Abstract
In this study, we investigated the composition of free amino acids and lactate (Lac) in polychaetes in river estuaries and inner bays using chromatographic techniques. Both l-amino acids and d-amino acids (d-asparagine, d-alanine (d-Ala), d-serine, d-aspartic acid, and d-proline (d-Pro)) were detected, indicating that polychaetes contain some d-amino acids. Some polychaete species exhibited notable amino acid levels, such as glycine in Capitellidae sp. and Thelepus sp., d-Pro in Glycera sp., and β-Ala in Scoletoma nipponica and Scoletoma sp.. High d-Lac levels were detected in Tylorrhynchus osawai and Hediste diadroma, (691 and 797 μmol/100 g-wet, respectively), with the d-form exceeding 98%. T. osawai was dominant in the upper tidal-sensitive zone, wherein other organisms were less abundant because of low salinity (3–8 PSU). Seasonal differences in the concentrations of components in T. osawai were observed, particularly a significant increase in d-Lac in the reproductive period. Notably, the d-Lac concentrations of T. osawai were higher upstream than downstream. Thus, d-Lac might be involved in strategies underlying adaptations to low salinity and reproductive activity. These results suggest that both the d-form of Lac and amino acids may play certain physiological roles in the life of polychaetes.
Similar content being viewed by others
Introduction
Amino acids are crucial for the sustenance of aquatic invertebrates. While l-amino acids are constituents of proteins, d-amino acids, mainly d-alanine (d-Ala), is involved in physiological functions such as osmoregulation in bivalves1 and crustaceans2,3,4, oogenesis in sea urchins5,6, molting of the Kuruma prawn7,8, and induction of tolerance to hypoxia in bivalves9. Among them, as osmoregulation is critical in aquatic invertebrates living in estuarine areas with highly variable salinity, d-Ala storage in the body is indispensable for their life.
Polychaetes are invertebrates inhabiting various locations, including marine, brackish, and freshwater environments and coastal sediments, estuaries, and tidal flats. Polychaetes are a dominant group of benthic organisms10,11. However, the biological mechanisms underlying the life cycle events of polychaetes, such as spawning, growth, and adaptation to a rapidly changing environment remain unknown. Data on the concentration and availability of d-amino acids and l-amino acids in polychaetes are scarce. Similar to other aquatic invertebrates, free d-amino acids may play important physiological and ecological roles in polychaetes inhabiting various environments.
Anaerobic environments are often formed in estuaries and inner bays12. l-Lactate (Lac) is the end product of anaerobic metabolism. Because polychaetes live at the bottom of sediments, which are highly anaerobic, l-Lac accumulates in polychaetes. Additionally, because the opposite enantiomer, d-Lac, is found in mammals and in the marine organism, octopus13,14, we speculated that both l-Lac and d-Lac are found in polychaetes, and their contents and the ratio may be altered ecologically by habitat and/or seasonal differences.
Thus, the purpose of this study was to characterize the concentration of amino acids and Lac in polychaete species living in different habitats, including several sites in Tokyo Bay, where some major rivers flow in, and Mangoku-ura Lagoon, a bag-shaped inner bay where no major river flows in. The obtained data can indicate the characteristics of d- and l-amino acids and Lac included in coastal polychaetes across various species and habitats. The findings are expected to assist in clarifying unexplained mechanisms underlying spawning, growth, and adaptation to a rapidly changing environment in polychaetes in future.
Considering the above background, we first investigated the presence and concentration of free d- and l-amino acids and -Lac in polychaetes inhabiting estuaries and inner bays, where highly environmental changes occur, by using high-performance liquid chromatography (HPLC) connected with mass-spectrometry or fluorescence detection.
Cluster and similarity of percentages (SIMPER) analysis were conducted to evaluate the similarity of concentrations and composition of these components among the polychaetes. By comparing the levels of free d- and l-amino acid and -Lac among 10 species of polychaetes, we found that Tylorrhynchus osawai had a d-Lac concentration approximately 10–100 times higher than that of other polychaetes. Therefore, we focused on T. osawai and conducted two subsequent experiments. First, we collected them “monthly for 5 months” from the Arakawa River that flows into Tokyo Bay and measured free d- and l-amino acid and -Lac concentrations. A hierarchical clustering heatmap analysis was conducted to identify components showing significant seasonal changes. Second, we collected them “at two points with different salinity levels” from the Arakawa River and, consequently, found that several components were significantly altered depending on the salinity of the habitat in T. osawai for the first time.
Methods
Collection and preparation of polychaete homogenates
No special permits were required to perform field research because the study sites were not privately owned or legislatively protected. Protected and/or endangered species were not included in this study.
Ten species of polychaetes were collected at low tide from the intertidal zone by digging into the sediment or mud using a shovel between October 2021 and May 2023 (Fig. 1, Supplementary Fig. S1, and Supplementary Tables S1 and S2). Marphysa sp. E (Eunicidae)15 and Arenicola brasiliensis (Arenicolidae) were collected from the tidal flats of Tokyo Bay, our main study sites. Hediste diadroma (Nereididae) and T. osawai (Nereididae) were collected from the estuary of the Tamagawa River and the tidal reach of the Arakawa River, respectively, both of which flow into the Tokyo Bay. Among the polychaetes examined in this study, T. osawai inhabited the most upstream areas of the tidal-sensitive zone. It is the most dominant polychaete species in these areas. We focused on T. osawai to obtain novel insights into the biochemical properties of polychaetes because they may possess mechanisms of tolerance toward low salinity, which may be related to osmolytes, as they dominate at 10% of the salinity of open seawater (3 PSU). To examine the effects of salinity on the component concentrations and composition of T. osawai, we collected polychaetes from two sites of the Arakawa River (upstream: approximately 16 km from the river mouth, 3 PSU; downstream: 8 km from the mouth, 8 PSU). T. osawai was collected continuously between January and May 2023 from upstream areas to examine the concentration changes from winter to spring to clarify whether the component concentrations and composition of T. osawai are constant or affected by their life cycles (such as growth and reproductive periods) and the season (such as high or low temperatures). Glycera sp. (Glyceridae), Marphysa sp. A (Eunicidae)15, Scoletoma nipponica (Lumbrineridae), Scoletoma sp. (Lumbrineridae), Capitellidae sp. (Capitellidae), and Thelepus sp. (Terebellidae) were mainly collected from the artificial tidal flat and around Setojima Island in the Mangoku-ura Lagoon, northern Japan. Mangoku-ura Lagoon is a bag-shaped inner bay, where the intertidal zone was lost owing to the land subsidence of approximately 80 cm caused during the Great East Japan Earthquake in 201116,17. Therefore, an artificial tidal flat (4 ha) was created at the mouth of the bay in 2016 for clam fishing. Mountain sand mixed with oyster shells was introduced into the tidal flat. The substrate of artificial tidal flats tends to be sandy mud mixed with oysters and clam shells. Setojima Island is located in the inner region of the bay with bottom sediment comprising soft mud. Various organisms, including polychaetes such as Marphysa sp, an asari clam Ruditapes philippinarum, and the Japanese mud shrimp Upogebia major18 inhabit these sites. Although the collection sites along the coast of Tokyo Bay are located around rivers and estuaries, the artificial tidal flat and Setojima Island is located on the Mangoku-ura Lagoon. The Mangoku-ura Lagoon is connected to the open sea by a narrow channel. The artificial tidal flat and Setojima Island are characterized by the absence of a major river inflow.
Collection sites of T. osawai at the Arakawa River. (a) Sampling site in the upstream region (scale bar: 1 m); (b) sampling site in the downstream region (scale bar: 1 m); (c) surface of the sediment and fracture surfaces of burrows of T. osawai (white square). The dug-up sediment is surrounded by a dashed yellow line, and the burrow is inhabited by T. osawai (white arrows). Numerous holes were observed in the surface sediment (red square, scale bar: 5 cm); (d) a magnified view of the region enclosed by the white frame. The white arrows indicate burrows, and the inner regions of the burrows (slightly brown zones) were more oxidative than the surrounding black zone (scale bar: 1 cm); (e) a magnified view of the holes on the sediment surface (red arrows, scale bar: 1 cm).
All polychaetes collected in this study were identifiable at the species level by morphological observations. Incomplete organisms (with bodies that were cut off at the time of collection and lacking heads or tails and only short fragments of which were available) and complete organisms that were challenging to identify were classified as belonging to the higher taxa to the extent that they could be identified.
Fresh living organisms were placed in plastic tubes filled with water collected at the collection sites and transported to the laboratory under refrigeration. For 1–2 d, the collected polychaetes were left in the local water and allowed to swim freely. Subsequently, they were stored at − 80 ℃ after fecal excretion. A piece of approximately 50–100 mg (excluding the head and feces) was cut from a thawed polychaete, placed into a 1.5-mL tube, and weighed. Ice-cold phosphate-buffered saline (PBS, 1.0 mL/100 mg sample) was added, and the sample was minced using a hand homogenizer and centrifuged at 13,200×g for 15 min at 4 ℃. The obtained supernatant was transferred to another 1.5-mL tube and diluted fivefold using PBS. The diluted supernatant (10 µL) was mixed with an organic solvent [CH3CN/CH3OH (1:1, v/v), 130 µL] in a 1.5-mL tube to prevent enzymatic reactions, vortexed for 1 min, and stored at − 80 ℃. Several samples were prepared in the same manner.
Biomass estimation at the Arakawa River
Biomass estimation was employed to investigate the relationships between the salinities and abundances of T. osawai at two sites in the Arakawa River with different salinities. Quadrats (dimension: 25 × 25 cm) were placed at three or four arbitrary points within each study area (approximately 10 × 50 m) up- and downstream in the Arakawa River and photographs were taken from just above each quadrat. The sediment in each quadrat was dug up and sieved. Only T. osawai was collected and counted. Five or six organisms from each quadrat were placed into plastic tubes identical to those described above and transported to the laboratory. Each organism was weighed, and the mean ± standard deviation (SD) was calculated. The number of holes inhabited by T. osawai in each quadrat was counted using the photographs. Based on the number of holes and average weight, the biomass of T. osawai per square meter was calculated.
Amino acid and Lac analysis
The reagents used to prepare and measure the samples are described in the Supplementary Information. The derivatization reagent used in amino acid analysis, (R)-CIMa-OSu, was synthesized in our laboratory as previously reported19.
In amino acid analysis, the frozen sample (diluted supernatant mixed with an organic solvent; kindly refer to the section Collection and preparation of polychaete homogenates) was thawed, derivatized with (R)-CIMa-OSu, purified using solid-phase extraction (SPE), and determined by using LC–MS/MS with minor modifications20.
In d- and l-Lac analysis, the thawed sample was fluorescence-derivatized with 4-nitro-7-piperazino-2,1,3-benzoxadiazole (NBD-PZ), subjected to SPE, and analyzed using column-switching HPLC-fluorescence detection21,22. A detailed explanation of the procedure is described in the Supplementary Information.
For analyzing an individual polychaete, samples were prepared in duplicates and each final solution was analyzed, with the mean taken as the component concentration of the polychaete. The concentrations of amino acids and Lac in each polychaete species are expressed as mean ± SD in µmol/100 g-wet.
Statistical analysis
Cluster analysis was conducted based on the concentrations of amino acids and Lac detected in the extracts of 78 individuals belonging to 10 species to investigate the biochemical characteristics of each polychaete species. The Bray–Curtis similarity index23 was calculated based on the concentrations logarithmically transformed using the natural logarithm functions (ln(x + 1)) of the components. We performed cluster analysis using the group average method. The significance of the differences between groups in the cluster analysis was evaluated using permutational multivariate analysis of variance24. Even if only one individual of a species was collected, or if a species was not collected continuously across seasons, it was still included in the cluster analysis. SIMPER analysis was also performed to evaluate the components that contributed to the separation of the groups by comparing the separated groups based on their similarity.
To investigate the monthly variation in the constituents of T. osawai, a hierarchical clustering heatmap analysis was conducted using the concentrations of amino acids and Lac detected in T. osawai collected in the upstream region of the Arakawa River between January and May 2023. If the peaks of the target compounds were not determined (N.D.), zero was substituted with the limit of detection. One of the three organisms collected in January (as marked with * in Fig. 4) exhibited low similarity to the other two organisms in the cluster analysis described above. Thus, the means of each target compound were obtained for two individuals. The concentration of each component of T. osawai collected from February to May was expressed as a relative value, with the mean concentration of each component of T. osawai collected in January (n = 2) defined as 1.0. Subsequently, these relative values were transformed logarithmically (ln(x + 1)) and used to calculate the Bray–Curtis similarity index23. Next, we performed a hierarchical clustering heatmap analysis using the group average method. These analysis were performed using the R software (version 4.2.1, The R Foundation, Vienna, Austria).
We used the Mann–Whitney U test to compare the concentrations of components between the collection sites of T. osawai, the up- and downstream areas. One-way analysis of variance, followed by the Bonferroni test, was used to compare the concentrations of the components based on the months in which T. osawai was collected. A P-value of < 0.05 indicated statistical significance. These two comparisons were performed using BellCurve for Excel (Social Survey Research Information, Tokyo, Japan). Bar graphs with dot plots were created using Prism 8 (GraphPad Software, Boston, MA, USA).
Results
Chromatograms and mass spectra of the components of polychaete extracts
Primary amino acids may be differentiated from secondary amino acids based on the fragmentation patterns of the mass spectra obtained by utilizing CIMa-OSu as a derivatization reagent19. Five d-amino acids (d-Asn, d-Ala, d-Ser, d-Asp, and d-Pro) were remarkably detected in polychaete extracts. A clear peak representing d-Pro was detected in the chromatogram of the Glycera sp. extract (Fig. 2a). The unique fragmentation pattern (de-PhCH2O product, m/z = 280) of CIMa-OSu coupled with Pro was obtained in the negative ion mode in collision-induced dissociation (CID) mass spectra (Fig. 2b). The fragment [M-H-43]− was not observed in the CID mass spectrum of d-Pro derived from Glycera sp. (Fig. 2b). These mass spectral data indicate the presence of a secondary amino acid (Fig. 2b)19. Extremely large peaks representing d-Ala and β-Ala were detected in the chromatograms of the extracts of T. osawai (Fig. 2c) and Scoletoma sp. (Supplementary Fig. S2a), respectively. Two marked fragments, [M-H-43]− (m/z = 319) and [M-H-194]− (de-CbzNHCH2NHCH3 fragment, m/z = 168), were detected in the negative ion mode (Fig. 2d and Supplementary Fig. S2b). These spectral data (Fig. 2d and Supplementary Fig. S2b) indicate the presence of Ala, which is a primary amino acid19.
Chromatograms and CID mass spectra. (a) Chromatogram of d- and l-Pro within the Glycera sp. extract; (b) negative CID mass spectrum of the CIMa derivative of d-Pro and the fragmentation pattern; (c) chromatogram of β-, d-, and l-Ala in the T. osawai extract; and (d) negative CID mass spectrum of the CIMa derivative of d-Ala and the fragmentation pattern. Chromatograms of d- and l-Lac within the (e) T. osawai extract and the derivatized structure of d-Lac and (f) Scoletoma sp. extract and the derivatized structure of l-Lac.
d,l-Lac in the polychaete extract was fluorescence-derivatized with NBD-PZ and detected using an octadecyl silica column (Supplementary Fig. S3a). d-Lac and l-Lac could be sufficiently separated through column-switching HPLC-fluorescence detection using a chiral column (Supplementary Fig. S3b). In the chromatogram of the T. osawai extract, the peak representing d-Lac was considerably larger than that representing l-Lac (Fig. 2e). The peaks representing d-Lac and l-Lac were detected in the chromatogram of the Scoletoma sp. extract (Fig. 2f). They were considerably smaller than those detected in the extract of T. osawai. Notably, the l-Lac peak was larger than the d-Lac peak.
Concentrations and ratios of the components of polychaete extracts
We calculated and compared the concentrations (mean ± SD) of the components present in the extract of each polychaete species without considering the periods and sites of sample collection to identify the characteristics of polychaete extract components. The concentrations of amino acids that act as osmolytes in various marine organisms25,26 are shown in Fig. 3, while those of the other amino acids are shown in Supplementary Fig. S4-1–4. The ratio of each amino acid to the total amino acids is shown in Supplementary Fig. S5, and the ratios of d-amino acids to d,l-amino acids are shown in Supplementary Fig. S6.
The concentration of glycine (Gly) was in the range 146–18,154 µmol/100 g-wet (Fig. 3a). Capitellidae sp. and Thelepus sp. contained particularly high concentrations of Gly, comprising approximately 80% of their total amino acid contents (Supplementary Fig. S5). d-Pro was detected only in three species of polychaetes: Glycera sp. (1625 µmol/100 g-wet, d% = 94), Marphysa sp. A (168.2 µmol/100 g-wet, d% = 84), and H. diadroma (13.88 µmol/100 g-wet, d% = 2.3, Fig. 3b and Supplementary Fig. S6). Conversely, l-Pro was detected in the extracts of all polychaetes in the range 31–1421 µmol/100 g-wet (Supplementary Fig. S4-2c). The concentrations of d-Ala and l-Ala in the polychaete extracts ranged from N.D. to 351 (Fig. 3c) and from 462 to 1486 µmol/100 g-wet (Supplementary Fig. S4-1c), respectively. The ratio of d-Ala to the total Ala ranged from 0 to 40% (Supplementary Fig. S6b). High concentration of β-Ala was detected in S. nipponica (733 µmol/100 g-wet) and Scoletoma sp. (587 µmol/100 g-wet, Fig. 3d). The d-Lac and l-Lac concentrations in the T. osawai extract were 691 and 14 (d% = 98), respectively, while those in the H. diadroma extract were 797 and 11 µmol/100 g-wet (d% = 99), respectively (Fig. 3e and f and Supplementary Fig. S6f). The d-Lac and l-Lac concentrations in the other polychaetes ranged from 2.00 to 51.3 and from 9.13 to 59.7 µmol/100 g-wet, respectively (Fig. 3e,f), and the ratio of d-Lac to total Lac ranged from 11 to 55% (Supplementary Fig. S6f).
Cluster and SIMPER analysis
We collected 78 polychaetes and divided them into eight groups (Groups A–H) by conducting cluster analysis using the concentrations of the components detected in each organism. The differences among the groups were statistically significant (Fig. 4, P = 0.002). Groups B (T. osawai), C (S. nipponica and Scoletoma sp.), and E (Capitellidae sp. and Thelepus sp.) were relatively well arranged. The results of SIMPER analysis are shown in Supplementary Table S3. A comparison of Groups B and C, between Groups B and E, and between Groups C and E showed that d-Lac in Group B, d-Ala and β-Ala in Group C, and Gly and d-Asn in Group E exhibited high contribution ratios.
Cluster analysis based on the concentrations of components detected in polychaetes. The organism marked with * was excluded from the hierarchical clustering heatmap analysis. See the Statistical analysis section for details. Cluster analysis was performed using the R software (version 4.2.1, https://www.r-project.org/).
Seasonal variations in the components of the T. osawai extracts
Mature T. osawai was present in the upstream region of the Arakawa River between late January and late February 2023. The degree of increase in the proportion of each component in the extract of T. osawai is shown in Fig. 5a. Between February and May, the proportions of l-Orn, l-Asp, and l-Arg increased more markedly than those of the other components. However, the degree of the increase varied considerably in each sampling month. The concentration of d-Lac increased in February and then decreased gradually (Fig. 5b), whereas that of l-Orn increased from February to May (Fig. 5c). The changes in the concentrations of the other components are shown in Supplementary Fig. S7.
(a) Hierarchical clustering heatmap. Horizontal axis: components detected in the extract of T. osawai; vertical axis: T. osawai collected monthly. Hierarchical clustering heatmap analysis was performed using the R software (version 4.2.1, https://www.r-project.org/). Changes in the concentrations of d-Lac (b) and l-Orn (c) in the extracts of T. osawai collected between January and May 2023 (mean ± SD [µmol/100 g-wet]).
Effects of different habitats on the components of the T. osawai extract
The up- and downstream salinities are 3 and 8 PSU in February and May 2023, respectively, i.e., the salinity in the upstream region is 10% of that of seawater. This area is the upper limit of the tidal area. Even the brackish water clam Corbicula japonica, which exhibits a high tolerance toward freshwater, does not inhabit this area. Conversely, the salinity in the downstream region is approximately threefold higher than that in the upstream region. Approximately 21–51 individuals were collected within a 25-cm quadrat upstream, with an average weight of 0.67 ± 0.26 g per individual and an average biomass of 3870 ± 1936 g/m2. Furthermore, 20–39 individuals were collected downstream, with an average weight of 0.36 ± 0.15 g per individual and an average biomass of 2446 ± 368 g/m2 (Supplementary Table S4).
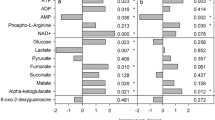
The d- and l-Lac concentrations in the extracts of T. osawai collected upstream tended to be higher than those collected downstream (Fig. 6a,b). Conversely, the concentrations of six amino acids (d-Ala, l-Ala, β-Ala, Gly, l-Asp, and l-Pro) were significantly higher in the samples collected downstream (Fig. 6c–h). Amino acids exhibiting no significant differences in concentration between the samples collected up- and downstream are shown in Supplementary Fig. S8.
Lac and amino acid concentrations in the extracts of T. osawai collected from the up- and downstream regions of the Arakawa River (mean ± SD [µmol/100 g-wet]). Horizontal axis: sampling sites, with Us: upstream region and Ds: downstream region, and the month of sample collection in 2023. The concentrations of Lac and amino acid in each organism are indicated by a dot. d-Lac (a), l-Lac (b), d-Ala (c), l-Ala (d), β-Ala (e), Gly (f), l-Asp (g), and l-Pro (h).
Discussion
This study is the first to reveal the concentrations of d- and l-amino acids in polychaetes inhabiting estuaries and inner bays by LC–MS/MS using an amino group-derivatization reagent, CIMa-OSu19. In contrast to their concentration in bivalves1 and crustaceans2,3,4, there is little information regarding the concentrations and roles of these amino acids in polychaetes. We determined the concentrations of d- and l-Lac in polychaetes owing to the scarcity of available data. Based on these quantitative data on the d- and l-amino acids and d- and l-Lac, we discussed the physiological functions of amino acids and Lac of polychaetes inhabiting areas that exhibit large environmental changes.
Polychaetes contained 27 amino acids, including Gly and five d-amino acids (d-Asn, d-Ala, d-Ser, d-Asp, and d-Pro), which are non-essential amino acids known as osmolytes that can be synthesized by marine organisms (Supplementary Fig. S9)25,26. We detected d-Pro only in three species of polychaetes (H. diadroma, Glycera sp., and Capitellidae sp.). Notably, Glycera sp. contained an extremely high concentration of d-Pro, with a higher d% than those exhibited by other marine organisms1, such as bivalves (6–238 µmol/100 g-wet), shrimps (0.6–3.7 µmol/100 g-wet), and crabs (3.0–12 µmol/100 g-wet). Although in 2004, Hoeger et al. reported high concentrations of β-Ala in Nereis japonica27, β-Ala was present in S. nipponica and Scoletoma sp. The three species of polychaetes, i.e., H. diadroma, Glycera sp., and Capitellidae sp., collected from the same sites (artificial tidal flat and around Setojima Island within the Mangoku-ura Lagoon) during approximately the same period (March to May 2023) contained low concentrations of β-Ala. Therefore, the high concentration of β-Ala in S. nipponica and Scoletoma sp. may not be because of the specificity of the collection site. Instead, the abundant β-Ala content may be attributed to the biochemical characteristics of Scoletoma sp. Perinereis aibuhitensis, a member of the Nereididae family, like T. osawai and H. diadroma, exhibits considerably high concentrations of d-Ala (3810 µmol/100 g-wet)26. Perhaps, P. aibuhitensis prefers areas with high salinity (approximately 20 PSU)28,29,30,31,32,33, as compared to T. osawai and H. diadroma (approximately between 1 and 10 PSU)34,35. Taken together with findings of Abe et al.26, our data suggest that d-Ala concentrations in polychaetes, within the same family, may differ significantly depending on their salinity preferences. Even compared to those of other marine organisms, such as crustaceans and bivalves (d-Ala: 343–2980 µmol/100 g-wet, l-Ala: 554–2358 µmol/100 g-wet, d% = 38–64)25,26, the d-Ala concentrations were lower in the polychaete species investigated in this study. Moreover, T. osawai and H. diadroma contained approximately 13–345-fold higher d-Lac concentrations than those of the other polychaetes. The extremely high d-Lac to d,l-Lac ratio (above 98%, Supplementary Fig. S6f) was not previously reported in a living species. Our observation of the burrows of T. osawai revealed holes opening on the surface of the muddy sediment (Fig. 1c,e) and light brown (oxidized) layers lining the burrows (Fig. 1c,d)36. Similar oxidized layers were also observed in the burrows of H. diadroma. During high tide, these holes supply oxygen through seawater and river water to T. osawai. Therefore, the possibility that only anaerobic metabolism is responsible for the high concentrations of d-Lac cannot be ruled out. In summary, a comparative analysis of 10 polychaete species revealed that some polychaete species have extremely high levels of amino acids and show considerable differences in the concentration and composition of d- and l-amino acids and Lac among species, which are large enough to be reflected in the dendrograms.
The next topic is T. osawai, which is characterized by extremely high concentrations of d-Lac, and we investigated the monthly variation of the d- and l-amino acids and Lac. T. osawai collected between January and May 2023 exhibited monthly variations in its d-Lac concentration, which increased in February and then gradually decreased. Hierarchical clustering heatmap analysis revealed that similar changes were observed in the concentrations of d-Lac, d-Ala, l-Ala, β-Ala, and Gly. T. osawai and H. diadroma exhibit reproductive swarming37,38, i.e., both mature males and females of these two polychaete species swim together at night at high tide, release eggs or sperms into the water, and subsequently die37. From an ecological perspective, T. osawai, which generally lives in burrows, requires considerable energy to get out of the burrow to swim and mate with other individuals. d-Lac and several amino acids may be involved in acquiring and storing energy for their living.
We found a seasonal alteration in l-Orn concentration in T. osawai. The proportion of l-Orn in T. osawai began to increase gradually in February, followed by a notable increase in April. l-Orn is an amino acid associated with the urea cycle39. However, limited data are available on nitrogen metabolism in marine invertebrates, including polychaetes such as T. osawai. Further studies on urea cycle-related components, such as ammonia and urea, should be conducted to obtain further insights into the nitrogen metabolism of T. osawai to determine the presence and seasonal variation of l-Orn in T. osawai.
Next, we focused on the salinity of the habitat of T. osawai. Both the weight of one individual and the amount of biomass per unit area tended to be higher upstream than downstream (Supplementary Table S4). The upstream region away from the mouth of the river, where T. osawai is abundant, has low salinity and is thus unsuitable for many marine organisms. Similar results have been reported by Hanafiah et al.28. The d-Lac concentration of T. osawai found in the upstream region is higher than that occurring in the downstream region, strongly suggesting that d-Lac might have a meaningful role in upstream habitation. Consequently, T. osawai may exhibit a different “osmotic adaptation system,” compared to that demonstrated by other organisms. While amino acids in other marine organisms help adapt to high salinity, d-Lac in T. osawai may be involved in its adaptation to low-salinity conditions. Therefore, we posit that d-Lac in T. osawai is utilized as an energy source for reproduction and is involved in adaptation to low-salinity upstream regions. These characteristics of T. osawai may be responsible for the expansion of its distribution area to specific niches.
We conclusively obtained highly reliable data regarding d-amino acids in polychaetes based on an LC–MS/MS analysis with unique fragmentation19. We found that a polychaete, T. osawai contained notable amounts of d-Lac. Component analysis of amino acids and Lac enantiomers can provide novel perspective regarding the life histories of polychaetes and their responses to environmental variations.
In future studies, we intend to perform exhaustive sampling in different seasons and environments to clarify the characteristics of the distributions of the d-forms and their functions in polychaetes. In addition to increasing the number of polychaete species, investigating changes in the concentrations and compositions of amino acids and Lac, along with the life histories from juvenile to mature, should be highly informative, particularly in elucidating the physiological functions of amino acids and Lac enantiomers in polychaetes.
Data availability
All data analyzed during this study are included in the published article and its Supplementary Information files.
References
Okuma, E., Watanabe, K. & Abe, H. Distribution of free d-amino acids in bivalve mollusks and the effects of physiological conditions on the levels of d- and l-alanine in the tissues of the hard clam, Meretrix lusoria. Fish. Sci. 64, 606–611 (1998).
Fujimori, T. & Abe, H. Physiological roles of free d- and l-alanine in the crayfish Procambarus clarkii with special reference to osmotic and anoxic stress responses. Comp. Biochem. Physiol. A 131, 893–900 (2002).
Okuma, E. & Abe, H. Total d-amino and other free amino acids increase in the muscle of crayfish during seawater acclimation. Comm. Biockem Phvsiol 1, 91–97 (1994).
Abe, H., Okuma, E., Amano, H., Noda, H. & Watanabe, K. Role of free d- and l-alanine in the Japanese mitten crab Eriocheir japonicus to intracellular osmoregulation during downstream spawning migration. Comp. Biochem. Physiol. A 123, 55–59 (1999).
Sato, M. Developmental changes in the content and physiological functions of d-amino acids in invertebrate. Nippon Suisan Gakkaishi 68, 909–910 (2002).
D’Aniello, A., Nardi, G., Cipollaro, M., Pischetola, M. & Padula, L. Occurrence of d-alanine in the eggs and the developing embryo of the sea urchin Paracentrotus lividus. Comp. Biochem. Physiol. B 97, 291–294 (1990).
Yoshikawa, N. Physiological function of free d-alanine in Kuruma Prawn Marsupenaeus japonicus. J. Soc. Jpn. Women Sci. 14, 16–20 (2014).
Yoshikawa, N., Ashida, W., Hamase, K. & Abe, H. HPLC determination of the distribution of d-amino acids and effects of ecdysis on alanine racemase activity in kuruma prawn Marsupenaeus japonicus. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 3283–3288 (2011).
Hochachka, P. W., Hashimoto, K., Abe, H. & Watabe, S. Living without oxygen (Teisanso Tekio No Seikagaku) (in Japanese). Koseisha Koseikaku, (ISBN 4-7699-0517-3) (1984).
Alvarez-Aguilar, A., Van Rensburg, H. & Simon, C. A. Impacts of alien polychaete species in marine ecosystems: A systematic review. J. Mar. Biol. Assoc. UK 102, 3–26 (2022).
Hutchings, P. Biodiversity and functioning of polychaetes in benthic sediments. Biodivers. Conserv. 7, 1133–1145 (1998).
Kodama, K. & Horiguchi, T. Effects of hypoxia on benthic organisms in Tokyo Bay, Japan: A review. Mar. Pollut. Bull. 63, 215–220 (2011).
Ohmori, S. et al. d-Lactate is present in much larger amount than l-lactate in cephalopods and gastropods. Zool. Sci. 14, 429–434 (1997).
Fujisawa, T., Akagi, S., Kawase, M., Yamamoto, M. & Ohmori, S. d-Lactate metabolism in starved Octopus ocellatus. J. Exp. Zool. A Comp. Exp. Biol. 303, 489–496 (2005).
Abe, H., Tanaka, M., Taru, M., Abe, S. & Nishigaki, A. Molecular evidence for the existence of five cryptic species within the Japanese species of Marphysa (Annelida: Eunicidae) known as “Iwa-mushi”. Plankton Benthos Res. 14, 303–314 (2019).
Okoshi, K. The effects of liquefaction, tsunami, and land subsidence on intertidal mollusks following the Great East Japan Earthquake in Ecological impacts of tsunamis on coastal ecosystems: Lessons from the Great East Japan Earthquake (eds. Urabe, J. & Nakashizuka, T.) 165–178 (Springer, 2016).
Okoshi, K. Effects of ground uplift, construction of an artificial tidal flat and tsunami seawalls on marine life and local residents following the 2011 Great East Japan Earthquake in Evolution of marine coastal ecosystems under the pressure of global changes. In Proceedings of Coast Bordeaux Symposium and of the 17th French-Japanese oceanography Symposium (eds. Ceccaldi, H.-J. et al.) 321–333 (Springer Nature, 2020).
Kitabatake, K., Izumi, K., Kondo, N. I. & Okoshi, K. Phylogeography and genetic diversity of the Japanese mud shrimp Upogebia major (Crustacea, Decapoda, Upogebiidae): Natural or anthropogenic dispersal?. ZooKeys 1182, 259–287 (2023).
Sakamoto, T. et al. Development of derivatization reagents bearing chiral 4-imidazolidinone for distinguishing primary amines from other amino acids and application to the liquid chromatography-tandem mass spectrometric analysis of miso. J. Chromatogr. A 1652, 462341 (2021).
Onozato, M., Nakanoue, H., Sakamoto, T., Umino, M. & Fukushima, T. Determination of d- and l-amino acids in garlic foodstuffs by liquid chromatography-tandem mass spectrometry. Molecules 28, 1773 (2023).
Onozato, M. et al. Serum d- and l-lactate, pyruvate and glucose levels in individuals with at-risk mental state and correlations with clinical symptoms. Early Interv. Psychiatry 14, 410–417 (2020).
Hasegawa, H. et al. Determination of serum d-lactic and l-lactic acids in normal subjects and diabetic patients by column-switching HPLC with pre-column fluorescence derivatization. Anal. Bioanal. Chem. 377, 886–891 (2003).
Bray, J. R. & Curtis, J. T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349 (1957).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26, 32–46 (2001).
Abe, H. Distribution of free d-amino acids and their biosyntheses and physiological roles in aquatic animals (in Japanese). Seikagaku 80, 308–315 (2008).
Abe, H. Distribution, metabolism and physiological functions of free d-amino acids in aquatic invertebrates (in Japanese). Vitamins 79, 79–86 (2005).
Hoeger, U. & Abe, H. β-Alanine and other free amino acids during salinity adaptation of the polychaete Nereis japonica. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 137, 161–171 (2004).
Hanafiah, Z., Sato, M., Nakashima, H. & Tosuji, H. Reproductive swarming of sympatric nereidid polychaetes in an estuary of the Omuta-gawa river in Kyushu, Japan, with special reference to simultaneous swarming of two Hediste species. Zool. Sci. 23, 205–217 (2006).
Chen, X. H. et al. First genetic assessment of brackish water polychaete Tylorrhynchus heterochaetus: Mitochondrial COI sequences reveal strong genetic differentiation and population expansion in samples collected from southeast China and north Vietnam. Zool. Res. 41, 61–69 (2020).
Kasahara, S., Tokoshima, S. & Nakamura, N. An ecological study on the swarming of the so-called Japanese palolo in Ashida river. J. Fac. Fish. Anim. Husb. Hiroshima Univ. 11, 79–89 (1972) (in Japanese with English abstract).
Gilbert, F. et al. Sediment reworking by the burrowing polychaete Hediste diversicolor modulated by environmental and biological factors across the temperate North Atlantic. A tribute to Gaston Desrosiers. J. Exp. Mar. Biol. Ecol. 541, 151588 (2021).
Kan, K., Kuroki, Y., Sato, M. & Tosuji, H. Larval recruitment process in the catadromous life history of Hediste diadroma (Nereididae, Annelida) in an estuary in Kagoshima Bay, Southern Japan. Plankton Benthos Res. 15, 30–43 (2020).
Sato, M. & Sattmann, H. Extirpation of Hediste japonica (Izuka, 1908) (Nereididae, Polychaeta) in central Japan, evidenced by a museum historical collection. Zool. Sci. 26, 369–372 (2009).
Fang, J. et al. Selectivity of Perinereis aibuhitensis (Polychaeta, Nereididae) feeding on sediment. Mar. Biol. Res. 14, 478–483 (2018).
Lv, F. et al. Effect of salinity on the growth performance, body composition, antioxidant indexes of Perinereis aibuhitensis and total nitrogen in the substrate. Agric. Sci. 8, 1239–1252 (2017).
Kikuchi, E. Effects of the brackish deposit-feeding polychaetes Notomastus sp. (Capitellidae) and Neanthes japonica (Izuka) (Nereidae) on sedimentary O2 consumption and CO2 production rates. J. Exp. Mar. Biol. Ecol. 114, 15–25 (1987).
Sato, M. Diversity of polychaetes and environments in tidal flats: A study on the Hesiste species group (Nereididae). Fossils 76, 122–133 (2004).
Kikuchi, H. Ecological study on the so-colled Japanese Palolo, Tylorrhychus heterochaetus Quatrefages. Bull. Fac. Lib. Arts Ibaraki Univ. Nat. Sci. 9, 25–37 (1959).
Walker, V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes. Metab. 11, 823–835 (2009).
Acknowledgements
The authors thank members Mr. K. Kitabatake, Mr. R. Fukumura, and Ms. K. Otsuka of the Okoshi Laboratory of Toho University for their assistance with sampling and biomass estimation. We also thank Ms. H. Saito for drawing the map. In addition, the authors would like to thank Editage (http://www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.O., K.O., A.N., and T.F.; Formal analysis: M.O. and W.S.; Resources and investigation: M.O., Y.O., K.O., A.N., T.S., and M.U.; Writing—review and editing: M.O., W.S., Y. O., K.O., A.N., and T.F.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onozato, M., Shinohara, W., Osaka, Y. et al. Characterization of polychaetes inhabiting estuaries and inner bays by composition analysis of amino acids and lactate enantiomers. Sci Rep 14, 5494 (2024). https://doi.org/10.1038/s41598-024-55861-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55861-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.