Abstract
Polychaetes can be successfully employed to recover otherwise wasted nutrients present in particulate organic matter (POM) of aquaculture effluents. The present study describes the fatty acid (FA) profile of four different polychaete species cultured in sand filters supplied with effluent water from a marine fish farm. The FA profile of cultured and wild Hediste diversicolor was compared and revealed a ≈ 24.2% dissimilarity, with cultured biomass displaying a higher content in two essential n-3 highly unsaturated FA (HUFA) (EPA [20:5 n-3] and DHA [22:6 n-3]—eicosapentaenoic and docosahexaenoic acid, respectively). The comparison of the FA profile of cultured H. diversicolor with that of other polychaete species whose larvae successfully settled on the sand filters (Diopatra neapolitana, Sabella cf. pavonina and Terebella lapidaria) revealed that their FA profile, which is here described for the first time, displayed high levels of EPA and DHA (≈ 1.5–4.8 and 1.0–1.1 µg mg−1 DW, respectively). The highest concentration of total FA per biomass of polychaete was recorded in H. diversicolor and T. lapidaria, with both species being the ones whose FA profiles revealed a lowest level of dissimilarity and more closely resembled that of the aquafeed used in the fish farm. In the present work it was demonstrated that it is possible to produce polychaetes biomass with high nutritional value through an eco-design concept such as integrated multi-trophic aquaculture (IMTA). Indeed, this framework promotes a cleaner production and, in this specific case, allowed to recover essential fatty acids that are commonly wasted in aquaculture effluents.
Similar content being viewed by others
Introduction
Aquaculture has grown globally 5.8% per year during the period 2001–2016 and continues to grow faster than any other food production sector1. In 2016, this industry produced 110.1 million tonnes of food fish and aquatic plants with an estimated value of USD 243.3 billion1. It is through the growth and development of this industry that can be possible to supplement human needs in n-3 highly unsaturated fatty acids (HUFA). A dose of 500 mg/day of eicosapentaenoic (20:5 n-3 [EPA]) and docosahexaenoic (22:6 n-3 [DHA]), n-3 HUFA, is recommended to reduce the risk of cardiovascular disease2,3,4,5. Based on this recommended dose to maintain a good cardiac wellness, there is a global requirement of approximately 0.4 million metric tonnes of n-3 HUFA per year5. Our needs in these essential fatty acids (EFA) are due to limitations that vertebrate species exhibit in the de novo synthesis of these molecules due to the lack of desaturases (Δ12 and Δ15) responsible to produce polyunsaturated fatty acids (PUFA) from oleic acid (18:1 n-9), thereby making their inclusion in the aquafeeds essential5,6,7. Marine fish for example incorporate in their tissues with little or no modification the fatty acids (FA) from lower trophic levels and, as such, some species may present well-defined FA signatures depending on their diet6. These EFA are included in formulated aquafeeds to satisfy the needs of cultured species, but especially so that these at the end of a productive cycle exhibit an optimal profile for human nutrition5. Presently, balanced aquafeeds are formulated using fish meal and fish oil (mainly for marine finfish and shrimp), two increasingly scarcer and costly marine based resources1. Their inclusion has been optimized over time and today´s formulas contain less than 10% and 20% of their protein and oil-based composition derived from these sources, respectively8, 9. Nonetheless, to sustain the expected global growth of aquaculture the search for new sources of EFA is of utmost importance.
Polychaete species can play a key-role on this quest for new sources of valuable EFA. These species can uptake nutrients present in aquaculture effluents in the form of particulate organic matter (POM) and, therefore, their culture under integrated multi-trophic aquaculture (IMTA) conditions has gained a growing attention. In marine IMTA systems, extractive organisms act at different trophic levels targeting the recovery of particulate organic matter (POM deposit feeders such as detritivores fish or invertebrates), dissolved organic matter (DOM filter feeders such as invertebrates) and dissolved inorganic nutrients (primary producers such as micro or macroalgae and halophytes)10,11,12,13,14,15,16,17. This concept enables POM-extractive organisms to incorporate otherwise wasted n-3 HUFA contained in the uneaten fraction of aquafeeds supplied to farmed species18,19,20. Indeed, as POM deposit feeders, polychaetes can play an important role in the recovery of these EFA (e.g., EPA [20:5 n-3] and DHA [22:6 n-3]). Species such as Hediste diversicolor6, 15, 21,22,23,24,25,26,27,28, Perinereis nuntia and P. helleri29, Nereis virens30, Abarenicolla pusilla31, Sabella spallanzanii32 and Arenicola marina27 have already been tested as IMTA extractive organisms. The ragworm H. diversicolor in particular revealed a significantly ability to retain valuable HUFA (such as EPA [20:5 n-3] and DHA [22:6 n-3]) from uneaten fish feeds that would otherwise be lost to the environment and negatively impact adjacent aquatic ecosystems6, 23. Some polychaete species evidenced de novo EFA biosynthesize, while their fat content also reflected the fat content of the diet33. These organisms are already known to play a central dietary role on the nutrition and production of some fish and crustacean species (e.g., soles, shrimps and crabs), being often used to trigger gonad maturation and spawning17, 34, 35. The development of production models that include polychaete species appears as an opportunity to meet the growing demand for these worm’s biomass. The potential market to produce for example H. diversicolor in polychaete assisted sand filters (PASF) under IMTA conditions (final productivities: 7000 ind. m−2 – 2300 g fresh weight biomass) was evaluated in approximately 90 € m−2 (if sold as bait)15. Unfortunately, there is no reference value available that may allow us to estimate what would be the tentative price of this DHA-rich polychaetes biomass if it was to be sold frozen (or dehydrated) and free of pathogens for premium aquafeeds formulation (e.g., finishing and breeding diets). The values of global harvest of polychaetes in 2016 (approx. 121,000 tonnes) are comparable to many of the world´s most important fisheries36. It has also already been acknowledged that their collection from the wild is likely insufficient to satisfy the global market demands (either as bait for sports fishing or as feed for aquaculture) and that this practice drives a multitude of negative environmental impacts37. Multiple objectives were target with the development of polychaete production models, such as the reduction of indiscriminate harvesting, reduction of imports of non-native species, development of new aquaculture products and the unravelling of new market and products17, 38, 39.
The present study evaluated the valorisation potential of several polychaete species produced through IMTA, a concept which promotes a cleaner production, as otherwise wasted nutrients can be converted into valuable polychaete biomass. This eco-design concept maximizes and diversifies production and increases efficiency in the use of nutrients, water and energy. Therefore, the first objective of the present study was to identify the FA profile of H. diversicolor stocked in tanks with a sand bed being supplied with an organic rich effluent from earthen ponds used for semi-intensive finfish grow-out and compare it with the FA profile of wild conspecifics. The FA profiles of H. diversicolor stocked in the tanks was also compared with that of the most representative polychaete species whose planktonic larvae successfully settled on the sand beds, namely Diopatra neapolitana, Sabella cf. pavonina and Terebella lapidaria. Finally, the FA profiles of cultured polychaetes were compared to that of the formulated aquafeed provided to the finfish in earthen ponds, in order to identify which species mimicked more closely the FA profile of the aquafeed, hence holding a greater potential to be more readily incorporated in its formulation.
Results
Comparison of fatty acid profiles of wild and IMTA-cultured Hediste diversicolor
The FA profiles of wild and IMTA-cultured H. diversicolor are detailed in Table 1 (FA from microbiome and iso and anteiso are presented in Supplementary Table S1). Significant differences were found between the FA profiles (ANOSIM test; R = 1; p = 0.008), with the SIMPER analysis 50% cut-off (Table 2) revealing an average dissimilarity of 24.2%. The higher content of alpha-linolenic (18:3 n-3 [ALA]), arachidonic (20:4 n-6 [ARA]) and adrenic (22:4 n-6 [AdA]) acids recorded in wild polychaetes biomass contributed greatly for these differences, as well as the higher content of linoleic acid (18:2 n-6 [LA]) and DHA (22:6 n-3) recorded in IMTA-cultured specimens. The 7,10,13-hexadecatrienoic acid (16:3 n-3) and gamma-linolenic acid (18:3 n-6) were identified only in wild polychaetes biomass, while DHA (22:6 n-3) was only identified in cultured polychaetes biomass. In general, IMTA-cultured polychaetes exhibited a FA profile with a higher unsaturated/saturated FA (UFA/SFA) ratio (Fig. 1a). By analysing the HUFA profile, it was also possible to verify that IMTA-cultured polychaetes exhibited a higher n-3/n-6 HUFA ratio, featuring an increment of n-3 HUFA (including EPA and DHA) and a reduction of n-6 HUFA (Fig. 1b,c, respectively).
Fatty acid profile of wild and IMTA-cultured Hediste diversicolor: (a) unsaturated and saturated fatty acids ratio (UFA/SFA); (b) n-3/n-6 highly unsaturated fatty acids ratio (n-3/n-6 HUFA); (c) sum of n-3 and n-6 highly unsaturated fatty acids content (∑n-3 and n-6 HUFA; values in µg mg−1 DW). Average values ± SD (n = 5).
Comparison of fatty acid profiles of different IMTA-cultured polychaete species
The FA profile of IMTA-cultured polychaetes H. diversicolor, D. neapolitana, S. cf. pavonina and T. lapidaria (Fig. 2) are summarized in Table 1. Apart from H. diversicolor, the FA profile of all other polychaete species is here described for the first time. A total of 22, 25 and 28 FA (excluding FA from microbiome and iso and anteiso—Supplementary Table S1) were identified for D. neapolitana, S. cf. pavonina and T. lapidaria, respectively. Significant differences were found between the FA profiles of the four IMTA-cultured polychaete species (ANOSIM test; R = 0.968; p = 0.001), with SIMPER analysis at a cut-off of 50% revealing the FA that most contributed to dissimilarities between them (Table 3). Terebella lapidaria exhibited the FA profile with the lowest dissimilarity for H. diversicolor, followed by S. cf. pavonina and D. neapolitana (Table 3). The polychaetes H. diversicolor and T. lapidaria exhibited the highest concentration of total FA per mg DW biomass. Palmitic (16:0), sum of oleic and vaccenic (18:1 n-9 and n-7), LA (18:2 n-6) and EPA (20:5 n-3) were the SFA, MUFA, PUFA and HUFA (respectively) that revealed the highest content for both polychaete species. The majority of these FA were also the ones most abundant for the other two polychaete species, except stearic (18:0) and 7,13-docosadienoate (22:2 n-9) which were the most abundant SFA and PUFA in the FA profile of D. neapolitana, and 5,13-docosadienoate (22:2 n-9) which was the most abundant PUFA in the FA profile of S. cf. pavonina. The concentration of DHA (22:6 n-3) was similar between the four IMTA-cultured polychaete species (0.99–1.10 µg mg−1 DW). Hediste diversicolor, D. neapolitana and T. lapidaria exhibited similar and higher UFA/SFA ratios (Fig. 3a). When analysing the HUFA profile, it was possible to verify that H. diversicolor and D. neapolitana exhibited the highest n-3/n-6 HUFA ratio (Fig. 3b), while the highest n-3 and n-6 HUFA contents were determined in H. diversicolor and T. lapidaria biomass (Fig. 3c).
Fatty acid profile of different IMTA-cultured polychaetes (Hediste diversicolor, Diopatra neapolitana, Sabella cf. pavonina and Terebella lapidaria): (a) unsaturated and saturated fatty acids ratio (UFA/SFA); (b) n-3/n-6 highly unsaturated fatty acids ratio (n-3/n-6 HUFA); (c) sum of n-3 and n-6 highly unsaturated fatty acids content (∑n-3 and n-6 HUFA; values in µg mg−1 DW). Average values ± SD (n = 5).
Comparison of fatty acid profiles of IMTA-cultured polychaete species and the aquafeed provided to fish in earthen ponds
The FA content of the aquafeed provided to the fish is detailed in Table 1. Palmitic acid (16:0), along with the sum of oleic and vaccenic acid (18:1 n-9 and n-7), LA (18:2 n-6) and DHA (22:6 n-3) were the SFA, MUFA, PUFA and HUFA (respectively) that exhibited the highest levels in the aquafeed.
The most represented UFA class in the aquafeed was MUFA (≈ 60.5% of all UFA), while in polychaetes the sum of PUFA and HUFA accounted for most UFA (53.2% for H. diversicolor, 83.5% for D. neapolitana, 51.4% for S. cf. pavonina and 54.3% for T. lapidaria). The FA profile of the aquafeed exhibited a content of n-3 HUFA (7.94 ± 0.49 µg mg−1 DW) similar to the one reported for H. diversicolor and, to a lesser extent, to the one reported for T. lapidaria. The levels of DHA (22:6 n-3) in the aquafeed per DW biomass was approximately 4-times higher than that recorded in all IMTA-cultured polychaete species. The EPA (20:5 n-3) content of all polychaete species, except S. cf. pavonina, was higher than the one present in the aquafeed. The principal coordinates analysis (PCO) revealed that the FA profiles that more closely resembled that of the aquafeed supplied to the fish being farm in earthen ponds were those of H. diversicolor and T. lapidaria (Fig. 4). The FA profile of D. neapolitana was the less similar to the aquafeed. The two PCO axis explained more than 87% of the variation recorded between samples from different groups.
Principal coordinates analysis (PCO) of common fatty acids present in the aquafeed supplied to fish being farmed and the four IMTA-cultured polychaetes (Hediste diversicolor, Diopatra neapolitana, Sabella cf. pavonina and Terebella lapidaria) (common with at least one of the species). Average values (± SD) (n = 5). ALA alpha-linolenic acid, ARA arachidonic acid, DHA docosahexaenoic acid, DPA docosapentaenoic acid, EPA eicosapentaenoic acid, ETA eicosatetraenoic acid, ETE eicosatrienoic acid, LA linoleic acid.
Discussion
The current scarcity of new sources of n-3 HUFA (mainly EPA and DHA) makes paramount the search for new ingredients from where these EFA can be derived5. Polychaetes are likely in the frontline of alternative sources of EFA that can be explored, namely through their integration in IMTA systems as extractive species to recover these valuable nutrients6, 23, 25, 26, 40.
Hediste diversicolor is well represented in multiple IMTA designs that have already featured its potential to recover nutrients from organic rich effluents6, 15, 23,24,25,26,27,28. The biomass of H. diversicolor, whose FA profile was evaluated in present work, was cultured in PASF installed to filter the effluent water of earthen ponds stocked with gilthead seabream (S. aurata) during 15 weeks27. From an initial inoculum of approximately 400 ind. m−2 a density of approximately 1000 ind. m−2 (2.5-fold increase) was achieved, with PASF contributing to retain with high efficiency the POM present in the aquaculture effluent (approx. 1.8 ± 1 mg L−1)27. The significant differences recorded between the FA profile of IMTA-cultured and wild H. diversicolor (with an overall dissimilarity of 24.2%) were mainly due to shifts in the concentration of common FA (e.g., ALA [18:3 n-3], ARA [20:4 n-6], AdA [22:4 n-6] and LA [18:2 n-6]). This dissimilarity was also due to the presence of less common FA, such as 7,10,13-hexadecatrienoic (16:3 n-3) and gamma-linolenic (18:3 n-6) which were only identified in wild polychaetes, and DHA (22:6 n-3) which was only identified in cultured polychaetes. In general, a total of 35 and 34 FA were identified in wild and IMTA-cultured H. diversicolor (respectively) (27 and 26 if FA from microbiome, Iso and anteiso are excluded). In the present study, it was not possible to conclude that the culture conditions benefit the enrichment of FA profile if evaluated only in terms of total FA content, as the values recorded for wild and IMTA-cultured polychaetes was very similar (≈ 41.6 and 39.8 µg mg−1 DW, respectively). Comparing the results recorded in the present study with previous ones which have characterised the FA profile of IMTA-cultured and wild H. diversicolor (Table 4), it is possible to verify that total FA content was slightly higher to that displayed by polychaetes supplied with the effluent water of a super-intensive farm of S. senegalensis (≈ 37.6 µg mg−1 DW)23, as well as that recorded for conspecifics supplied with processed water from a S. aurata RAS (27.1 µg mg−1 DW)6. On the other side, Wang et al.25 reported a slightly higher FA content (56.9 µg mg−1 DW) in polychaetes filtering the effluent water from a salmon smolt facility. Conversely to our results, these studies reported increases between 30 and 50% in total FA content of cultured organisms in respect to wild conspecifics (24.4, 17.8 and 41.6 µg mg−1 DW, respectively). Pajand et al.24 also reported a higher FA content (109.9 mg g−1 DW) for H. diversicolor that filtered the effluent water of beluga sturgeon (Huso huso), although no comparison was performed with the FA profile of wild conspecifics. Different size classes of H. diversicolor can present different FA profiles (< 30 mm: ≈ 25.4, 30–50 mm: 27.3 and > 50 mm: ≈ 37.6 µg mg−1 DW)15. In the present study the FA characterisation was performed in specimens with a size > 40 mm and differences recorded with the above-mentioned studies could also be due to different maturation stages and not solely a consequence of contrasting culture conditions (environmental and effluent water nutrient load). The higher concentration of MUFA detected in IMTA-cultured biomass, may be likely a consequence of the FA profile exhibited by the main source of nutrients present in effluent water (the aquafeed provided to S. aurata). Pajand et al.24 obtained a similar result with MUFA and HUFA being the most and least represented FA classes, respectively, in H. diversicolor (≈ 39.4% and 4.6% of total FA, respectively) reflecting the formulation of the aquafeed supplied to H. huso (≈ 40.5% and 0.6% of total FA, respectively) (Table 4). Bischoff et al.6 and Marques et al.23 verified that HUFA was the major FA class in IMTA cultured polychaetes (≈ 34% and 37.8% of total FA, respectively) when aquafeeds being supplied to fish displayed a higher proportion of HUFA (24% and 20–28% of total FA, respectively) (Table 4). From H. diversicolor production under the culture conditions tested in the present work, it can be predicted a generation of approximately 39.8 g of total FA per kg DW biomass, of which ≈ 6.6 g corresponded to n-3 HUFA (≈ 4.8 g EPA and 1.0 g DHA). The levels of EPA and DHA measured in IMTA-cultured specimens in the present study differed from the values reported by Marques et al.23, as well as those reported by Pajand et al.24 (Table 4). These differences likely reflect different culture conditions, mainly the intensification of fish culture conditions and different aquafeeds formulation. In the present work, IMTA-cultured polychaetes displayed EPA (20:5 n-3), DHA (22:6 n-3), ALA (18:3 n-3) and ARA (20:4 n-6), with only DHA not being detected in wild conspecifics. Marques et al.23 did not detect ALA in IMTA-cultured specimens, nor DHA in wild H. diversicolor. Bischoff et al.6 reported that wild specimens did not exhibit any detectable levels of DHA, ALA and ARA. These finds are likely explained by the seasonal shifts in the lipid content and FA profile that H. diversicolor is known to display, with maximum level of lipid content being detected in the winter (19.3% DW) and the lowest during the summer (6.6% DW)34.
In this study it was also possible to compare the FA profile of H.diversicolor with that of other polychaete species (D. neapolitana, S. cf. pavonina and T. lapidaria) which adapted to the conditions in PASF and were identified as potential candidates to integrate IMTA designs as extractive species27. The planktonic larvae of the three polychaete species mentioned above successfully colonized the sand beds of PASF, most likely because the substrate of these filters provided the specific cues required for their larvae to settle and metamorphose (e.g., free FA have been suggested to favour the settlement of some species41). To date, the performance of D. neapolitana had never been tested under an IMTA framework. The adults of this species can present sizes ranging between 150 and 500 mm in length, being one of the species most intensively harvested in the coastal lagoon where the present study was performed42. This polychaete reveals an iteroparous reproduction behaviour with a discontinuous reproductive season (spawning: March–July; resting: August–September)43. From the four polychaete species whose FA profiles were evaluated in the present work, it was D. neapolitana that exhibited the lowest content of total FA with the n-3/n-6 HUFA ratio being similar to that of H. diversicolor. Despite this similarity, in overall, D. neapolitana was the species whose FA profile showed a greater dissimilarity to that of H. diversicolor. An analysis of D. neapolitana productivity in terms of FA profile allowed us to estimate the generation of approximately 12.5 g of total FA per Kg DW biomass produced, of which approximately 40% corresponded to n-3 HUFA (including EPA and DHA). As the FA profile of wild specimens of D. neapolitana has never been determined, it is impossible to verify if IMTA conditions enhance their value in EFA. Previous studies showed that this species reveals a lower capacity to grow in highly organic enriched areas44, a fact that may constraint its use in more intensive IMTA systems. The development of sustainable production models for D. neapolitana is paramount43, as the level of exploitation (eventually even overexploitation) and inherent digging activity may result in an enhanced biodiversity loss in the benthos42. In terms of bioremediation, it must be highlighted that these organisms are ecosystem engineers that stabilise the sediment with the tubes they secrete and thus increase the structural complexity and biodiversity of their habitat43, 45, 46, a feature that may contribute for a less pronounced bioturbation. For this reason, this species is likely less suitable to promote safeguard the functionality of PASF tested in present work, as these required complete percolation of water through the substrate27.
The species S. cf. pavonina inhabits the tubes that the worm secretes, and it feeds by using crowns of ciliated filaments on their heads47. This polychaete can achieve a size of 270 mm, with an additional 45 mm of its branchial crown48, 49. It displays a filter feeding behaviour and is a gonochoristic broadcaster that displays an annual reproductive cycle (spawning period in May/June)49. There is no evidence of this species having ever been included in IMTA designs as an extractive species. The total FA content detected in this polychaete species was lower than that of H. diversicolor, being also the species which exhibited the lowest n-3/n-6 HUFA ratio. Sabella cf. pavonina exhibited a FA profile slightly more similar to that of H. diversicolor than the one observed for D. neapolitana. An analysis of S. cf. pavonina productivity in terms of FA profile allowed us to estimate the generation of approximately 25.2 g of total FA per Kg DW biomass produced, of which approximately 10% corresponded to n-3 HUFA (including EPA and DHA). It is known that this species can filter more than 70 L of seawater per hour50. However, no major enhancement of bioturbation in PASF could be perceived for this tubiculous polychaete, which, like D. neapolitana and for the same reasons, does not appear to be a species indicated to promote the functionality of PASF.
Concerning the polychaete T. lapidaria, this species can achieve a size of 100 mm51 and is characterized by the presence of a feed collecting apparatus formed by numerous tentacles that secrete mucus to trap different feed items52. Until the present study, it has never been considered for culture or tested using IMTA conditions. The high culture density recorded in the present study at the end of experimental period (> 4000 ind. m−2) revealed the great potential that this worm presents to adapt to these systems27. The results here reported are the first FA characterization for T. lapidaria. This polychaete species exhibited the most similar FA profile to H. diversicolor, concerning total FA content and FA composition. Despite having a concentration of n-3 HUFA similar to H. diversicolor and D. neapolitana, this species exhibited the lowest n-3/n-6 HUFA ratio, due to the fact that it has a concentration of n-6 HUFA higher than all other polychaete species tested in the present work. An analysis of T. lapidaria productivity in terms of FA profile allowed us to estimate the generation of approximately 36.2 g of total FA per Kg DW biomass produced, of which approximately 15% correspond to n-3 HUFA (including EPA and DHA).
In the evaluation of which of the four IMTA-cultured polychaete species exhibited the FA profile that most resemble that of aquafeed formula (diet supplied to S. aurata produced in earthen ponds) it was concluded that H. diversicolor and T. lapidaria were the species whose FA profile displayed the highest similarity. These polychaetes revealed the higher contents of n-3 and n-6 HUFA in their composition. This allowed us to assume that both species featured an EFA profile more suitable to be integrated in premium aquafeeds formulation. Here it is important to bear in mind that the differences in n-3 and n-6 FA profile exhibited by the different polychaete species may likely be explained by its contrasting abilities to produce FA de novo.
The four polychaete species display different feeding habits and explore different trophic niches. Hediste diversicolor is considered a discrete motile polychaete, classified as an active predator53. This omnivorous species may exhibit a deposit-feeding behaviour and mainly consumes organic matter from substrate53, 54. Diopatra neapolitana is considered a discrete motile polychaete, omnivorous, a scavenger and detritus feeder39, 53, 55. Terebella lapidaria is sessile or a discretely motile polychaete and a surface deposit feeder53 that traps detritus, including unicellular algae (e.g., diatoms), and various small invertebrates (including larvae) with the mucus secreted by its tentacles, which transfers feed to its mouth52, 53. This species also benefits from sediment enrichment in POM derived from fish production56. The polychaete S. cf. pavonina is a sessile species that display filter feeding behaviour and can feed both on phytoplankton (e.g., pelagic diatoms, dinoflagellates, other unicellular algae), small invertebrates (including larvae) and POM dissolved in water column, thus contributing to making the water clearer32, 48, 53. Due to these different feeding habits, the nutrition of the four polychaete species surveyed may be more or less on dependent on the POM fraction derived from uneaten fish feed.
The present work demonstrated that it is possible to produce polychaetes biomass with high nutritional value through an eco-design concept such as IMTA, a framework that promotes a cleaner production and, in this case, allowed to recover EFA commonly wasted in aquaculture effluents. The potential of using H. diversicolor to recover nutrients, namely EFA, present in the effluent water of earthen ponds used for finfish aquaculture was confirmed. It was also shown that it is feasible to co-culture several other polychaete species in deep sand beds stocked with H. diversicolor through the natural settling of planktonic larvae (e.g., D. neapolitana, S.cf. pavonina and T. lapidaria). All species displayed different FA profiles, but all hold the potential to recover available nutrients in the effluent water and give origin to value-added biomass, rich in EFA (namely n-3 HUFA, such as EPA and DHA). The FA profile of D. neapolitana, S. cf. pavonina and T. lapidaria was described here for the first time demonstrating that it is feasible to diversify the polychaete species to be included in PASF. As polychaetes with planktonic larvae will likely always appear in IMTA designs similar to the ones described in the present work, further studies are necessary to maximize the polyculture potential of marine polychaetes using PASF.
Material and methods
Experimental set-up
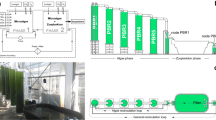
The biomass of polychaetes whose FA profiles were evaluated in present work resulted from an IMTA study performed at AlgaPlus (40° 36′ 43″ N, 8° 40′ 43″ W), an aquaculture company operating in Ria de Aveiro coastal Lagoon watershed area (western Atlantic coast of Portugal)27. The present study used the POM fraction of the effluent water from a semi-intensive production pond stocked with gilthead seabream (Sparus aurata). Approximately ≈ 12.000 fish with an average weight of 400 g were stocked, being fed twice a day (specific feeding rate ≈ 1.2% day−1) on a commercial diet with 43% crude protein, 17% crude fat and 10% crude fibre (Standard orange 4; Sorgal). The effluent water was pumped to 5 tanks arranged in a parallel set-up. Each tank had a volume of 0.1 m3, a surface area of 0.3 m2 and its bottom was covered by a 200-mm tall sand bed (0.7–1 mm grain size). A 100 mm water column was used on each tank by placing an external standpipe regulating the water level. The standpipe was also connected to a bottom draining pipe that allowed full water percolation through the sand bed. Each tank received a water flow of 25 L h−1 (0.5 tank volume renewal h−1). An image of the experimental set-up is presented in Fig. 5. The experimental trial was run for 15 weeks, from (July 2017 to November 2017) and no additional feed was supplied to the tanks with the sand bed besides the fish farm effluent. The characterisation of the environmental (Temp., oxygen, pH, salinity) and water composition (SPM, POM, TN, DIN, TP and DIP) conditions of effluent filtered by PASF, as well as the efficiency of bioremediation and productivity achieved in these systems are described in detail in Jerónimo et al.27.
Polychaetes stocking and sampling
Wild specimens of H. diversicolor were collected at Ria de Aveiro (40° 47′ 23″ N, 8° 40′ 23″ W) by local fisherman and each of the 5 tanks with a sand bed was inoculated with 440 ind. m−2 (≈ 167 g FW m−2). As the effluent originated from earthen ponds supplied by the coastal lagoon (Ria de Aveiro) was not pre-filtered, the presence of other polychaete species, namely in their larv planktonic phase was expected to co-occur in the experimental units. At the end of the experimental period, polychaetes were collected with hand core samples (Ø 75 mm, 150 mm depth; N = 5). Specimens were sorted in situ and transported to the laboratory for taxonomic identification while alive and further processing. All specimens were depurated overnight in aerated containers holding pre-combusted sterilized sand and artificial seawater to safeguard empty guts and no potential bias of FA analysis. Following depuration, all polychaetes were freeze-dried and stored at − 80 °C before further analysis.
The FA profiles of H. diversicolor stocked in the tanks was also compared with that of other polychaete species whose planktonic larvae successfully settled on the sand beds, namely Diopatra neapolitana (Onuphidae), Sabella cf. pavonina (Sabellidae) and Terebella lapidaria (Terebellidae). For each species, a composite sample per tank was used for FA analysis. The same procedure was applied to generate 5 composite samples of wild specimens of H. diversicolor from the collection site at Ria de Aveiro. For the species H. diversicolor, D. neapolitana and S. cf. pavonina 5 polychaetes were considered for each composite sample, while for T. lapidaria 20 polychaetes were considered due to the lower size of their specimens. In addition, 5 samples of fish feed were freeze dried and stored at − 80 °C before FA analysis.
FA extraction and analysis
The FA content was quantified by screening the fatty acid methyl esters (FAME) obtained through gas chromatography-mass spectrometry (GC–MS) following a well-established method currently on use in our laboratory57,58,59. To prepare the FAME all freeze-dried samples were powdered and homogeneized. Then, 1 mL of n-hexane containing 10 µg mL−1 of the internal standard C19:0 was added to 10 mg of biomass. Then, 200 µl of methalonic (MeOH) KOH solution (2 M) was added, and the tube was sealed and mixed vigorously in a vortex shaker for 2 min. Following this procedure, 2 ml of saturated NaCl solution (aqueous solution of 1 g NaCl in 100 mL Milli-Q water) was added to the tube, and the mixture was centrifuged for 5 min at 2000 rpm. Following centrifugation, 20 µL of the organic phase was transferred into another tube, was dried under a stream of nitrogen gas and store at − 20 °C until FAME analysis. Immediately before analysis, the FAME were dissolved in 100 µL of hexane and 2 µL of this solution was analysed by gas chromatography–mass spectrometry system (GC–MS) (Agilent Technologies, USA) connected to an Agilent 5973 Network Mass Selective Detector (70 eVand and m/z 50–550 in a 1 s cycle), and equipped with a DB-FFAP column (30 m long, 0.32 mm internal diameter, and 0.25 μm film thickness) (J & W Scientific, Folsom, CA).The oven temperature programmed were as follows: (1) initial temperature of 80 °C for 3 min; (2) linear increase to 160 °C (25 °C min−1); (3) linear increase to 210 °C (2 °C min−1); (4) linear increase to 250 °C (30 °C min−1); (5) standing at 250 °C for 10 min. The temperatures of injector and detector were 220 and 280 °C, respectively. Helium was used as the carrier gas (1.7 mL min−1). The FA content of the fish feed was determined, using 1 mL of n-hexane containing 0.75 µg mL−1 of internal standard (C19:0) added to 15 µg of the lipid extract. All remaining procedures were identical as described above. The FA identification was performed by matching with a previously injected standards mixture (Supelco37 Component FAME Mix, Sigma-Aldrich), as well as by comparing each MS spectrum with a database (AOCS lipid library). The FA content (µg mg−1 dry weight, DW) in the samples analysed was calculated based on an external calibration curve using a certified standard mixture (Supelco37 Component FAME Mix, Sigma-Aldrich) and C19:0 as internal standard. The FA 18:4 n-3, 22:3 n-6, 22:4 n-6, 22:5 n-3, 22:5 n-6, 16:3 n-3, 24:2, 13-methyl-14:0 iso, 14-methyl-15:0 iso &13-methyl-15:0 anteiso, 14-methyl-16:0 anteiso, 10-methyl-16:0, 7-methyl-hexadec-6-enoate and 16-methyl-17:0 iso were determined based on the reference values of the FA 18:3 n-3, 22:2, 23:0, 22:6 n-3, 22:6 n-3, 16:0, 24:1 n-9, 15:0, 16:0, 17:0, 17:0, 17:0 and18:0, respectively. In the present study, PUFA were defined as all FA with two or more double bounds, while HUFA refers to all FA with four or more double bonds.
Statistical analysis
Statistical analysis was performed using PRIMER v6 with the PERMANOVA + add-on. In order to ascertain differences in the FA content (µg mg−1 DW) of wild and cultured H. diversicolor, a 1-way analysis of similarities (ANOSIM) was performed on a resemblance matrix produced using Bray Curtis similarity coefficient of data previously transformed using the formula log (x + 1). A SIMPER analysis was also performed to evaluate which FA contributed the most to the dissimilarities recorded between samples mentioned above until a total of 50% cumulative dissimilarity was achieved. A 1-way ANOSIM and SIMPER analysis using the same criteria was used to highlight the differences in FA profiles between stocked H. diversicolor and other polychaete species whose planktonic larvae successfully settled in the experimental units (D. neapolitana, S. cf. pavonina and T. lapidaria). To determine which species displayed the FA profile that most closely resembled the FA source (aquafeed provided to fish), resemblance matrixes of the 16 most common FA between feed and polychaetes (common with at least one species) were prepared using Bray Curtis similarity coefficient of the data previously log (x + 1) transformed and then a principal coordinates analysis (PCO) plot was performed.
Those FA known to be related to the microbiome (15:0, 17:0, 17:1 n-8 and 17:1 n-9) and others (13-methyl-14:0, 14-methyl-15:0 + 13-methyl-15:0, 10-methyl-16:0, 7-methyl-hexadec-6-enoate, 16-methyl-17:0) were not included in the above-mentioned analysis. For a detailed description of all the statistical methods referred employed above please see Anderson et al.60.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Material files.
References
FAO. The State of World Fisheries and Aquaculture—Meeting the Sustainable Development Goals (Food and Agriculture Organization of the United Nations, 2018).
Gebauer, S., Psota, T., Harris, W. & Kris-Etherton, P. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 83, 1526S-1535S (2006).
Aranceta, J. & Pérez-Rodrigo, C. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: A systematic review. Br. J. Nutr. 107, S8–S22 (2012).
EFSA. Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 10(7), 1–48 (2012).
Tocher, D. R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449, 94–107 (2015).
Bischoff, A. A., Fink, P. & Waller, U. The fatty acid composition of Nereis diversicolor cultured in an integrated recirculated system: Possible implications for aquaculture. Aquaculture 296, 271–276 (2009).
Sargent, J. R., Tocher, D. R. & Bell, J. G. The Lipids. In Fish Nutrition 3rd edn (eds Halver, J. E. & Hardy, R. W.) 181–257 (Academic Press, 2003).
Ytrestøyl, T., Aas, T. S. & Åsgård, T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 448, 365–374 (2015).
Stevens, J. R., Newton, R. W., Tlusty, M. & Little, D. C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 90, 115–124 (2018).
Barrington, K., Chopin, T. & Robinson, S. Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. In Integrated Mariculture: A Global Review (ed. D. Soto). FAO Fish. Aquac. Tech. Pap. 529, 7–46 (2009).
Chopin, T. et al. Multitrophic integration for sustainable marine aquaculture. Encycl. Ecol. 1–5, 2463–2475 (2008).
Chopin, T. et al. Integrating seaweeds into marine aquaculture systems: A key towards sustainability. J. Appl. Phycol. 37, 975–986 (2001).
Custódio, M., Villasante, S., Cremades, J., Calado, R. & Lillebø, A. I. Unravelling the potential of halophytes for marine integrated multi-trophic aquaculture (IMTA)—A perspective on performance, opportunities and challenges. Aquac. Environ. Interact. 9, 445–460 (2017).
Gunning, D., Maguire, J. & Burnell, G. The development of sustainable saltwater-based food production systems: A review of established and novel concepts. Water 8, 598 (2016).
Marques, B., Calado, R. & Lillebø, A. I. New species for the biomitigation of a super-intensive marine fish farm effluent: Combined use of polychaete-assisted sand filters and halophyte aquaponics. Sci. Total Environ. 599–600, 1922–1928 (2017).
Nardelli, A. E., Chiozzini, V. G., Braga, E. S. & Chow, F. Integrated multi-trophic farming system between the green seaweed Ulva lactuca, mussel, and fish: A production and bioremediation solution. J. Appl. Phycol. 31, 847–856 (2019).
Pombo, A. et al. Insight into aquaculture’s potential of marine annelid worms and ecological concerns: a review. Rev. Aquac. 12, 107–121 (2018).
Schneider, O., Sereti, V., Eding, E. H. & Verreth, J. A. J. Analysis of nutrient flows in integrated intensive aquaculture systems. Aquac. Eng. 32, 379–401 (2005).
Wang, X., Olsen, L. M., Reitan, K. I. & Olsen, Y. Discharge of nutrient wastes from salmon farms:Eenvironmental effects, and potential for integrated multi-trophic aquaculture. Aquac. Environ. Interact. 2, 267–283 (2012).
Endut, A., Jusoh, A. & Ali, N. Nitrogen budget and effluent nitrogen components in aquaponics recirculation system. Desalin. Water Treat. 52, 744–752 (2014).
Bergström, P., Hällmark, N., Larsson, K. J. & Lindegarth, M. Biodeposits from Mytilus edulis: A potentially high-quality food source for the polychaete, Hediste diversicolor. Aquac. Int. 27, 89–104 (2019).
Bergström, P. et al. Testing the potential for improving quality of sediments impacted by mussel farms using bioturbating polychaete worms. Aquac. Res. 48, 161–176 (2017).
Marques, B. et al. Adding value to ragworms (Hediste diversicolor) through the bioremediation of a super-intensive marine fish farm. Aquac. Environ. Interact. 10, 79–88 (2018).
Pajand, Z. O., Soltani, M., Bahmani, M. & Kamali, A. The role of polychaete Nereis diversicolor in bioremediation of wastewater and its growth performance and fatty acid composition in an integrated culture system with Huso huso (Linnaeus, 1758). Aquac. Res. 48, 5271–5279 (2017).
Wang, H. et al. Growth and nutritional composition of the polychaete Hediste diversicolor (OF Müller, 1776) cultivated on waste from land-based salmon smolt aquaculture. Aquaculture 502, 232–241 (2019).
Yousefi-Garakouei, M., Kamali, A. & Soltani, M. Effects of rearing density on growth, fatty acid profile and bioremediation ability of polychaete Nereis diversicolor in an integrated aquaculture system with rainbow trout (Oncorhynchus mykiss). Aquac. Res. 50, 725–735 (2019).
Jerónimo, D., Lillebø, A. I., Santos, A., Cremades, J. & Calado, R. Performance of polychaete assisted sand filters under contrasting nutrient loads in an integrated multi-trophic aquaculture (IMTA) system. Sci. Rep. 10, 1–10 (2020).
Jerónimo, D., Lillebø, A. I., Cremades, J., Cartaxana, P. & Calado, R. Recovering wasted nutrients from shrimp farming through the combined culture of polychaetes and halophytes. Sci. Rep. 11, 6587 (2021).
Palmer, P. J. Polychaete-assisted sand filters. Aquaculture 306, 369–377 (2010).
Brown, N., Eddy, S. & Plaud, S. Utilization of waste from a marine recirculating fish culture system as a feed source for the polychaete worm, Nereis virens. Aquaculture 322–323, 177–183 (2011).
Gómez, S., Hurtado, C. F., Orellana, J., Valenzuela-Olea, G. & Turner, A. Abarenicola pusilla (Quatrefages, 1866): A novel species for fish waste bioremediation from marine recirculating aquaculture systems. Aquac. Res. 49, 1363–1367 (2017).
Giangrande, A. et al. Utilization of the filter feeder polychaete Sabella spallanzanii Gmelin (Sabellidae) as bioremediator in aquaculture. Aquac. Int. 13, 129–136 (2005).
Santos, A. et al. Effect of three diets on the growth and fatty acid profile of the common ragworm Hediste diversicolor (O.F. Müller, 1776). Aquaculture 465, 37–42 (2016).
Luis, O. J. & Passos, A. M. Seasonal changes in lipid content and composition of the polychaete Nereis (Hediste) diversicolor. Comp. Biochem. Physiol. 111B, 579–586 (1995).
Luis, O. J. & Ponte, A. C. Control of reproduction of the shrimp Penaeus kerathurus held in captivity. J. World Aquac. Soc. 24, 31–39 (1993).
Watson, G. J., Murray, J. M., Schaefer, M. & Bonner, A. Bait worms: A valuable and important fishery with implications for fisheries and conservation management. Fish Fish. 18, 374–388 (2016).
Fidalgo e Costa, P. et al. The market features of imported non-indigenous polychaetes in Portugal and consequent ecological concerns. Sci. Mar. 70, 287–292 (2006).
Olive, P. J. W. Polychaete aquaculture and polychaete science: A mutual synergism. Hydrobiologia 402, 175–183 (1999).
Xenarios, S., Queiroga, H., Lillebø, A. & Aleixo, A. Introducing a regulatory policy framework of bait fishing in European coastal lagoons: The case of Ria de Aveiro in Portugal. Fishes 3, 2 (2018).
Palmer, P. J., Wang, S., Houlihan, A. & Brock, I. Nutritional status of a nereidid polychaete cultured in sand filters of mariculture wastewater. Aquac. Nutr. 20, 675–691 (2014).
Qian, P. Reproductive strategies and developmental patterns in annelids. Hydrobiologia 402, 239–253 (1999).
Cunha, T., Hall, A. & Queiroga, H. Estimation of the Diopatra neapolitana annual harvest resulting from digging activity in Canal de Mira, Ria de Aveiro. Fish. Res. 76, 56–66 (2005).
Arias, A., Paxton, H. & Budaeva, N. Redescription and biology of Diopatra neapolitana (Annelida: Onuphidae), a protandric hermaphrodite with external spermaducal papillae. Estuar. Coast. Shelf Sci. 174, 1–17 (2016).
Carregosa, V. et al. Physiological and biochemical responses of the Polychaete Diopatra neapolitana to organic matter enrichment. Aquat. Toxicol. 155, 32–42 (2014).
Bailey-Brock, J. H. Ecology of the tube-building polychaete Diopatra leuckarti Kinberg, 1865 (Onuphidae) in Hawaii: Community structure, and sediment stabilizing properties. Zool. J. Linn. Soc. 1865, 191–199 (1984).
Thomsen, M. S., Muth, M. F. & McGlathery, K. J. Tube-forming polychaetes enhance invertebrate diversity and abundance in sandy sediments of Mozambique, Africa. Afr. J. Mar. Sci. 33, 327–332 (2011).
Wells, G. P. On the behaviour of sabella. R. Soc. 138, 278–299 (1951).
Knight-jones, P. & Perkins, T. H. A revision of Sabella, Bispira and Stylomma (Polychaeta: Sabellidae). Zool. J. Linn. Soc. 123, 385–467 (1998).
Murray, J. M., Watson, G. J., Giangrande, A., Bentley, M. G. & Farrell, P. Reproductive biology and population ecology of the marine fan worm Sabella pavonina (savigny) (polychaeta: Sabellidae). Invertebr. Reprod. Dev. 55, 183–196 (2011).
Dales, R. P. Some quantitative aspects of feeding in Sabellid and Serpulid fan worms. J. Mar. Biol. Assoc. UK 36, 309–316 (1956).
Rouse, G. W. & Pleijel, F. Polychaetes (ed. Westheide, W.) (Oxford University Press, 2001).
Sutton, M. F. The feeding mechanism, functional morphology and histology of the alimentary canal of Terebella lapidaria L. (Polychaeta). Proc. Zool. Soc. Lond. 129, 487–523 (1957).
Fauchald, K. & Jumars, P. A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. 17, 193–284 (1979).
Reise, K. Spatial configurations generated by motile benthic polychaetes. Helgoländer Wiss. Meeresunters. 32, 55–72 (1979).
Daǧli, E., Ergen, Z. & Çinar, M. E. One-year observation on the population structure of Diopatra neapolitana Delle Chiaje (Polychaeta: Onuphidae) in Izmir Bay (Aegean Sea, eastern Mediterranean). Mar. Ecol. 26, 265–272 (2005).
Carvalho, S., Barata, M., Gaspar, M. B., Pousão-Ferreira, P. & Cancela da Fonseca, L. Enrichment of aquaculture earthen ponds with Hediste diversicolor: Consequences for benthic dynamics and natural productivity. Aquaculture 262, 227–236 (2007).
Aued-Pimentel, S., Lago, J. H. G., Chaves, M. H. & Kumagai, E. E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 1054, 235–239 (2004).
Maciel, E. et al. Polar lipidome profiling of Salicornia ramosissima and Halimione portulacoides and the relevance of lipidomics for the valorization of halophytes. Phytochemistry 153, 94–101 (2018).
Melo, T. et al. Lipidomics as a new approach for the bioprospecting of marine macroalgae—unraveling the polar lipid and fatty acid composition of Chondrus crispus. Algal Res. 8, 181–191 (2015).
Anderson, M. J., Gorley, R. N. & Clarke, K. R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. 1–214.
Acknowledgements
This work was financially supported by project “AquaMMIn-Development and validation of a modular integrated multitrophic aquaculture system for marine and brackish water species” (MAR-02.01.01-FEAMP-0038) co-funded by Portugal 2020 and the European Union through Mar2020, the Operational Programme (OP) for the European Maritime and Fisheries Fund (EMFF) in Portugal and by the Integrated Programme of SR&TD “SmartBioR-Smart Valorization of Endogenous Marine Biological Resources Under a Changing Climate” (Centro-01-0145-FEDER-000018), co-funded by Centro 2020 program, Portugal 2020, European Union, through the European Regional Development Fund. Daniel Jerónimo acknowledges FCT (Portuguese Foundation for Science and Technology) by financially supporting his PhD grant (PD/BD/127989/2016). Thanks for financial support are also due to CESAM (UIDB/50017/2020 + UIDP/50017/2020), to FCT/MEC through national funds, and the cofounding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020. The authors would also like to thank Rui Pereira and Helena Abreu for providing the conditions to perform this study at AlgaPlus facilities. Thanks are also due to Ascensão A. Ravara, Luísa Marques and Andreia Santos for the technical support provided in the identification of polychaetes and to Luísa Marques and Elisabete da Costa for FA profile characterization performed in the fish feed.
Author information
Authors and Affiliations
Contributions
D.J., R.C., A.I.L. and J.C. conceived and designed the experiment; D.J. and E.M. performed the experiment; D.J., M.R.D., E.M. analysed the data; E.M., M.R.D., A.I.L. and R.C. contributed with reagents/materials/analysis tools; D.J. lead the manuscript writing and all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jerónimo, D., Lillebø, A.I., Maciel, E. et al. Unravelling the fatty acid profiles of different polychaete species cultured under integrated multi-trophic aquaculture (IMTA). Sci Rep 11, 10812 (2021). https://doi.org/10.1038/s41598-021-90185-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90185-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.