC patients. Moreover, the NRS-2002 is an effective, convenient, and economical marker that provides a reference for prognostic stratification and new treatment strategies for NPC patients.
Data availability
The raw data supporting the conclusions of this article will be made available by the corresponding author (email address: houtao@csu.edu.cn) without undue reservation.
References
Chen, Y. P. et al. Nasopharyngeal carcinoma. Lancet 394, 64–80 (2019).
Li, J. Y., Chen, Y. P., Li, Y. Q., Liu, N. & Ma, J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol. Cancer 20, 27 (2021).
Zhang, Y. et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N. Engl. J. Med. 381, 1124–1135 (2019).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011).
Zhao, R., Liang, Z., Chen, K. & Zhu, X. Nomogram based on inflammatory biomarkers and nutritional indicators for predicting overall survival in locoregionally advanced nasopharyngeal carcinoma. J. Inflamm. Res. 15, 2971–2981 (2022).
Xiong, Y. et al. Prognostic efficacy of the combination of the pretreatment systemic immune-inflammation index and Epstein–Barr virus DNA status in locally advanced nasopharyngeal carcinoma patients. J. Cancer 12, 2275–2284 (2021).
Feng, Y. et al. Systemic inflammation response index is a predictor of poor survival in locally advanced nasopharyngeal carcinoma: A propensity score matching study. Front. Oncol. 10, 575417 (2020).
Jeng, L. B., Chan, W. L. & Teng, C. F. Prognostic significance of serum albumin level and albumin-based mono- and combination biomarkers in patients with hepatocellular carcinoma. Cancers (Basel) 15, 1005 (2023).
Ji, S. et al. A combined immune prognostic index in esophageal squamous cell carcinoma patients treated with anti-PD-1 therapy. Ther. Adv. Med. Oncol. 15, 17588359231174868 (2023).
Zhang, C. L. et al. Research progress and value of albumin-related inflammatory markers in the prognosis of non-small cell lung cancer: A review of clinical evidence. Ann. Med. 55, 1294–1307 (2023).
Liang, X. et al. The prognostic value of new index (LANR) composed of pre-operative lymphocytes, albumin, and neutrophils in patients with resectable colorectal cancer. Front. Oncol. 11, 610264 (2021).
Li, W. F. et al. Locoregional extension patterns of nasopharyngeal carcinoma and suggestions for clinical target volume delineation. Chin. J. Cancer 31, 579–587 (2012).
Qu, J. et al. Characterization and clinical verification of immune-related genes in hepatocellular carcinoma to aid prognosis evaluation and immunotherapy. BMC Cancer 23, 549 (2023).
Chatziioannou, E. et al. Deep learning-based scoring of tumour-infiltrating lymphocytes is prognostic in primary melanoma and predictive to PD-1 checkpoint inhibition in melanoma metastases. EBioMedicine 93, 104644 (2023).
Qiu, J. et al. Higher radiation dose on immune cells is associated with radiation-induced lymphopenia and worse prognosis in patients with locally advanced esophageal squamous cell carcinoma. Front. Immunol. 14, 1066255 (2023).
Li, S. et al. Severe lymphopenia as a prognostic factor in rectal cancer patients receiving adjuvant chemoradiotherapy: A retrospective study. Sci. Rep. 13, 7566 (2023).
Aizawa, M., Yabusaki, H., Matsuki, A., Bamba, T. & Nakagawa, S. Predictive significance of surgery-induced lymphopenia on the survival after curative resection for locally advanced gastric cancer: A retrospective cohort analysis. World J. Surg. Oncol. 21, 7 (2023).
Tamura, K. et al. Activated neutrophils inhibit chemotactic migration of activated T lymphocytes to CXCL11 by multiple mechanisms. Cell Immunol. 384, 104663 (2023).
Simoncello, F. et al. CXCL5-mediated accumulation of mature neutrophils in lung cancer tissues impairs the differentiation program of anticancer CD8 T cells and limits the efficacy of checkpoint inhibitors. Oncoimmunology 11, 2059876 (2022).
Petersson, J. et al. Bone marrow neutrophils of multiple myeloma patients exhibit myeloid-derived suppressor cell activity. J. Immunol. Res. 2021, 6344344 (2021).
Gao, F. et al. Neutrophils regulate tumor angiogenesis in oral squamous cell carcinoma and the role of Chemerin. Int. Immunopharmacol. 121, 110540 (2023).
Rubenich, D. S. et al. Tumor-neutrophil crosstalk promotes in vitro and in vivo glioblastoma progression. Front. Immunol. 14, 1183465 (2023).
Yi, X. et al. The relationship between inflammatory response markers and the prognosis of non-muscle invasive bladder cancer and the development of a nomogram model. Front. Oncol. 13, 1189086 (2023).
Luo, H. et al. Combination of neutrophil/lymphocyte ratio and albumin/bilirubin grade as a prognostic predictor for hepatocellular carcinoma patients undergoing curative hepatectomy. BMC Gastroenterol. 23, 162 (2023).
Zhang, J. et al. Single and combined use of the platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and systemic immune-inflammation index in gastric cancer diagnosis. Front. Oncol. 13, 1143154 (2023).
Chiang, C. L. et al. Prognostic factors for overall survival in nasopharyngeal cancer and implication for TNM staging by UICC: A systematic review of the literature. Front. Oncol. 11, 703995 (2021).
Zhao, L., Chen, H., Hu, B., Zhang, H. & Lin, Q. Prognostic significance of Ki67 expression and the derived neutrophil-lymphocyte ratio in nasopharyngeal carcinoma. Cancer Manag. Res. 10, 1919–1926 (2018).
He, Q. et al. T-cells infiltration mediates the association between neutrophil/lymphocyte ratio and survival in gastric cancer. Cancer Med. 12, 15893–15902 (2023).
Erul, E. et al. Evaluation of sarcopenia as a prognostic biomarker in locally advanced head and neck squamous cell carcinoma. Biomark. Med. 17, 87–99 (2023).
McGovern, J., Dolan, R. D., Skipworth, R. J., Laird, B. J. & McMillan, D. C. Cancer cachexia: A nutritional or a systemic inflammatory syndrome? Br. J. Cancer 127, 379–382 (2022).
Oymak, E., Guler, O. C. & Onal, C. Prognostic significance of albumin and globulin levels in cervical cancer patients treated with chemoradiotherapy. Int. J. Gynecol. Cancer 33, 19–25 (2023).
Tsai, M. H. et al. Clinical impact of albumin in advanced head and neck cancer patients with free flap reconstruction—A retrospective study. PeerJ 6, e4490 (2018).
Yang, H. et al. Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: A meta-analysis and systematic review. Clin. Otolaryngol. 45, 167–176 (2020).
Onuma, S. et al. Clinical effects of the neutrophil-to-lymphocyte ratio/serum albumin ratio in patients with gastric cancer after gastrectomy. J. Pers. Med. 13, 432 (2023).
Hashimoto, I. et al. Clinical significance of neutrophil-to-lymphocyte ratio/serum albumin ratio in patients with metastatic gastric or gastroesophageal junction cancer administered trifluridine/tipiracil. Anticancer Res. 43, 1689–1697 (2023).
Funding
This work was supported by Natural Science Foundation of Hunan Province, 2022JJ40682, 2021JJ40882, National Natural Science Foundation of China, 81802476, Science and Technology Program of Hunan Province, 2021SK53515.
Author information
Authors and Affiliations
Contributions
S.J.Z.: Conceptualization, methodology, and writing-original draft. J.L.: data collection, and methodology. Z.C. data collection and software. Y.H.-F. and Y.C.-X.: resources, data curation and writing-review and editing. X.L.-L. and C.H.-H.: investigation. T.H.: resources, software, conceptualization, writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, S., Chen, Z., Ling, J. et al. Nomograms based on the lymphocyte–albumin–neutrophil ratio (LANR) for predicting the prognosis of nasopharyngeal carcinoma patients after definitive radiotherapy. Sci Rep 14, 5388 (2024). https://doi.org/10.1038/s41598-024-56043-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56043-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.